Abstract
Artificial nitrogen oxide (NOx) emissions due to the combustion of fossil fuels constitute more than 75% of the total NOx emissions. Given the continuous reinforcement of NOx emission standards worldwide, the development of environmentally and economically friendly NOx reduction techniques has attracted much attention. This study investigates the selective non-catalytic reduction (SNCR) of NOx by methane, ammonia, and urea in the presence of sodium carbonate and methanol and the concomitant generation of N2O. In addition, the SNCR mechanism is explored using a chemical modeling software (CHEMKIN III). Under optimal conditions, NOx reduction efficiencies of 80–85%, 66–68%, and 32–34% are achieved for ammonia, urea, and methane, respectively. The N2O levels generated using methane (18–21 ppm) were significantly lower than those generated using urea and ammonia. Addition of sodium carbonate and methanol increased the NOx reduction efficiency by methane to ≥40% and 60%, respectively. For the former, the N2O level and reaction temperature further decreased to 2–3 ppm and 850–900 °C, respectively. The experimental results were well consistent with simulations, and the minor discrepancies were attributed to microscopic variables. Thus, our work provides essential guidelines for selecting the best available NOx control technology.
1. Introduction
Nitrogen oxides (NOx), along with sulfur oxides and dust, are representative air pollutants predominantly produced through the combustion of fossil fuels and are precursors of particulate matter [1,2]. Artificial NOx emissions due to fossil fuel combustion represent more than 75% of the total NOx emissions [3], which highlights the need for effective NOx abatement methods.
Among the NOx reduction technologies applied to various industrial processes involving combustion, selective catalytic reduction (SCR) and selective non-catalytic reduction (SNCR) are particularly noteworthy post-treatment prevention methods [4,5]. Although SCR processes are more efficient than SNCR processes [6], the latter offer the benefits of lower installation costs, convenience, and broad applicability, and are therefore more widely used in industry [7]. Given that SNCR has been used as the best available control technology and best available technique in numerous countries [6,7,8], many researchers have attempted to improve its NOx reduction efficiency by altering the reaction conditions, e.g., through the introduction of auxiliary agents and additives [9,10,11].
In SNCR, the choice of the reducing agent directly impacts the NOx reduction efficiency. Amines and cyanides are predominantly used as the reducing agents; ammonia and urea have also been used in general industrial processes [12]. Given the continuous reinforcement of NOx emission standards worldwide and the issues associated with existing reducing agents, the development of environmentally and economically friendly NOx reduction methods has drawn much attention [13,14]. Although ammonia and urea are the most widely used reducing agents, ammonia slip caused by unreacted reducing agents adversely affects human health and corrodes process equipment [15]. In addition, ammonia is classified as a toxic chemical in South Korea, and this limits the use of high ammonia concentrations [16]. In view of its low cost, urea is widely used in small businesses instead of ammonia. However, large quantities of N2O, a potent greenhouse gas [17], are generated, often failing to meet the environmental standards because of its low NOx reduction effectiveness [18].
Herein, gas fuel (CH4) typically used in the combustion process was employed as a direct reducing agent to overcome the problems of high cost, ammonia slip, facility site problems, and greenhouse gas generation. The use of carbon-containing gases as reducing agents [19] and the injection of additional CH-based additives into existing reducing agents [19,20] during SNCR have been extensively investigated. Although CH-based reducing agents have lower efficiencies than existing reducing agents, no site is required in the former case, as fuels that are used in the combustion process are directly added as reducing agents and do not cause severe issues such as ammonia slip [20,21]. Most studies on NOx reduction technologies have primarily derived conclusions through the analysis of experimental results, whereas only few works have analyzed the SNCR mechanism.
To bridge this gap, this study investigates the reduction of NOx by methane, ammonia, and urea and the concomitant generation of N2O. In addition, the SNCR mechanism is probed using a chemical modeling software (CHEMKIN III).
2. Materials and Methods
2.1. Experimental Setup
Lab-scale experiments were performed in a cylindrical stainless steel (304 3/4H SS) reactor with a width of 42.6 mm and a length of 850 mm inside a heating furnace operated at a maximum temperature of 1150 °C (Figure 1). The gaseous reactants were mixed in the mixing chamber and then introduced into the reactor through the preheater. The temperature of preheater was set to 750~800 °C. For efficient mixing, the mixing chamber utilized a tube of 20 mm in diameter and 5 m in length. Three thermocouples were installed inside the reactor to measure and adjust the reaction temperature. The injected reducing agent was sprayed at a set flow rate using a mass flow controller.
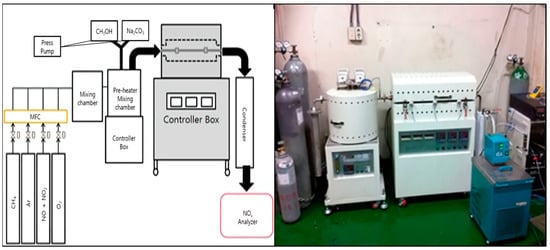
Figure 1.
Schematic of the experimental procedure and the lab-scale SNCR process.
Among the gases emitted after SNCR, NOx and O2 were quantified using a portable flue gas analyzer (GreenLineMK2, Il-Sang Co., Ltd., Korea) of the electrochemical electrode cell type, whereas N2O was quantified using the instrument (Hymeth, Kinsco Co., Ltd., Seoul, Korea) employed in the NDIR method.
2.2. Experimental and Modeling Conditions
The reactor temperature ranged from 800 to 1150 °C and was varied in steps of 25 °C for a detailed analysis of temperature change effects. The concentration of the introduced NOx was fixed at 1000 ppm. To simulate the actual process, a mixture of NO (90 vol%) and NO2 (10 vol%) was introduced into the system, where the O2 level ranged from 0 to 10 vol%. The normalized stoichiometric ratio (NSR) of the reducing agents ranged from 1.0 to 2.0, and the NSR of the additionally injected additives relative to the reducing agents ranged from 0 to 0.3. The total reaction time was set to 1.0 s, and the flow velocity inside the reactor ranged from 21 to 27 L/min. The flow rate and flow velocity were adjusted in real time using MFC. CHEMKIN III modeling was performed to analyze the experimental results under conditions similar to those used in the experiments. CHEMKIN III is a software whose purpose is to facilitate the formation, solution, and interpretation of problems involving elementary gas-phase chemical kinetics. The thermodynamic data pertaining to substances related to each unit reaction were extracted from NASA CEA code [22,23] and GRI Mech 3.0 code [24,25] databases. Table 1 lists the experimental and modeling conditions.

Table 1.
Experimental and modeling conditions.
3. Results and Discussion
3.1. Reactor Temperature Profile
Prior to experiments, a temperature profile test was conducted to confirm the uniformity of the temperature distribution inside the reactor. As temperature conditions significantly influence chemical reactions occurring during the SNCR, a uniform setting is required [26]. When the temperature distribution was examined at the same temperature setting by selecting three measurement points inside the reactor and two measurement points outside the reactor, points 2, 3, and 4 inside the reactor exhibited differences of 30, 10, and 20 °C, respectively. These temperature variations were attributed to a discrepancy between the temperature of the gas at the point of introduction (point 1) and that of the mixture at point 2. Moreover, the “end effect” [27], which was observed at both ends of the reactor, and the “wall effect”, which occurred on the reactor wall for points 3 and 4, may have contributed to the observed temperature discrepancies [28]. “End effect” and “wall effect” are phenomena in which the temperature decreases at the beginning and end of the reactor due to movement of fluid inside the reactor and the reactor temperature. These effects were accounted for, and the temperatures at the three points were suitably altered for the experiment. Figure 2 shows the data measured at each temperature.
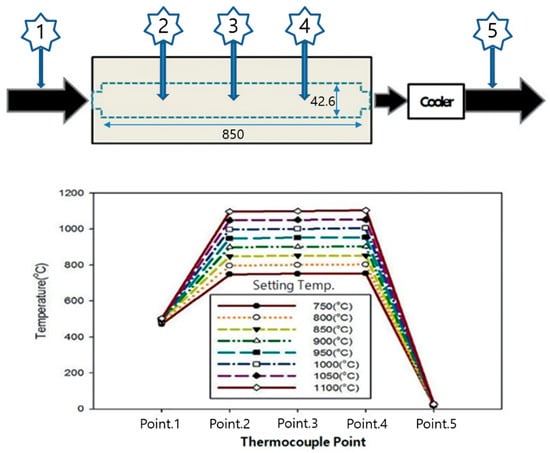
Figure 2.
Temperature profile within the SNCR reactor.
3.2. NOx Reduction Performances of Ammonia, Urea, and Methane
NOx reduction efficiencies were analyzed for reductant (ammonia, urea, and methane) NSRs of 1.0–3.0 (Figure 3). The NOx reduction efficiency equaled 80–85% for ammonia at an NSR of 1.5, 66–68% for urea at an NSR of 1.5, and 32–34% for methane at an NSR of 2. Moreover, the primary reactions affecting NOx reduction in the reactor during reducing agent injection were identified as reactions (1) and (2) for ammonia and urea and as reactions (3) and (4) for methane [29,30]:
NH3 + NO + 0.25O2 → N2 + 1.5H2O
(NH2)2CO + 2NO + 0.5O2 → 2N2 + CO2 + 2H2O
CH4 + 4NO2 → 4NO + CO2 + 2H2O
CH4 + 4NO → 2N2 + CO2 + 2H2O
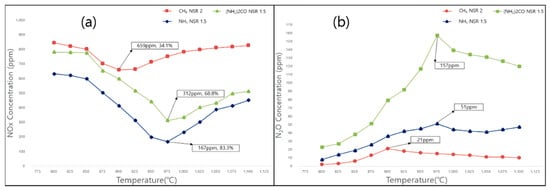
Figure 3.
Effects of temperature on the levels of (a) NOx and (b) N2O obtained under optimal conditions (NO = 900 ppm, NO2 = 100 ppm, O2 = 7%, NH3 and (NH2)2CO NSR = 1.5, CH4 NSR = 2) for (NH2)2CO, NH3, and CH4 as reducing agents.
The N2O levels generated using methane (18–21 ppm) were significantly lower than those generated using urea (157 ppm) and ammonia (51 ppm). In addition, the range of activation temperatures for methane (875–925 °C) was 50–80 °C lower than those for ammonia and urea.
Methane exhibited a lower NOx reduction efficiency than ammonia and urea but generated a significantly smaller amount of N2O and offered the benefit of a lower (by ~80 °C) reaction temperature range. Considering that N2O is a potent greenhouse gas, it is necessary to develop methods of increasing the NOx reduction efficiency, such as for the injection of additives to reducing agents and the adjustment of operating variables [31,32,33]. These methods are expected to be used as alternatives for existing reducing agents.
3.3. CH4-SNCR in the Presence of Additives (Na2CO3 and CH3OH)
Numerous studies have attempted to reduce the range of activation temperatures and increase the NOx reduction efficiency by injecting supplementary additives into the primary reducing agents used in the SNCR process, namely ammonia and urea [10,11]. In this section, methane, which exhibited a low NOx reduction efficiency compared to urea and ammonia, was used as a direct reducing agent in the presence of sodium carbonate and methanol as additives, and the effects of these additives were analyzed (Figure 4).
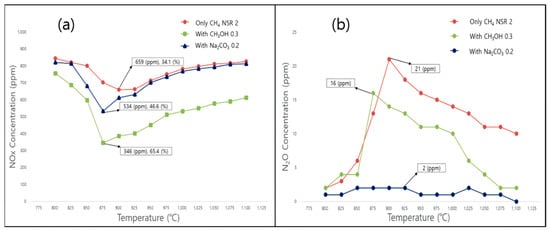
Figure 4.
Effects of additives and temperature on (a) NOx removal and (b) N2O formation for CH4 as the reducing agent under optimal conditions (NO = 900 ppm, NO2 = 100 ppm, O2 = 7 vol%, CH4 NSR = 2.0, CH3OH/CH4 = 0.3, Na2CO3/CH4 = 0.2).
When methanol was injected at a ratio of 0.3, the NOx reduction efficiency marginally increased to 60%, and the reaction temperature range decreased by 25 °C compared to that observed in the methane-only scenario. The N2O concentration (16 ppm) obtained in the presence of methanol was also lower than that obtained without methanol (21 ppm). The following primary reactions occurred in the presence of methanol [34]:
CH3OH + O → CH3 + O2 + H
CH3 + NO → HCN + H2O
HCN + OH → HNCO + H
HNCO + OH → NCO + H2O
NCO + NO → N2 + CO2
NCO + NO → N2O + CO
HCN + O → CN + OH
CN + N2O + H → HCN + NO + N
NOx reduction was affected by reaction (6), which involves the separation of CH3 from methanol [34]. The increase in the N2O reduction efficiency was relatively minor compared to the significant increase in the NOx reduction efficiency, which was attributed to the simultaneous occurrence of N2O generation in reaction (10) and its reduction in reaction (12). As simultaneous reactions are affected by reaction conditions, our results suggest that N2O can be further generated depending on the reaction conditions. CH compounds as reducing agents are known to increase the reaction efficiency and induce reactions at low temperatures by accelerating the reactions of OH radicals [35].
Na-containing additives are known to accelerate the generation of hydrogen, hydrogen peroxide, hydrocarbons, and alcohols, and induce NOx reduction [36,37]. In particular, the addition of Na-based additives to reducing agents lowers the reaction temperature and extends the range of the activation temperature [37]. In the present method, the injection of sodium carbonate (additive/CH4 = 0.2) increased the NOx reduction efficiency by ~40%. Moreover, this agent efficiently promoted N2O reduction, generating only 2–3 ppm of N2O. This increased efficiency was attributed to reaction (19), in which Na generated by the decomposition of sodium carbonate combines with N2O to generate NaO and N2. However, significantly lower quantities of N2O were generated at temperatures of 900 °C and higher. The primary reactions occurring in the SNCR reactor during the injection of sodium carbonate were identified as follows [36,37]:
Na2CO3 → Na2O + CO2
Na2O + H2O → 2NaOH
NaOH + O2 → NaO2 + OH
NaOH + M → Na + OH + M
NaOH + H → Na + H2O
NaO2 + M → Na + O2 + M
Na + N2O → NaO + N2
Na + NO2 → NaO + NO
NaO + H2O → NaOH + OH
NaO + NH3 + M → NaOH + NH2 + M
3.4. Reaction Modeling
CHEMKIN III was used for the theoretical analysis of experimental results. Among the reaction models available in CHEMKIN III, the SENKIN reaction model uses the thermodynamic properties (entropy, enthalpy, and heat capacity) of chemical species participating in reactions and the reaction mechanisms composed of unit reactions. Moreover, the SENKIN model derives experimental results in advance, enabling the examination of chemical changes with reaction time [38,39]. In this section, after the optimal efficiency response in the experiment was compared with the modeling results, reaction pathways were constructed using the Fortran (Ver. 95, IBM, ISO/IEC 1539-1:1997) program based on sensitivity analysis to investigate the reducing agents and additive reactions.
Figure 5 shows the comparative data results of experiments and modeling. When methane was used as the reducing agent, the experimental and simulated trends in the NOx reduction efficiency, N2O generation, temperatures for improved efficiency, and reaction time agreed with each other. However, the experimental efficiencies were lower than the simulated ones by about 4.21% (optimal efficiency condition). This discrepancy was attributed to the action of microscopic variables in the experiment and agreed with other studies, wherein modeling and experiments were performed simultaneously [40,41].
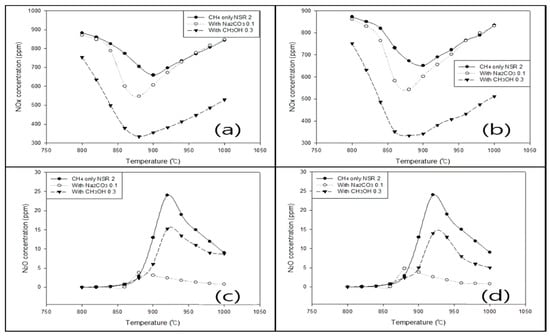
Figure 5.
(a,c) Experimental and (b,d) simulated (identical conditions) effects of temperature and additives on the levels of (a,b) NOx and (c,d) N2O.
3.4.1. Sensitivity Analysis Using CHEMKIN III
Sensitivity analysis is a good method of obtaining chemically essential reactions from target reactions and has been used for process design, effect analysis, and device improvement [42]. Herein, sensitivity analysis was conducted for 645 reactions used in modeling to identify the reactions with the minimal effect on N2O generation and NOx reduction in the case of methane. The effect of the rate constant of each reaction on NOx and N2O concentrations was examined using the following equation [43,44]:
Sj.i = ΔZj/Δki
Here, Zj is the concentration under condition j, ki is the rate constant of reaction i, and Sj.i is the sensitivity [42]. A negative result of sensitivity analysis represents a reduction in NOx and N2O concentrations, whereas a positive result represents an increase in these concentrations. When methane was used in the absence of additives, the primary reactions were identified as follows:
CH4 + O2 → CH3 + HO2
CH4 + OH → CH3 + H2O
CH3 + O2 → CH2O + OH
CH3 + CH3(M) → C2H6(M)
CH3 + O2 → CH3O + O
Reactions (25)–(27) exhibited high sensitivity to NOx generation, NOx reduction and N2O generation, and N2O reduction, respectively. In particular, reaction (26) significantly affected both NOx reduction and N2O generation. This suggests that nitrogen and NOx, which were separated during NOx decomposition by the reducing agent, were converted into N2O, which resulted in simultaneous NOx reduction and N2O generation. As simultaneous reactions are significantly affected by external reaction conditions such as temperature, oxygen concentration, and reaction time, we decided to comprehensively examine the external conditions when CH-based reducing agents were used for N2O reduction.
Sensitivity analysis for NO and N2O reactions showed that the most important reactions could be selected as CH3 + O2 = CH2O + OH, CH3 + CH3(M) = C2H6(M), and CH3 + O2 = CH3O + O used only CH4 in 645 reactions (Figure 6).
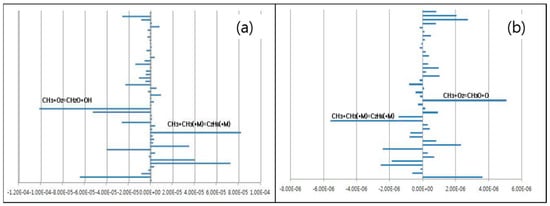
Figure 6.
Sensitivity coefficients of (a) NOx and (b) N2O in CH4 SNCR reactions (NO = 900 ppm, NO2 = 100 ppm, O2 = 7 vol%, CH4 NSR = 2).
3.4.2. Identification of CH4-SNCR Reaction Pathways Using Fortran
Reaction pathways were constructed by integrating the results derived from CHEMKIN III using Fortran (Ver. 95, IBM, ISO/IEC 1539-1:1997). Overall, an integral reaction flow analysis was constructed using methane as the reducing agent, and methane oxidation and NOx removal reactions were simulated separately. Finally, the major reaction pathways were constructed by applying the sensitivity constants obtained in Section 3.4.1 and compared with those determined for urea.
The oxidation reactions of methane to CH2O and HCO are the primary reactions occurring during methane oxidation. Subsequently, HCO is decomposed into HCN and HCCO, converted into NCO via reactions with N and C, and finally generates CO2 and N2, thereby reducing NOx. Figure 7 shows the CH4 oxidation reaction, NOx reduction reaction, and the total reaction flow for CH4 as a reducing agent.
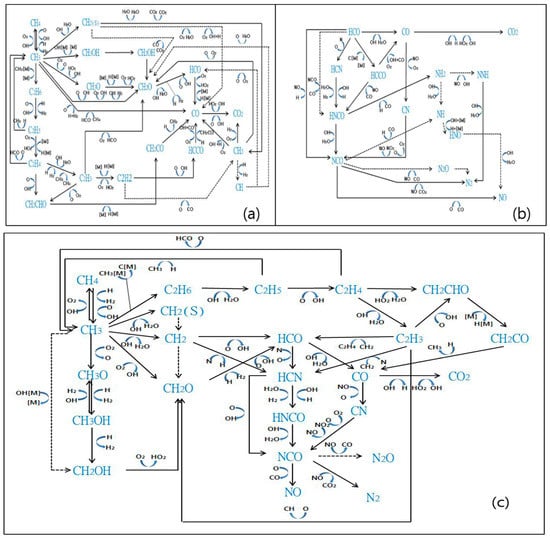
Figure 7.
Reactions occurring during SNCR with CH4 as the reducing agent (NO = 900 ppm, NO2 = 100 ppm, O2 = 7 vol%, CH4 NSR = 2, temperature = 900 °C). (a) CH4 oxidation reactions, (b) NOx reactions, (c) overall pathways.
The primary reaction pathways (Figure 8) were used to analyze the mechanism of NOx reduction by methane and urea. Urea generated ammonia and HNCO, and the former afforded NH2 and an O or OH radical. NH2 is an essential element of SNCR, as it reacts with NO to generate N2 [45]. In addition, HNCO reacts with the H radical to furnish NH2 or NCO, and NCO is finally converted into N2O or N2 to achieve the reduction of NOx.
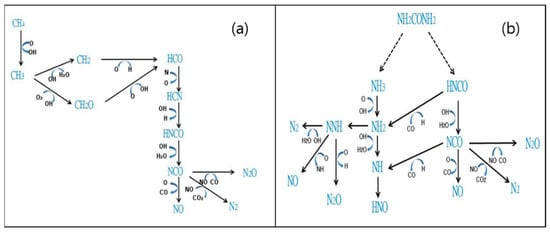
Figure 8.
Comparison of (a) CH4 and (b) (NH2)2CO primary pathways (NO = 900 ppm, NO2 = 100 ppm, O2 = 7 vol%, CH4 NSR = 2, temperature = 900 °C).
Unlike those obtained for urea, the modeling results obtained for methane as a reducing agent indicated only one primary reaction path, according to which CH4 is converted into HCN through reactions ((26)–(31)), and the generated NCO reacts with an O radical to afford NO, N2, and N2O.
CH4 + O → CH3 + OH
CH3 + OH → CH2 + H2O
CH3 + O2 → CH2O + OH
CH2 + O → HCO + H
CH2O + O → HCO + OH
HCO + N → HCN + O
4. Conclusions
The effects of reducing agents (ammonia, urea, and methane) on the SNCR process were determined experimentally and simulated using modeling software. In addition, the NOx reduction and N2O generation characteristics were analyzed in the presence of sodium carbonate and methanol as supplementary additives.
NOx reduction efficiencies of 80–85%, 76–78%, and 32–34% were obtained for ammonia, urea, and methane, respectively. Although methane exhibited the lowest NOx reduction efficiency, its reaction temperature range (850–950 °C) was approximately 80 °C lower than that of the other reducing agents. Moreover, the N2O level (21 ppm) observed in the case of methane was four times lower than that observed for ammonia and more than 15 times lower than that observed for urea. When additives were injected (CH4 NSR = 0.2) to improve the NOx reduction efficiency of CH4, the use of methanol increased this efficiency to ≥60%, while the use of sodium carbonate increased the efficiency to 40%. Moreover, the addition of methanol significantly reduced N2O emissions. Chemical modeling revealed that N2O reduction was caused by the chain reaction of Na. The reaction tree constructed for the use of methanol revealed that CH2O and HCN were generated as intermediates and were eventually converted into NCO, which, in turn, reduced NOx and generated N2O.
This study comprehensively analyzed various reducing agents and additives used in the SNCR process. The CH4-SNCR process cannot be defined as the NOx reduction technology with the highest efficiency. However, considering the constantly changing technology and the diverse conditions at various industrial combustion facilities, we believe that our findings will serve as guidelines for selecting the best available control technology toward reducing air pollution, including NOx pollution.
Author Contributions
Conceptualization, P.-M.P. and J.-I.D.; validation, Y.-K.P. and J.-I.D.; formal analysis, P.-M.P.; investigation, P.-M.P. and Y.-K.P.; resources, P.-M.P.; data curation, P.-M.P.; writing—original draft preparation, P.-M.P. and Y.-K.P.; writing—review and editing, P.-M.P.; visualization, J.-I.D.; supervision, J.-I.D.; project administration, P.-M.P.; funding acquisition, P.-M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Institute of Environmental Research (NIER) funded by the Ministry of Environment (MOE) of the Republic of Korea, grant number NIER-2019-01-01-022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is contained within this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amoatey, P.; Omidvarborna, H.; Baawain, M.S.; Al-Mamun, A. Emissions and exposure assessments of SOX, NOX, PM10/2.5 and trace metals from oil industries: A review study (2000–2018). Process Saf. Environ. Prot. 2019, 123, 215–228. [Google Scholar] [CrossRef]
- Munawer, M.E. Human health and environmental impacts of coal combustion and post-combustion wastes. J. Sustain. Min. 2018, 17, 87–96. [Google Scholar] [CrossRef]
- Deng, J.; Wang, X.; Wei, Z.; Wang, L.; Wang, C.; Chen, Z. A review of NOx and SOx emission reduction technologies for marine diesel engines and the potential evaluation of liquefied natural gas fuelled vessels. Sci. Total Environ. 2021, 766, 144319. [Google Scholar] [CrossRef] [PubMed]
- Rolón, B.G.; Hernández, C.M. Effect of additives for the reduction of NOx in the corrosion of overheating pipes. Eng. Fail. Anal. 2020, 118, 104902. [Google Scholar] [CrossRef]
- Van Caneghem, J.; De Greef, J.; Block, C.; Vandecasteele, C. NOx reduction in waste incinerators by selective catalytic reduction (SCR) instead of selective non catalytic reduction (SNCR) compared from a life cycle perspective: A case study. J. Clean. Prod. 2016, 112, 4452–4460. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, P.; Chen, Y.; Wang, Y.; Ding, Q.; Sui, Z.; Chen, H.; Shen, Z.; Wu, X. A basic comprehensive study on synergetic effects among the metal oxides in CeO2-WO3/TiO2 NH3-SCR catalyst. Chem. Eng. J. 2021, 421, 127833. [Google Scholar] [CrossRef]
- Lee, G.-W.; Shon, B.-H.; Yoo, J.-G.; Jung, J.-H.; Oh, K.-J. The influence of mixing between NH3 and NO for a De-NOx reaction in the SNCR process. J. Ind. Eng. Chem. 2008, 14, 457–467. [Google Scholar] [CrossRef]
- Wielgosiński, G.; Czerwińska, J.; Szymańska, O.; Bujak, J. Simultaneous NOx and dioxin removal in the SNCR process. Sustainability 2020, 12, 5766. [Google Scholar] [CrossRef]
- Bae, S.W.; Roh, S.A.; Kim, S.D. NO removal by reducing agents and additives in the selective non-catalytic reduction (SNCR) process. Chemosphere 2006, 65, 170–175. [Google Scholar] [CrossRef]
- Cai, J.; Zheng, W.; Wang, Q. Effects of hydrogen peroxide, sodium carbonate, and ethanol additives on the urea-based SNCR process. Sci. Total Environ. 2021, 772, 145551. [Google Scholar] [CrossRef]
- Daood, S.S.; Yelland, T.S.; Nimmo, W. Selective non-catalytic reduction–Fe-based additive hybrid technology. Fuel 2017, 208, 353–362. [Google Scholar] [CrossRef]
- Ayoub, M.; Irfan, M.F.; Yoo, K.-S. Surfactants as additives for NOx reduction during SNCR process with urea solution as reducing agent. Energy Convers. Manag. 2011, 52, 3083–3088. [Google Scholar] [CrossRef]
- Gholami, F.; Tomas, M.; Gholami, Z.; Vakili, M. Technologies for the nitrogen oxides reduction from flue gas: A review. Sci. Total Environ. 2020, 714, 136712. [Google Scholar] [CrossRef] [PubMed]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NO abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef] [PubMed]
- Wypiór, T.; Krzyżyńska, R. Effect of ammonia and ammonium compounds on wet-limestone flue gas desulfurization process from a coal-based power plant—Preliminary industrial scale study. Fuel 2020, 281, 118564. [Google Scholar] [CrossRef]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Shin, Y.; Jung, Y.; Cho, C.P.; Pyo, Y.D.; Jang, J.; Kim, G.; Kim, T.M. NOx abatement and N2O formation over urea-SCR systems with zeolite supported Fe and Cu catalysts in a nonroad diesel engine. Chem. Eng. J. 2020, 381, 122751. [Google Scholar] [CrossRef]
- Javed, M.T.; Irfan, N.; Gibbs, B.M. Control of combustion-generated nitrogen oxides by selective non-catalytic reduction. J. Environ. Manag. 2007, 83, 251–289. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, N.; Yang, J.; Xu, B. Experimental and modeling study of the effect of CH4 and pulverized coal on selective non-catalytic reduction process. Chemosphere 2008, 73, 650–656. [Google Scholar] [CrossRef]
- Yao, T.; Duan, Y.; Yang, Z.; Li, Y.; Wang, L.; Zhu, C.; Zhou, Q.; Zhang, J.; She, M.; Liu, M. Experimental characterization of enhanced SNCR process with carbonaceous gas additives. Chemosphere 2017, 177, 149–156. [Google Scholar] [CrossRef]
- Świeboda, T.; Krzyżyńska, R.; Bryszewska-Mazurek, A.; Mazurek, W.; Czapliński, T.; Przygoda, A. Advanced approach to modeling of pulverized coal boilers for SNCR process optimization-review and recommendations. Int. J. Thermofluids 2020, 7–8, 100051. [Google Scholar] [CrossRef]
- Liu, Q.; Proust, C.; Gomez, F.; Luart, D.; Len, C. The prediction multi-phase, multi reactant equilibria by minimizing the Gibbs energy of the system: Review of available techniques and proposal of a new method based on a Monte Carlo technique. Chem. Eng. Sci. 2020, 216, 115433. [Google Scholar] [CrossRef]
- Reynier, P.; D’Ammando, G.; Bruno, D. Review: Modelling chemical kinetics and convective heating in giant planet entries. Prog. Aerosp. Sci. 2018, 96, 1–22. [Google Scholar] [CrossRef]
- Liu, Z.; Consalvi, J.-L.; Kong, W. An exponential integrator with Schur–Krylov approximation to accelerate combustion chemistry computation. Combust. Flame 2019, 203, 180–189. [Google Scholar] [CrossRef]
- Wang, Y.; Movaghar, A.; Wang, Z.; Liu, Z.; Sun, W.; Egolfopoulos, F.N.; Chen, Z. Laminar flame speeds of methane/air mixtures at engine conditions: Performance of different kinetic models and power-law correlations. Combust. Flame 2020, 218, 101–108. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, X.; Li, T.; Li, S. Effect of HCl and CO on nitrogen oxide formation mechanisms within the temperature window of SNCR. Fuel 2020, 267, 117231. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Z.; Zhang, L.; Jiang, H.; Lu, Y.; Chen, J.; Zhang, G. Study of the wall effect of the sample position well of the Frisch-grid ionization chamber. Appl. Radiat. Isot. 2017, 125, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, R.; Wang, S.; Chen, Q.; Yang, H. End wall effect on particle motion in a chute flow. Particuology 2021, 54, 102–108. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef] [Green Version]
- Si, M.; Shen, B.; Adwek, G.; Xiong, L.; Liu, L.; Yuan, P.; Gao, H.; Liang, C.; Guo, Q. Review on the NO removal from flue gas by oxidation methods. J. Environ. Sci. 2021, 101, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, E.; Lassaletta, L.; Sanz-Cobena, A.; Garnier, J.; Vallejo, A. The potential of organic fertilizers and water management to reduce N2O emissions in Mediterranean climate cropping systems. A. review. Agric. Ecosyst. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef] [Green Version]
- Strokal, M.; Kroeze, C. Nitrous oxide (N2O) emissions from human waste in 1970–2050. Curr. Opin. Environ. Sustain. 2014, 9–10, 108–121. [Google Scholar] [CrossRef]
- Winiwarter, W.; Klimont, Z. The role of N-gases (N2O, NOx, NH3) in cost-effective strategies to reduce greenhouse gas emissions and air pollution in Europe. Curr. Opin. Environ. Sustain. 2011, 3, 438–445. [Google Scholar] [CrossRef]
- Cao, Q.; Wu, S.; Lui, H.; Liu, D.; Qiu, P. Experimental and modeling study of the effects of multicomponent gas additives on selective non-catalytic reduction process. Chemosphere 2009, 76, 1199–1205. [Google Scholar] [CrossRef]
- Adelman, B.J.; Beutel, T.; Lei, G.-D.; Sachtler, W.M.H. On the mechanism of selective NOx reduction with alkanes over Cu/ZSM-5. Appl. Catal. B 1996, 11, L1–L9. [Google Scholar] [CrossRef]
- Niu, S.; Han, K.; Lu, C. An experimental study on the effect of operating parameters and sodium additive on the NOxOUT process. Process Saf. Environ. Prot. 2011, 89, 121–126. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, J.; Zhou, Z.; Chen, Z.; Liu, J.; Cen, K. Action of oxygen and sodium carbonate in the urea-SNCR process. Combust. Flame 2009, 156, 1785–1790. [Google Scholar] [CrossRef]
- An, S.; Jung, J.C. Kinetic modeling of thermal reactor in Claus process using CHEMKIN-PRO software. Case Stud. Therm. Eng. 2020, 21, 100694. [Google Scholar] [CrossRef]
- Schwer, D.A.; Tolsma, J.E.; Green, W.H.; Barton, P.I. On upgrading the numerics in combustion chemistry codes. Combust. Flame 2002, 128, 270–291. [Google Scholar] [CrossRef]
- Lijuan, C.; Zhijun, L.; Li, H.; Boxi, S.; Lingya, Z.; Yue, W. Influence of CO2 concentration and inlet temperature on adsorption path of lean NOx trap. Energy Procedia 2019, 158, 4383–4388. [Google Scholar] [CrossRef]
- Xi, S.; Xue, J.; Wang, F.; Li, X. Reduction of large-size combustion mechanisms of n-decane and n-dodecane with an improved sensitivity analysis method. Combust. Flame 2020, 222, 326–335. [Google Scholar] [CrossRef]
- Ali, G.; Zhang, T.; Wu, W.; Zhou, Y. Effect of hydrogen addition on NOx formation mechanism and pathways in MILD combustion of H2-rich low calorific value fuels. Int. J. Hydrogen Energy 2020, 45, 9200–9210. [Google Scholar] [CrossRef]
- Chemkin Theory Manual, 2016, ANSYS Reaction Design. Available online: https://personal.ems.psu.edu/~radovic/ChemKin_Theory_PaSR.pdf (accessed on 18 August 2021).
- Zhou, D.; Yang, W.; Li, J.; Tay, K.L.; Kraft, M. Combustion modeling in RCCI engines with a hybrid characteristic time combustion and closed reactor model. Appl. Energy 2018, 227, 665–671. [Google Scholar] [CrossRef]
- Samu, V.; Varga, T.; Rahinov, I.; Cheskis, S.; Turányi, T. Determination of rate parameters based on NH2 concentration profiles measured in ammonia-doped methane–air flames. Fuel 2018, 212, 679–683. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).