Winter Air Pollution and Genotoxic Effects in Children Living in a Highly Polluted Urban Area
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Comet Assay in Salivary Leukocytes
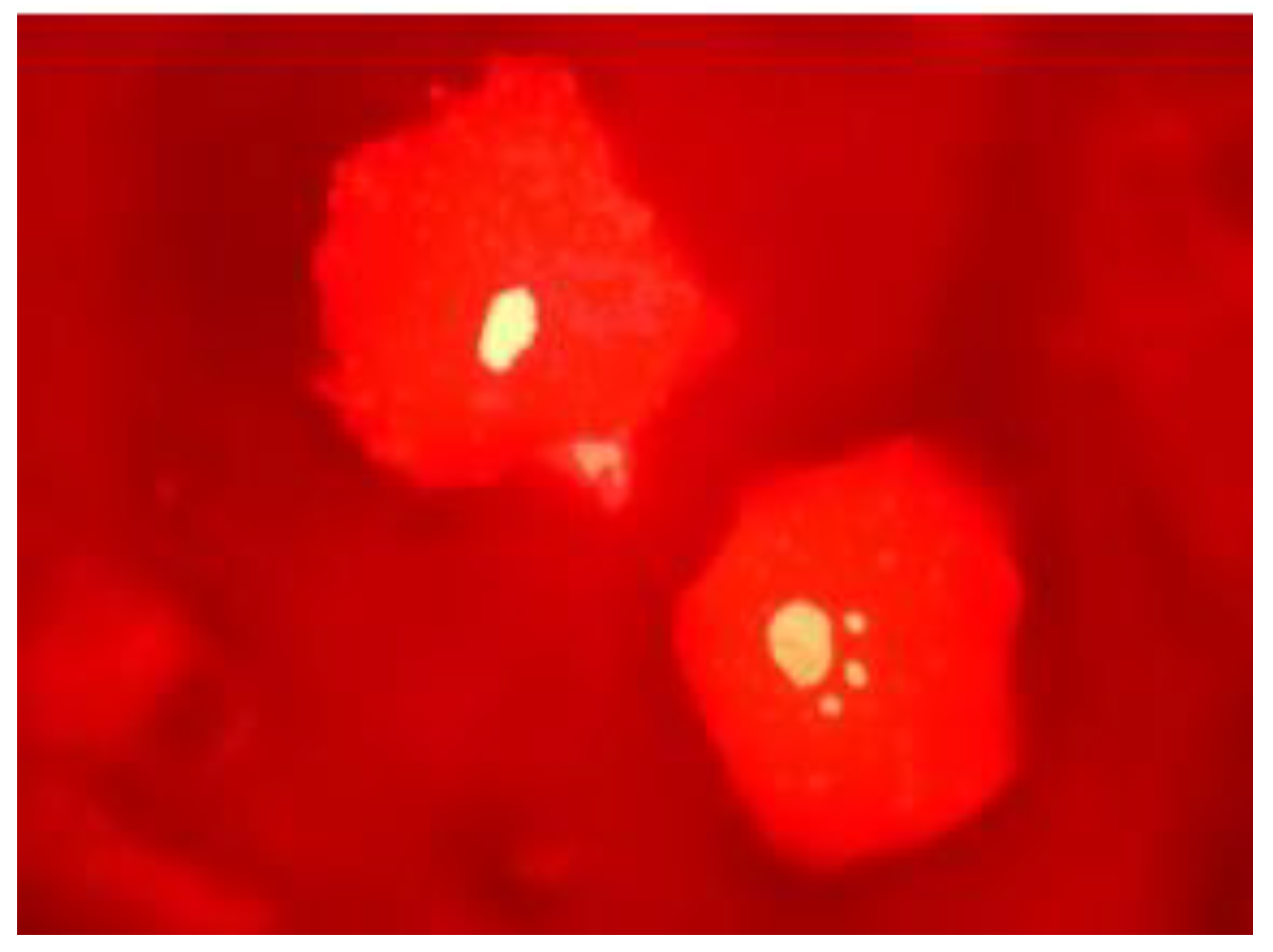
2.3. Micronucleus Test in Buccal Cells
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Outdoor Air Pollution; IARC Publications: Lion, France, 2016; Volume 109, ISBN 9789283201755. [Google Scholar]
- EAA. Air Quality in Europe 2019. European Environment Agency, Technical Report No 10/2019; Luxembourg: Publications Office of the European Union: Copenhagen, Denmark, 2019. [Google Scholar]
- EAA. Air Quality in Europe 2020. European Environment Agency, Technical Report No 9/2020; Luxembourg: Publications Office of the European Union: Copenhagen, Denmark, 2020. [Google Scholar]
- WHO. Air Quality Guidelines: Global Update 2005—Particulate Matter, Ozone, Nitrogen Dioxide and Sulphur Dioxide; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2006. [Google Scholar]
- Wang, Q.; Li, J.; Yang, J.; Chen, Y.; Li, Y.; Li, S.; Xie, C.; Chen, C.; Wang, L.; Wang, L.; et al. Seasonal characterization of aerosol composition and sources in a polluted city in Central China. Chemosphere 2020, 258, 127310. [Google Scholar] [CrossRef] [PubMed]
- Pongpiachan, S.; Hattayanone, M.; Suttinun, O.; Khumsup, C.; Kittikoon, I.; Hiruyatrakul, P.; Cao, J. Assessing human exposure to PM10-bound polycyclic aromatic hydrocarbons during fireworks displays. Atmos. Pollut. Res. 2017, 8, 816–827. [Google Scholar] [CrossRef]
- Pongpiachan, S.; Kositanont, C.; Palakun, J.; Liu, S.; Ho, K.F.; Cao, J. Effects of day-of-week trends and vehicle types on PM 2.5-bounded carbonaceous compositions. Sci. Total Environ. 2015, 532, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Topinka, J.; Rossner, P., Jr.; Milcova, A.; Schmuczerova, J.; Pencikova, K.; Rossnerova, A.; Ambrož, A.; Štolcpartová, J.; Bendl, J.; Hovorka, J.; et al. Day-to-day variability of toxic events induced by organic compounds bound to size segregated atmospheric aerosol. Environ. Pollut. 2015, 202, 135–145. [Google Scholar] [CrossRef]
- Srimuruganandam, B.; Nagendra, S.S. Source characterization of PM10 and PM2.5 mass using a chemical mass balance model at urban roadside. Sci. Total Environ. 2012, 433, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.G.; Gualtieri, M.; Ferrero, L.; Lo Porto, C.; Udisti, R.; Bolzacchini, E.; Camatini, M. Seasonal variations in chemical composition and in vitro biological effects of fine PM from Milan. Chemosphere 2010, 78, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Pey, J.; Querol, X.; Alastuey, A. Discriminating the regional and urban contributions in the North Western Mediterranean: PM levels and composition. Atmos. Environ. 2010, 44, 1587–1596. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Feretti, D.; Pedrazzani, R.; Ceretti, E.; Dal Grande, M.; Zerbini, I.; Viola, G.C.V.; Gelatti, U.; Donato, F.; Zani, C. “Risk is in the air”: Polycyclic aromatic hydrocarbons, metals and mutagenicity of atmospheric particulate matter in a town of Northern Italy (Respira study). Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 842, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Bonetta, S.; Bonetta, S.; Schilirò, T.; Ceretti, E.; Feretti, D.; Covolo, L.; Vannini, S.; Villarini, M.; Moretti, M.; Verani, M.; et al. Mutagenic and genotoxic effects induced by PM0.5 of different Italian towns in human cells and bacteria: The MAPEC_LIFE study. Environ. Pollut. 2019, 245, 1124–1135. [Google Scholar] [CrossRef]
- Palacio, I.C.; Barros, S.B.M.; Roubucek, D.A. Water-soluble and organic extracts of airborne particulate matter induce micronuclei in human lung epithelial A549 cells. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 812, 1–11. [Google Scholar] [CrossRef]
- Ceretti, E.; Zani, C.; Zerbini, I.; Viola, G.; Moretti, M.; Villarini, M.; Dominici, L.; Monarca, S.; Ferettia, D. Monitoring of volatile and non-volatile urban air genotoxins using bacteria, human cells and plants. Chemosphere 2015, 120, 221–229. [Google Scholar]
- Dumax-Vorzet, A.F.; Tate, M.; Walmsley, R.; Elder, R.H.; Povey, A.C. Cytotoxicity and genotoxicity of urban particulate matter in mammalian cells. Mutagenesis 2015, 30, 621–633. [Google Scholar] [CrossRef]
- Traversi, D.; Cervella, P.; Gilli, G. Evaluating the genotoxicity of urban PM2.5 using PCR-based methods in human lung cells and the Salmonella TA98 reverse test. Environ. Sci. Pollut. Res. Int. 2015, 22, 1279–1289. [Google Scholar] [CrossRef]
- Lepers, C.; Dergham, M.; Armand, L.; Billet, S.; Verdin, A.; Andre, V. Mutagenicity and clastogenicity of native airborne particulate matter samples collected under industrial, urban or rural influence. Toxicol. In Vitro 2014, 28, 866–874. [Google Scholar] [CrossRef]
- De Brito, K.C.; Lemos, C.T.; Rocha, J.A.; Mielli, A.C.; Matzenbacher, C.; Vargas, V.M. Comparative genotoxicity of airborne particulate matter (PM2.5) using Salmonella, plants and mammalian cells. Ecotoxicol. Environ. Saf. 2013, 94, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.T.; Coronas, M.V.; Rocha, J.A.; Vargas, V.M. Mutagenicity of particulate matter fractions in areas under the impact of urban and industrial activities. Chemosphere 2012, 89, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Claxton, L.D.; Woodall, G.M. A review of the mutagenicity and rodent carcinogenicity of ambient air. Mutat. Res. 2007, 636, 36–94. [Google Scholar] [CrossRef]
- Coronas, M.V.; Rocha, J.A.; Salvadori, D.M.; Vargas, V.M. Evaluation of area contaminated by wood treatment activities: Genetic markers in the environment and in the child population. Chemosphere 2016, 144, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Bonassi, S.; Knudsen, L.E.; Sram, R.J.; Holland, N.; Ugolini, D.; Merlo, D.F. Children’s exposure to environmental pollutants and biomarkers of genetic damage. I. Overview and critical issues. Mutat. Res. 2006, 612, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Ugolini, D.; Bonassi, S.; Fucic, A.; Holland, N.; Knudsen, L.E.; Šrám, R.J.; Ceppi, M.; Bocchini, V.; Merlo, D.F. Children’s exposure to environmental pollutants and biomarkers of genetic damage. II. Results of a comprehensive literature search and meta-analysis. Mutat. Res. 2006, 612, 14–39. [Google Scholar] [CrossRef] [PubMed]
- Szeto, Y.T.; Benzie, I.F.; Collins, A.R.; Choi, S.W.; Cheng, C.Y.; Yow, C.M.; Tse, M.M. A buccal cell model comet assay: Development and evaluation for human biomonitoring and nutritional studies. Mutat. Res. 2005, 578, 371–381. [Google Scholar] [CrossRef]
- Gajski, G.; Gerić, M.; Oreščanin, V.; Garaj-Vrhovac, V. Cytogenetic status of healthy children assessed with the alkaline comet assay and the cytokinesis-block micronucleus cytome assay. Mutat. Res. 2013, 750, 55–62. [Google Scholar] [CrossRef]
- Wild, C.P.; Kleinjans, J. Children and increased susceptibility to environmental carcinogens: Evidence or empathy? Cancer Epidemiol. Biomark. Prev. 2003, 2, 1389–1394. [Google Scholar]
- Feretti, D.; Ceretti, E.; De Donno, A.; Moretti, M.; Carducci, A.; Bonetta, S.; Marrese, M.R.; Bonette, A.; Covolo, L.; Bagordo, F.; et al. Monitoring air pollution effects on children for supporting public health policy: The protocol of the prospective cohort MAPEC study. BMJ Open 2014, 4, e006096. [Google Scholar] [CrossRef]
- Zani, C.; Donato, F.; Grioni, S.; Viola, G.C.V.; Ceretti, E.; Feretti, D.; Festa, A.; Bonizzoni, S.; Bonnetti, A.; Monarca, S.; et al. Feasibility and reliability of a questionnaire for evaluation of the exposure to indoor and outdoor air pollutants, diet and physical activity in 6-8-year-old children. Ann. Ig. 2015, 27, 646–656. [Google Scholar]
- Agnoli, C.; Krogh, V.; Grioni, S.; Sieri, S.; Palli, D.; Masala, G.; Sacerdote, C.; Vineis, P.; Tumino, R.; Frasca, G.; et al. A Priori–Defined Dietary Patterns Are Associated with Reduced Risk of Stroke in a Large Italian Cohort. J. Nutr. 2011, 141, 1552–1558. [Google Scholar] [CrossRef]
- Zani, C.; Ceretti, E.; Grioni, S.; Viola, G.C.V.; Donato, F.; Feretti, D.; Festa, A.; Bonizzoni, S.; Bonnetti, A.; Monarca, S.; et al. Are 6–8 years old Italian children moving away from the Mediterranean diet? Ann. Ig. 2016, 28, 339–348. [Google Scholar]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Thomas, P.; Fenech, M. Buccal Micronucleus Cytome Assay. In DNA Damage Detection In Situ, Ex Vivo, and In Vivo. Met Mol Biology (Methods and Protocols); Didenko, V., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 235–248. [Google Scholar]
- Bolognesi, C.; Knasmueller, S.; Nersesyan, A.; Thomas, P.; Fenech, M. The HUMNxl scoring criteria for different cell types and nuclear anomalies in the buccal micronucleus cytome assay—An update and expanded photogallery. Mutat. Res. 2013, 753, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Bonassi, S.; Coskun, E.; Ceppi, M.; Lando, C.; Bolognesi, C.; Burgaz, S.; Holland, N.; Kirsh-Volders, M.; Knasmueller, S.; Zeiger, E.; et al. The HUman MicroNucleus project on eXfoLiated buccal cells (HUMNXL): The role of life-style, host factors, occupational exposures, health status, and assay protocol. Mutat. Res. Mutat. Res. 2011, 728, 88–97. [Google Scholar] [CrossRef]
- Karahalil, B.; Karakaya, A.E.; Burgaz, S. The micronucleus assay in exfoliated buccal cells: Application to occupational exposure to polycyclic aromatic hydrocarbons. Mutat. Res. 1999, 442, 29–35. [Google Scholar] [CrossRef]
- Villarini, M.; Levorato, S.; Salvatori, T.; Ceretti, E.; Bonetta, S.; Carducci, A.; Grassi, T.; Vannini, S.; Donato, F.; Bonetta, S.; et al. Buccal micronucleus cytome assay in primary school children: A descriptive analysis of the MAPEC_LIFE multicenter cohort study. Int. J. Hyg. Environ. Health 2018, 221, 883–892. [Google Scholar] [CrossRef]
- Ceretti, E.; Feretti, D.; Viola, G.C.V.; Zerbini, I.; Limina, R.M.; Zani, C.; Capelli, M.; Lamera, R.; Donato, F.; Gelatti, U. DNA Damage in Buccal Mucosa Cells of Pre-School Children Exposed to High Levels of Urban Air Pollutants. PLoS ONE 2014, 9, e96524. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, T.; Roy, S.; Basu, C.; Ganguly, S.; Ray, M.R.; Lahiri, P. Air pollution in Calcutta elicits adverse pulmonary reaction in children. Indian J. Med. Res. 2000, 112, 21–26. [Google Scholar] [PubMed]
- Demircigil, G.Ç.; Erdem, O.; Gaga, E.O.; Altuğ, H.; Demirel, G.; Özden, Ö.; Arı, A.; Örnektekin, S.; Döğeroğlu, T.; van Doorm, W.; et al. Cytogenetic biomonitoring of primary school children exposed to air pollutants: Micronuclei analysis of buccal epithelial cells. Environ. Sci. Pollut. Res. 2014, 21, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Mergener, M.; Rhoden, C.R.; Amantéa, S.L. Nuclear abnormalities in cells from nasal epithelium: A promising assay to evaluate DNA damage related to air pollution in infants. J. Pediatr. 2014, 90, 632–636. [Google Scholar] [CrossRef][Green Version]
- Silva da Silva, C.; Marzari Rossato, J.; Vaz Rocha, J.A.; Ferrão Vargas, V.M. Characterization of an area of reference for inhalable particulate matter (PM2.5) associated with genetic biomonitoring in children. Mutat. Res. 2015, 778, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, D.; Sposito, J.C.V.; Crispim, B.; Nascimento, A.; Barufatti Grisolia, A. Genotoxic and mutagenic effects of passive smoking and urban air pollutants in buccal mucosa cells of children enrolled in public school. Toxicol. Mech. Meth. 2017, 27, 346–351. [Google Scholar] [CrossRef]
- Huen, K.; Gunn, L.; Duramad, P.; Jeng, M.; Scalf, R.; Holland, N. Application of a geographic information system to explore associations between air pollution and micronucleus frequencies in African American children and adults. Environ. Mol. Mutagen. 2006, 47, 236–246. [Google Scholar] [CrossRef]
- Sisenando, H.A.; de Batistuzzo, S.R.; Artaxo, P.; Saldiva, P.H.; de Souza Hacon, S. Micronucleus frequency in children exposed to biomass burning in the Brazilian Legal Amazon region: A control case study. BMC Oral Health 2012, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Marcon, A.; Fracasso, M.E.; Marchetti, P.; Doria, D.; Girardi, P.; Guarda, L.; Pesce, G.; Pironi, V.; Ricci, P.; De Marco, R. Outdoor Formaldehyde and NO2Exposures and Markers of Genotoxicity in Children Living Near Chipboard Industries. Environ. Health Perspect. 2014, 122, 639–645. [Google Scholar] [CrossRef]
- Panico, A.; Grassi, T.; Bagordo, F.; Idolo, A.; Serio, F.; Tumolo, M.R.; De Giorgi, M.; Guido, M.; Tutino, M.; De Donno, A. Micronucleus Frequency in Exfoliated Buccal Cells of Children Living in an Industrialized Area of Apulia (Italy). Int. J. Environ. Res. Public Health 2020, 17, 1208. [Google Scholar] [CrossRef]
- Lemos, A.T.; Lemos, C.T.; Coronas, M.V.; Rocha, J.R.D.; Vargas, V.M.F. Integrated study of genotoxicity biomarkers in schoolchildren and inhalable particles in areas under petrochemical influence. Environ. Res. 2020, 188, 109443. [Google Scholar] [CrossRef]
- Holland, N.; Bolognesi, C.; Kirsch-Volders, M.; Bonassi, S.; Zeiger, E.; Knasmueller, S.; Fenech, M. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: The HUMN project perspective on current status and knowledge gaps. Mutat. Res. 2008, 659, 93–108. [Google Scholar] [CrossRef]
- Ceretti, E.; Donato, F.; Zani, C.; Villarini, M.; Verani, M.; De Donno, A.; Bonetta, S.; Feretti, D.; Carducci, A.; Idolo, A.; et al. Results from the European Union MAPEC_LIFE cohort study on air pollution and chromosomal damage in children: Are public health policies sufficiently protective? Environ. Sci. Eur. 2020, 32, 74. [Google Scholar] [CrossRef]
- Idolo, A.; Grassi, T.; Bagordo, F.; Panico, A.; De Giorgi, M.; Serio, F.; Guido, M.; Piscitelli, P.; De Filippis, G.; Raho, A.; et al. Micronuclei in Exfoliated Buccal Cells of Children Living in a Cluster Area of Salento (Southern Italy) with a High Incidence of Lung Cancer: The IMP AIR Study. Int. J. Environ. Res. Public Health 2018, 15, 1659. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan-Gordo, C.; Fthenou, E.; Pedersen, M.; Espinosa, A.; Chatzi, L.; Beelen, R.; Chalkiadaki, G.; Decordier, I.; Hoek, G.; Merlo, M.F.; et al. Outdoor air pollution exposures and micronuclei frequencies in lymphocytes from pregnant women and newborns in Crete, Greece (Rhea cohort). Environ. Res. 2015, 143, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Zani, C.; Ceretti, E.; Zerbini, I.; Viola, G.C.V.; Donato, F.; Gelatti, U.; Feretti, D. Comet Test in Saliva Leukocytes of Pre-School Children Exposed to Air Pollution in North Italy: The Respira Study. Int. J. Environ. Res. Public Health 2020, 17, 3276. [Google Scholar] [CrossRef] [PubMed]
- Zani, C.; Donato, F.; Ceretti, E.; Pedrazzani, R.; Zerbini, I.; Gelatti, U.; Feretti, D. Genotoxic Activity of Particulate Matter and In Vivo Tests in Children Exposed to Air Pollution. Int. J. Environ. Res. Public Health 2021, 18, 5345. [Google Scholar] [CrossRef]
- Billet, S.; Landkocz, Y.; Martin, P.; Verdin, A.; Ledoux, F.; Lepers, C.; André, V.; Cazier, F.; Sichel, F.; Shirali, P.; et al. Chemical characterization of fine and ultrafine PM, direct and indirect genotoxicity of PM and their organic extracts on pulmonary cells. J. Environ. Sci. 2018, 71, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Valle-Hernández, B.L.; Mugica-Alvarez, V.; Salinas-Talavera, E.; Amador-Muñoz, O.; Murillo-Tovar, M.A.; Villalobos-Pietrini, R.; De Vizcaya-Ruíz, A. Temporal variation of nitro-polycyclic aromatic hydrocarbons in PM10 and PM2.5 collected in Northern Mexico City. Sci. Total Environ. 2010, 408, 5429–5438. [Google Scholar] [CrossRef]
- Slezakova, K.; Castro, D.; Pereira, M.C.; Moralis, S.; Delerue-Matos, C.; Alvim-Ferraz, M.C. Influence of traffic emissions on the carcinogenic polycyclic aromatic hydrocarbons in outdoor breathable particles. J. Air Waste Manag. Assoc. 2010, 60, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Slezakova, K.; Delerue-Matos, C.; Pereira, M.C.; Morais, S. Children environmental exposure to particulate matter and polycyclic aromatic hydrocarbons and biomonitoring in school environments: A review on indoor and outdoor exposure levels, major sources and health impacts. Environ. Int. 2019, 124, 180–204. [Google Scholar] [CrossRef] [PubMed]
- Bagordo, F.; MAPEC_LIFE Study Group; De Donno, A.; Grassi, T.; Guido, M.; DeVoti, G.; Ceretti, E.; Zani, C.; Feretti, D.; Villarini, M.; et al. Lifestyles and socio-cultural factors among children aged 6-8 years from five Italian towns: The MAPEC_LIFE study cohort. BMC Public Health 2017, 17, 233. [Google Scholar] [CrossRef]

| N. | % | |
|---|---|---|
| Gender | ||
| Male | 80 | 45.0 |
| Female | 100 | 55.0 |
| Mother of Italian nationality | ||
| No | 31 | 17.2 |
| Yes | 149 | 82.8 |
| Father of Italian nationality | ||
| No | 24 | 13.3 |
| Yes | 156 | 86.7 |
| Mother degree | ||
| No | 102 | 56.6 |
| Yes | 78 | 43.4 |
| Father degree | ||
| No | 113 | 62.8 |
| Yes | 67 | 37.2 |
| BMI categories | ||
| Normal weight (BMI < 17.4) | 138 | 76.7 |
| Overweight/obese (BMI ≥ 17.4) | 42 | 23.3 |
| Adherence to Mediterranean diet | ||
| No (IMI < 6) | 151 | 83.9 |
| Yes (IMI ≥ 6) | 29 | 16.1 |
| Exposure to second-hand smoke | ||
| No | 159 | 88.3 |
| Yes | 21 | 11.7 |
| Children’s outdoor sport | ||
| No | 117 | 65.0 |
| Yes | 63 | 35.0 |
| Winter Season 1 | Winter Season 2 | Difference with Wilcoxon Test p | |
|---|---|---|---|
| MN test | |||
| Number of subjects | 180 | 180 | |
| MN frequency (%) | |||
| Mean ± SD | 0.51 ± 0.59 | 0.40 ± 0.52 | 0.06 |
| Median | 0.5 | 0.0 | |
| Range | 0.0–2.5 | 0.0–2.0 | |
| N. (%) children with at least one MN | 104 (57.8) | 86 (47.8) | 0.05 |
| N. (%) children with at least one MN in all two seasons | 51 (28.3) | ||
| N. and (%) of children without MN in any season | 41 (22.8) | ||
| Comet test | |||
| Number of subjects | 71 | 71 | |
| Visual score (arbitrary unit) | |||
| Mean ± SD | 173.2 ± 50.8 | 208.8 ± 67.6 | 0.009 |
| Median | 162.0 | 193.0 | |
| Range | 111.1–327.2 | 114.0–349.3 | |
| Pollutants | Winter Season 1 (Mean ± SD) | Winter Season 2 (Mean ± SD) | p |
|---|---|---|---|
| PM0.5 (mg/m3) | 14.14 ± 4.68 | 15.62 ± 4.80 | 0.001 |
| PAH (ng/m3) | 7.96 ± 4.60 | 6.52 ± 1.15 | <0.001 |
| NitroPAH (ng/m3) | 0.09 ± 0.03 | 0.04 ± 0.002 | <0.001 |
| cPAH (ng/m3) | 4.23 ± 3.47 | 3.47 ± 0.54 | <0.001 |
| BaP (ng/m3) | 0.81 ± 0.48 | 0.65 ± 0.14 | <0.001 |
| IRR | 95% CIs | p Value | |
|---|---|---|---|
| MN TEST | |||
| PM0.5 | 0.95 | 0.92; 0.98 | 0.009 |
| PAH | 0.97 | 0.93; 1.04 | 0.08 |
| cPAH | 0.94 | 0.88; 1.01 | 0.07 |
| BaP | 0.74 | 0.52; 1.05 | 0.09 |
| COMET TEST | Coeff. | 95% CIs | p Value |
| PM0.5 | −0.003 | −0.017; 0.010 | 0.67 |
| PAH | −0.0004 | −0.020; 0.019 | 0.96 |
| cPAH | −0.0002 | −0.38; 0.38 | 1.0 |
| BaP | −0.00002 | −0.18; 0.18 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zani, C.; Ceretti, E.; Feretti, D.; Villarini, M.; Moretti, M.; Verani, M.; De Donno, A.; Bonetta, S.; Buschini, A.; Bonetti, A.; et al. Winter Air Pollution and Genotoxic Effects in Children Living in a Highly Polluted Urban Area. Atmosphere 2021, 12, 1191. https://doi.org/10.3390/atmos12091191
Zani C, Ceretti E, Feretti D, Villarini M, Moretti M, Verani M, De Donno A, Bonetta S, Buschini A, Bonetti A, et al. Winter Air Pollution and Genotoxic Effects in Children Living in a Highly Polluted Urban Area. Atmosphere. 2021; 12(9):1191. https://doi.org/10.3390/atmos12091191
Chicago/Turabian StyleZani, Claudia, Elisabetta Ceretti, Donatella Feretti, Milena Villarini, Massimo Moretti, Marco Verani, Antonella De Donno, Sara Bonetta, Annamaria Buschini, Alberto Bonetti, and et al. 2021. "Winter Air Pollution and Genotoxic Effects in Children Living in a Highly Polluted Urban Area" Atmosphere 12, no. 9: 1191. https://doi.org/10.3390/atmos12091191
APA StyleZani, C., Ceretti, E., Feretti, D., Villarini, M., Moretti, M., Verani, M., De Donno, A., Bonetta, S., Buschini, A., Bonetti, A., Bonizzoni, S., Gelatti, U., & on behalf of the MAPEC-LIFE Study Group. (2021). Winter Air Pollution and Genotoxic Effects in Children Living in a Highly Polluted Urban Area. Atmosphere, 12(9), 1191. https://doi.org/10.3390/atmos12091191











