Potential of Chamomile recutita Plant Material to Inhibit Urease Activity and Reduce NH3 Volatilization in Two Agricultural Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Experimental Design
2.3. Docking Study
2.4. Statistical Analysis
3. Results
3.1. Soil NH3 Volatilization
3.2. Urea Hydrolysis and Urease Activity
3.3. Concentration of NH4+ and NO3−
3.4. Enzyme Docking Study
4. Discussion
4.1. Urease Inhibition by Chamomile Recutita Plant Materials and NBPT in Two Soils
4.2. Possible Underlying Mechanisms for Plant Materials Retarding Urease Activities and NH3 Volatilization in Tested Soils
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Wang, G.; Guo, T.; Xing, Y.; Zhang, F. Effects of plastic mulch and nitrogen fertilizer on the soil microbial community, enzymatic activity and yield performance in a dryland maize cropping system. Eur. J. Soil Sci. 2021, 72, 400–412. [Google Scholar] [CrossRef]
- Hojito, M.; Hayashi, K.; Matsuura, S. Ammonia exchange on grasslands in an intensive dairying region in central Japan. Jpn. J. Soil Sci. Plant Nutr. 2010, 56, 503–511. [Google Scholar] [CrossRef]
- Dawar, K.; Zaman, M.; Rowarth, J.S.; Blennerhassett, J.; Turnbull, M.H. Urease inhibitor reduces N losses and improves plant-bioavailability of urea applied in fine particle and granular forms under field conditions. Agric. Ecosyst. Environ. 2011, 144, 41–50. [Google Scholar] [CrossRef]
- Arango, J.; Moreta, D.; Nunez, J.; Hartmann, K.; Dominguez, M.; Ishitani, M.; Rao, I. Developing methods to evaluate phenotypic variability in biological nitrification inhibition (BNI) capacity of Brachiaria grasses. Trop. Grassl. 2014, 2, 6–8. [Google Scholar] [CrossRef]
- Boyer, E.W.; Goodale, C.L.; Jaworski, N.A.; Howarth, R.W. Anthropogenic nitrogen sources and relationships to riverine nitrogen export in the northeastern USA. Biogeochemistry 2002, 57, 137–169. [Google Scholar] [CrossRef]
- Arora, K.; Srivastava, A. Nitrogen losses due to nitrification: Plant based remedial prospects. Int. J. Bioassays 2013, 2, 984–991. [Google Scholar]
- Qiu, H.; Sun, D.; Gunatilake, S.R.; She, J.; Mlsna, T.E. Analysis of trace dicyandiamide in stream water using solid phase extraction and liquid chromatography UV spectrometry. J. Environ. Sci. 2015, 35, 38–42. [Google Scholar] [CrossRef]
- Hendrickson, L.L.; Douglass, E.A. Metabolism of the urease inhibitor N-(n-butyl) thiophosphoric triamide (NBPT) in soils. Soil Biol. Biochem. 1993, 25, 1613–1618. [Google Scholar] [CrossRef]
- Cantarella, H.; Trivelin, P.C.O.; Contin, T.L.M.; Dias, F.L.F.; Rossetto, R.; Marcelino, R.; Coimbra, R.B.; Quaggio, J.A. Ammonia volatilisation from urease inhibitor-treated urea applied to sugarcane trash blankets. Sci. Agric. 2008, 65, 397–401. [Google Scholar] [CrossRef]
- Sutton, M.A.; Bleeker, A.; Howard, C.M.; Bekunda, M.; Grizzetti, B.; de Vries, W.; van Grinsven, H.J.M.; Abrol, Y.P.; Adhya, T.K.; Billen, G.; et al. Our Nutrient World: The Challenge to Produce More Food and Energy with Less Pollution. 2013, pp. 1–114. Available online: https://www.unep.org/resources/report/our-nutrient-world-challenge-produce-more-food-and-energy-less-pollution (accessed on 10 September 2021).
- Patra, D.D.; Kiran, U.; Pande, P. Urease and nitrification retardation properties in natural essential oils and their byproducts. Commun. Soil Sci. Plant Anal. 2006, 37, 1663–1673. [Google Scholar] [CrossRef]
- Suescun, F.; Paulino, L.; Zagal, E.; Ovalle, C.; Munoz, C. Plant extracts from the Mediterranean zone of Chile potentially affect soil microbial activity related to N transformations: A laboratory experiment. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2012, 62, 556–564. [Google Scholar] [CrossRef]
- Fonseca, J.M.; Rushing, J.W.; Rajapakse, N.C.; Thomas, R.L.; Riley, M.B. Potential implications of medicinal plant production in controlled environments: The case of feverfew (Tanacetum parthenium). HortScience 2006, 41, 531–535. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Hurtado, M.D.P.; Ono, H.; Moreta, D.E.; Salcedo, A.F.; Yoshihashi, A.T.; Ishikawa, T.; Ishitani, M.; Ohnishi-Kameyama, M.; et al. Evidence for biological nitrification inhibition in Brachiaria pastures. Proc. Nat. Acad. Sci. USA 2009, 106, 17302–17307. [Google Scholar] [CrossRef]
- DeLuca, T.; Nilsson, M.C.; Zackrisson, O. Nitrogen mineralization and phenol accumulation along a fire chronosequence in northern Sweden. Oecologia 2002, 133, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.K.; Manzoor, M. Effect of soil-applied calcium carbide and plant derivatives on nitrification inhibition and plant growth promotion. Int. J. Environ. Sci. Technol. 2013, 10, 961–972. [Google Scholar] [CrossRef][Green Version]
- Opoku, A.; Chaves, B.; De Neve, S. Neem seed oil: A potent nitrification inhibitor to control nitrate leaching after incorporation of crop residues. Biol. Agric. Hortic. 2014, 30, 145–152. [Google Scholar] [CrossRef]
- Ram, M.; Patra, D.D.; Subrahmaniyam, K.; Singh, D.V. Nitrification inhibitory properties in Mentha-spent and pyrethrum flowers. J. Indian. Soc. Soil Sci. 1993, 41, 176–177. [Google Scholar]
- Mohanty, S.; Patra, A.K.; Chhonkar, P.K. Neem (Azadirachta indica) seed kernel powder retards urease and nitrification activities in different soils at contrasting moisture and temperature regimes. Bioresour. Technol. 2008, 99, 894–899. [Google Scholar] [CrossRef]
- Northup, R.R.; Yu, Z.; Dahlgren, R.A.; Vogt, K.A. Polyphenol control of nitrogen release from pine litter. Nature 1995, 377, 227–229. [Google Scholar] [CrossRef]
- Schmidt, B.M. Responsible use of medicinal plants for cosmetics. HortScience 2012, 47, 985–991. [Google Scholar] [CrossRef]
- Yunfeng, S.; Lili, Z.; Muqiu, Z.; Xiaoxiong, X. Inhibitory effects of aqueous extracts of tropical forest plants on urea hydrolysis and nitrification in soil. Appl. Mech. Mater. 2014, 587–589, 877–881. [Google Scholar]
- Tabatabai, M.A. Soil enzymes. In Method of Soil Analysis, Part 2-Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Eds.; Marcel Dekker Press: New York, NY, USA, 1 January 1994; pp. 775–833. [Google Scholar]
- Mulvaney, R.L.; Bremner, J.M. A modified diacetylmonoxime method for colorimetric determination of urea in soil extracts. Commun. Soil Sci. Plan. 1979, 10, 163–1170. [Google Scholar] [CrossRef]
- Balasubramanian, A.K.; Ponnuraj, J. Crystal structure of the first plant urease from jack bean: 83 years of journey from its first crystal to molecular structure. Mol. Biol. 2010, 400, 274–283. [Google Scholar] [CrossRef]
- Musiani, F.; Arnofi, E.; Casadio, R.; Ciurli, S. Structure-based computational study of the catalytic and inhibition mechanisms of urease. J. Biol. Inorg. Chem. 2001, 6, 300–314. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Chhonkar, P.K. Adsorption of urease on clays saturated with different cations. J. Indian Soc. Soil. Sci. 1982, 30, 27–32. [Google Scholar]
- Kumar, V.; Wagenet, R.J. Urease activity and kinetics of urea transformation in soils. Soil Sci. 1984, 137, 263–269. [Google Scholar] [CrossRef]
- Kumar, U.; Jain, M.C.; Kumar, S.; Pathak, H.; Majumdar, D. Role of nitrification inhibitors on nitrous oxide emissions in a fertilized alluvial clay loam under different moisture regimes. Curr. Sci. 2000, 79, 224–228. [Google Scholar]
- Zantua, M.I.; Bremner, J.M. Production and persistence of urease activity in soil. Soil Biol. Biochem. 1976, 8, 369–374. [Google Scholar] [CrossRef]
- Patra, A.K.; Burford, J.R.; Rego, T.J. Volatilization losses of surface applied urea nitrogen from Vertisols in the Indian semi-arid tropics. Biol. Fert. Soils 1996, 22, 345–349. [Google Scholar] [CrossRef]
- Marinari, S.; Masciandaro, G.; Ceccanti, B.; Grego, S. Influence of organic and mineral fertilisers on soil biological and physical properties. Bioresour. Technol. 2000, 72, 9–17. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Relationships between soil urease activity and other properties of some tropical wetland rice soils. Fert. Res. 1983, 8, 123–127. [Google Scholar] [CrossRef]
- Singh, J.; Kunhikrishnan, A.; Bolan, N.S.; Saggar, S. Impact of urease inhibitor on ammonia and nitrous oxide emissions from temperate pasture soil cores receiving urea fertilizer and cattle urine. Sci. Total Environ. 2013, 465, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Suter, H.; Sultana, H.; Turner, D.; Davies, R.; Walker, C.; Chen, D.L. Influence of urea fertiliser formulation, urease inhibitor and season on ammonia loss from ryegrass. Nutr. Cycl. Agroecosyst. 2013, 95, 175–185. [Google Scholar] [CrossRef]
- Sherlock, R.R.; Black, A.S.; Smith, N.P. Micro-environment soil pH around broadcast urea granules and its relationship to ammonia volatilization. In Nitrogen Cycling in Temperate Agricultural Systems; Bacon, P.E., Evans, J., Stonier, R.R., Taylor, A.C., Eds.; Australian Society of Soil Science: Wagga, Australia, 1987; pp. 316–326. [Google Scholar]
- Rauha, J.P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Lodhi, M.A.K.; Killingbeck, K.T. Allelopathic inhibition of nitrification and nitrifying bacteria in a ponderosa pine (Pinus ponderosa Dougl.) community. Am. J. Bot. 1980, 76, 1423–1429. [Google Scholar] [CrossRef]
- Vandevivere, P.; Ficara, E.; Terras, C.; Julies, E.; Versraete, W. Copper-mediated selective removal of nitrification inhibitors from industrial wastewaters. Environ. Sci. Technol. 1998, 32, 1000–1006. [Google Scholar] [CrossRef]
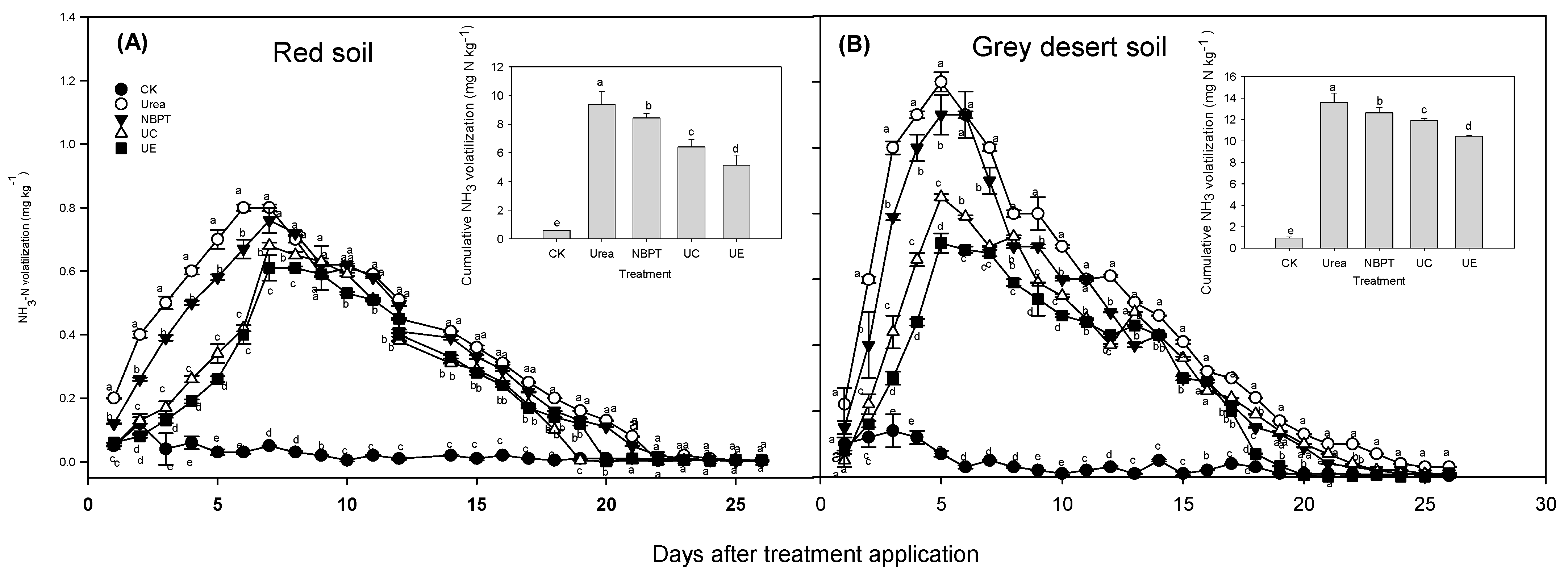
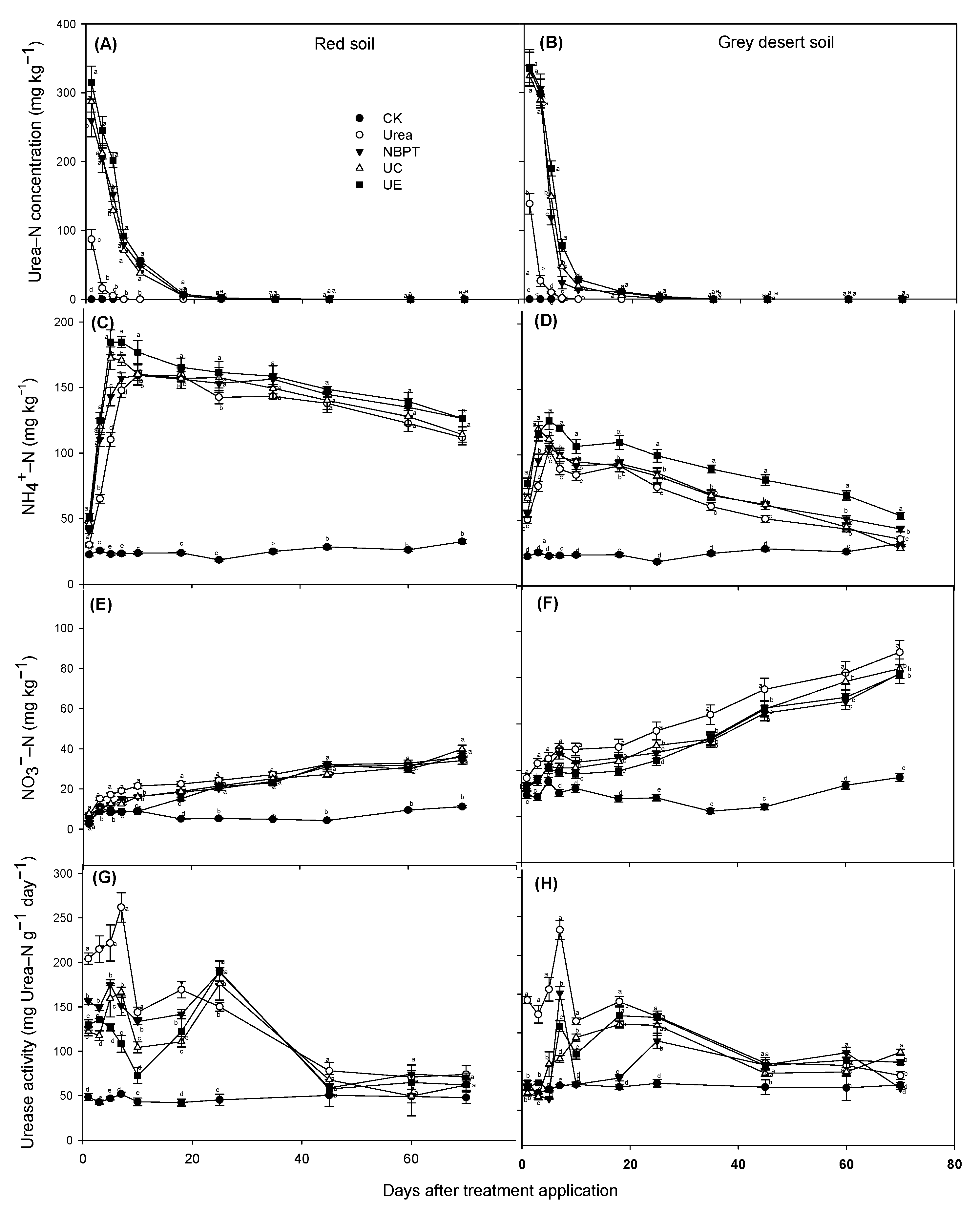
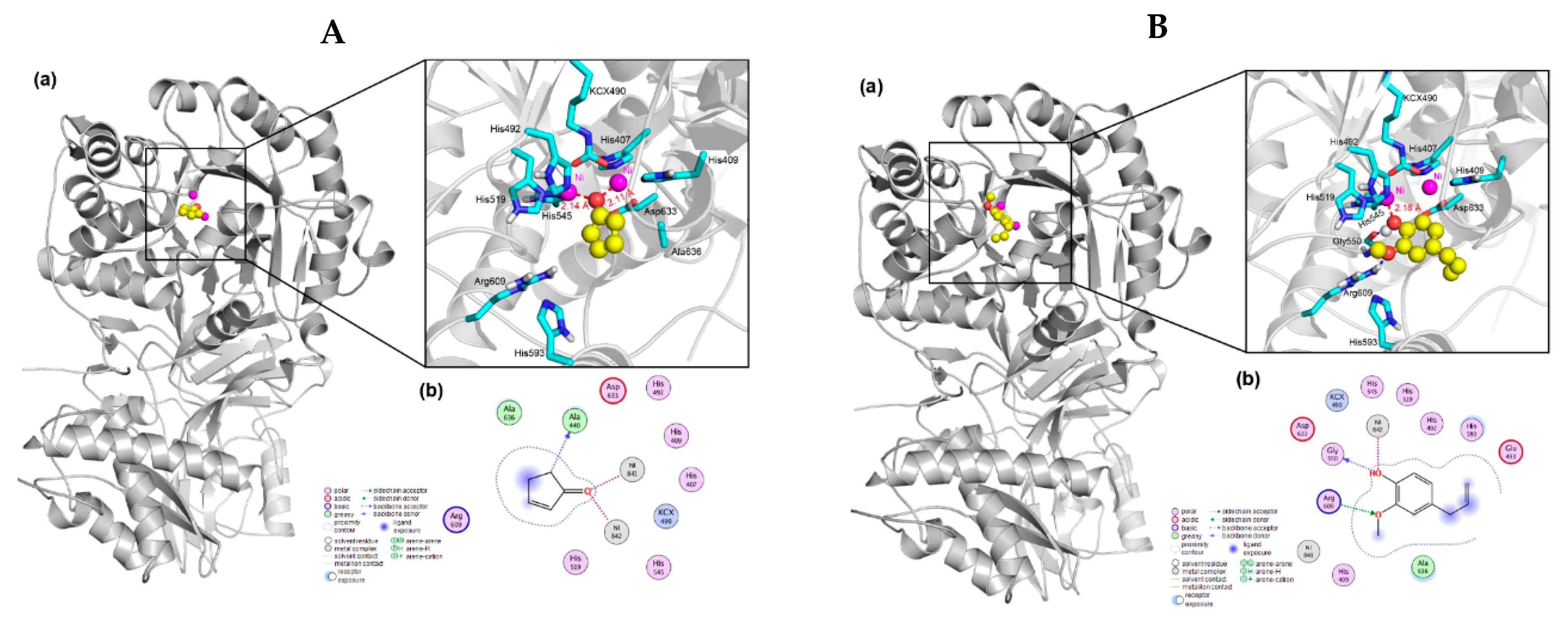
| Soil Types | MAT (°C) | MAP (mm) | pH (Kcl/Cacl) | TP (g kg−1) | TN (g kg−1) | Available N (mg kg−1) | Organic Carbon (g kg−1) | Urease Activity (mg Urea–N kg−1 h−1) | Electrical Conductivity (μ S cm−1) |
|---|---|---|---|---|---|---|---|---|---|
| Red soil | 17.8 | 1665 | 4.7 | 1.2 | 2.06 | 70.8 | 10.8 | 36.7 | 60.1 |
| Grey desert soil | 8.0–8.5 | 180–270 | 8.0 | 0.48 | 2.02 | 64.3 | 15.7 | 48.6 | 125.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, S.; Luo, J.; Lindsey, S.; Wang, L.; Zhang, L.; Shi, Y. Potential of Chamomile recutita Plant Material to Inhibit Urease Activity and Reduce NH3 Volatilization in Two Agricultural Soils. Atmosphere 2021, 12, 1223. https://doi.org/10.3390/atmos12091223
Li J, Wang S, Luo J, Lindsey S, Wang L, Zhang L, Shi Y. Potential of Chamomile recutita Plant Material to Inhibit Urease Activity and Reduce NH3 Volatilization in Two Agricultural Soils. Atmosphere. 2021; 12(9):1223. https://doi.org/10.3390/atmos12091223
Chicago/Turabian StyleLi, Jie, Shuai Wang, Jiafa Luo, Stuart Lindsey, Lingli Wang, Lei Zhang, and Yuanliang Shi. 2021. "Potential of Chamomile recutita Plant Material to Inhibit Urease Activity and Reduce NH3 Volatilization in Two Agricultural Soils" Atmosphere 12, no. 9: 1223. https://doi.org/10.3390/atmos12091223
APA StyleLi, J., Wang, S., Luo, J., Lindsey, S., Wang, L., Zhang, L., & Shi, Y. (2021). Potential of Chamomile recutita Plant Material to Inhibit Urease Activity and Reduce NH3 Volatilization in Two Agricultural Soils. Atmosphere, 12(9), 1223. https://doi.org/10.3390/atmos12091223







