Abstract
Intensive care units (ICUs) are special areas in hospitals for patients with severe and life-threatening diseases. ICUs are of several categories, such as neonatal ICUs, cardiac ICUs, neurological ICUs, surgical ICUs, etc. The ICUs’ patients may show a high susceptibility for hospital-acquired infections (HAIs) depending on underlying disease, duration of stay and treatment. ICUs are considered potential reservoirs for (opportunistic) pathogenic microbial strains and the risk of acquiring infection in these hospital environments is higher than in others. Several studies show the role of inanimate surface and equipment contamination in the transmission of pathogens to ICU patients. The aim of this study is to describe the results of 124 sampling campaigns performed during 12 years of microbiological surveillance of five ICUs of different categories, for an overall number of 714 samples (232 from air and 482 from surface), to analyze their trends and to elaborate suggestions to improve ICUs’ environmental quality and patients’ safety.
1. Introduction
In the last few decades hospitals had to face several difficult challenges: an increasing proportion of immunologically vulnerable patients, rapidly evolving medical technologies and healthcare models, budget restrictions, and new health emergencies [1]. All these features interfere with healthcare and can also modify the risk of acquiring healthcare-associated infections (HCAIs).
Among hospital’s wards, intensive care units (ICU) are one of the areas more involved in these challenges, since they are specialized divisions, which provide close monitoring and support to threatened or failing vital functions in critically ill patients who suffer from illnesses with the potential to endanger life, and perform adequate diagnostic measures and medical or surgical therapies to improve outcome [2]. These units include several categories: neonatal ICUs, cardiac ICUs, neurological ICUs, surgical ICUs, medical ICUs, etc. Each of them has different characteristics and requirements, depending on the type of patient and disease.
The ICUs in hospitals have been the subject of many studies in the last two decades. Particular attention has been devoted to the role of infection control, but also to the built environment requirements, and also considering the increased demand emerging from the recent COVID-19 global pandemic.
Depending on the underlying disease, duration of stay and treatment, patients admitted to these units may show higher susceptibility to HAIs than healthy individuals. Some peculiar risk factors are the frequency of contact with healthcare workers (in particular with their hands), the number of colonized or infected patients in the same ward and the lack of compliance with infection prevention guidelines [3]. ICUs’ rooms layout can also affect the risk of infection [3], and the patient’s safety in a broad sense [4]. A recent review [5], analyzing the role of rooms design, shows that although several studies report a protective effect of single-bed ICU rooms versus patients’ antibiotic-resistant infections during their stay [6,7], or to nosocomial infection rate compared to a multibed unit [8], other studies demonstrated no association [9] or weaker associations [10], suggesting that the main benefit of the unit design is to facilitate appropriate personnel behavior and that, consequently, design features are subordinate to more primary drivers of infection control, such as personnel behaviors [11].
Regardless, ICUs’ environments are considered potential reservoirs for (opportunistic) pathogenic microbial strains [12] able to survive and multiply on the medical equipment and in the surrounding environment [13,14]. In fact, several vehicles (i.e., surfaces, equipment, hospital textiles, air, etc.) can be a source of microorganisms and infections [15,16,17,18]. For example, some non-invasive devices, such as electrical equipment and devices that are difficult to clean due to their irregular shape, have been reported as a source for infection [12]. Soiled or contaminated bed linen and pajamas, or privacy curtains, can also spread microorganisms during their handling [19]. The same infected patients can act as a source of microorganisms and, in some studies, the surfaces close to the patient, frequently touched by them, resulted in being heavily contaminated [20,21]. It has been reported that the risk of acquiring a nosocomial infection increased significantly when the total microbial burden of the surface exceeded 500 CFU/100 cm2 [18]. For these reasons ICUs are included among the hospital environments at high risk and classified as ISO class 8 [22], comparable to a grade C–D clean room following EU Guidelines to Good Manufacturing Practice Medical Products for Human and Veterinary Use [23]. For these environments a heating, ventilation and air conditioning (HVAC) system is recommended, equipped with positive or neutral pressure and different level of air filtration depending on the type of ICU (general, burn, neonate, etc.) and the Country [24].
In the ICUs, microbial concentrations in air and on surfaces must meet specific requirements to guarantee the safety of patients, medical staff and visitors [4].
In particular, national and international guidelines and regulations report standards on the level of contamination of air and surfaces [11,25,26,27,28].
Different strategies have been adopted to evaluate the environmental biocontamination. The US Centers for Disease Control and Prevention recommend environmental sampling only to support an investigation of an outbreak of disease or infections where environmental reservoirs or fomites are epidemiologically implicated in disease transmission [29]. Other researchers recommend a periodic monitoring of high-risk environments to verify the absence of anomalies in the air treatment systems and the level of application of all the cleaning procedures, especially for protected areas [30,31]. Regarding the sampling method, several researchers prefer the sampling of surfaces rather than active air sampling, due to the higher reliability of results and the lower costs of investigations [31]. The active sampling provides information about the concentration of viable particles in the air [30], whereas surface sampling may be more sensitive for some microorganisms (e.g., molds), because they may settle on surfaces and remain for a long time, especially on electric devices [32]. However, which microbiological environmental sampling approach to prefer is an unresolved issue; the lack of standardized protocols and reference values for environmental surveillance, recognized at national and international level, leave the choice to each hospital in terms of where, when, why and how to detect environmental microorganisms.
In this paper we describe the trend of environmental bacterial pollution observed in some ICUs of hospital buildings of the city of Rome (Italy), considering their activities, layout and structural characteristics, to evaluate changes in bacterial pollution among and within ICUs over time and to suggest preventive actions and design solutions to improve the hygienic standards and the safety of patients and healthcare workers.
2. Materials and Methods
2.1. Setting
Microbial monitoring data refers to 5 ICUs (ICU 1-Medical, ICU 2-Surgical, ICU 3-Neurological, ICU 4-Cardiac, ICU 5-Neonatal), situated in different hospital buildings. Each ICU has different number of beds, rooms and room layout (beds/room). In all ICUs, air is supplied by a centralized HVAC system without air recirculation and running continuously, equipped with high-efficiency particulate air filters, with the exception of one of them (Neonatal ICU) which is equipped with a localized HVAC system. Table 1 shows the main characteristics of the involved ICUs.

Table 1.
ICUs involved in the investigation. (#: number).
2.2. Air Sampling
Data on microbial air sampling span over thirteen years (2009–2021) of surveillance. They have been collected in the patient area of the ICU rooms with the presence of patients only, after at least one hour from the morning cleaning activities and using an active sampler (Surface Air System Super ISO sampler (VWR International PBI, Milan Italy), with 55 mm diameter RODAC plates, a flow rate of 100 L/min, for a suction volume of 200 L. Results are expressed as colony-forming units (CFU)/m3. Tryptic Soy Agar (TSA) has been used for total mesophilic bacterial count. After field sampling, TSA agar plates have been incubated at 36° (±1) for 48 h. The results are interpreted, in accordance with Rocha et al. (2012) [33] as follows: very clean (<10 CFU/m3); clean (10–100 CFU/m3); acceptable (101–200 CFU/m3); contaminated (>200 CFU/m3).
2.3. Surface Sampling
It refers to a selection of the more frequently touched or the more neglected surfaces during cleaning activities. In detail, they are the following: cardiac monitor, medication cart, windowsill, headboard, bed lamp, baby incubator and, in some cases: infusion pump, wardrobe, bedside table. Sampling was carried out using 24 cm2 RODAC plates containing TSA, with a neutralizer added to inhibit the residual activity of the disinfectant used on the surfaces during sanitization. The sampling methods used for Rodac plates were as recommended by the European Standard—International Organization for Standardization (EN ISO) 14698-1:2004 [34]. After field sampling, TSA agar plates were incubated at 36° (±1) for 48 h. The results are interpreted, in accordance with CCLIN South-West 2016 and ISPESL 2009 guidelines [35,36,37,38], as follows: very clean (≤5 CFU/plate); clean (6–25 CFU/plate); acceptable (26–50 CFU/plate); contaminated (>50 CFu/plate). The contaminated level is unacceptable and requires a revision of the cleaning protocol [37,38].
Throughout the years air and surfaces samples have been collected by the same operators.
2.4. Microclimatic Monitoring
In order to have some indication about the HVACs functioning, microclimatic monitoring, based on measurements of air temperature, relative humidity and air velocity, has been performed in each sampling.
In order to evaluate the patients’ thermal comfort and to detect possible air drafts, the microclimatic parameters were measured next to the patient’s bed in the case of a single-bed room, or next to one of the beds in the case of multi-bed rooms, far from openings and sources of irradiation.
The measurement of the microclimatic parameters lasted about 30 min and was carried out in parallel with the microbiological sampling. The instruments automatically calculated the average values for each investigated parameter. A datalogger Delta OHM to measure temperature and relative humidity and a “Testo” anemometer to measure air velocity were used.
2.5. Statistical Analysis
Excel package (Microsoft Office, Microsoft Corporation, Redmond, WA, USA) was used for the statistical evaluations. Descriptive statistical analysis was performed to obtain mean, standard deviation, median and quartiles. Differences between the results recorded over the thirteen years of study (2009–2021) were evaluated using the analysis of variance. Chi-squared (X2) test for trend was used to assess variations in the percentage of contaminated samples over time. p-values of 0.05 were considered to indicate significance.
3. Results
Overall, 714 samples have been collected in the ICUs’ patients’ rooms during the period of exam. In particular, 232 samples regarded air and 482 were carried out on surfaces. The sample distribution varied among ICUs and over time, depending on specific requests and problems occurred along the years. Air and surface samplings results will be described separately.
3.1. Air Sampling Results
Table 2 shows the number of air samples and the mean level of bacterial contamination (SD, median and quartiles) obtained in each ICU.

Table 2.
Number of air samples and bacterial contamination (CFU/m3) in each ICUs. (#: number).
ICU 4 and ICU 5 showed an average bacterial level of > 200 CFU/m3, with variability of values, moving from 6–11 CFU/m3 to 972 CFU/m3 in both cases, as shown Table 2. In total, 69/232 air samples (29.7%) showed a level of contamination > 200 CFU/m3. Forty-two of them (60.9%) came from ICU 4 and ICU 5.
Figure 1 shows the distribution of all air samples by level of contamination in the investigated years. The percentage of contaminated samples varies over time, moving from a minimum value of 6.7% in 2015 to the highest of 65.0% in 2013.
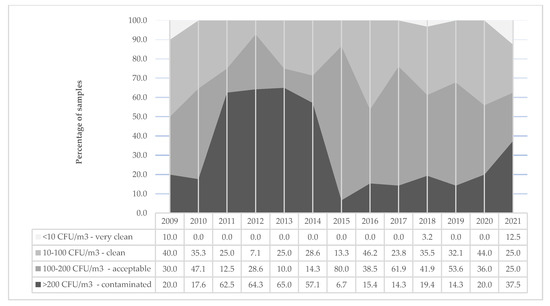
Figure 1.
Distribution (%) of air samples by level of contamination over years (2009–2021).
A clear distinction before and after 2015 in contaminated samples is observed. This difference justifies the overall significant decreasing trend in the percentage of contaminate samples (>200 CFU/m3) during years (X2 for trend = 11.6; p < 0.05). To understand if the observed decreasing trend is attributable to a general improvement of all ICUs, this analysis is stratified by ICU, analyzing six subsequent two-year periods (2009–2020) (Table 3). The number of contaminated samples (≥200 CFU/m3) on the total number of samples per ICUs along the years shows a significant decreased trend in ICU 5 (X2 for trend = 18.8; p < 0.005) only.

Table 3.
Temporal trend of contaminated air samples in each ICUs.
3.2. Surfaces Sampling Results
Table 4 reports the distribution of surface samples and average CFU/plate collected in each ICU in the study period. Median values and quartiles are also reported. The mean values reported underline that, in the average, all ICUs surfaces results were acceptable (>25–50 CFU/plate).

Table 4.
Number of surface samples and average level of microbial contamination of surfaces (CFU/plate) by ICU. (#: number).
In this case, ICU 4 also shows the highest mean and median value of CFU/plate and a high range of values (min–max: 1–195 CFU/plate). ICU 4 is followed by ICU 1 that shows similar problem, but of lesser entity (mean: 37.2 CFU/plate; median: 27 CFU/plate; min–max: 1–145 CFU/plate).
Figure 2 shows the distribution of all surface samples by level of contamination over time. Overall, 135 surfaces (28%), resulted as contaminated (>50 CFU/plate). Their percentage changes over time, moving from a minimum value of 15.6% in 2011 to the highest of 41.3% in 2018. A similar trend is observed in acceptable samples (>25–50 CFU/plate).
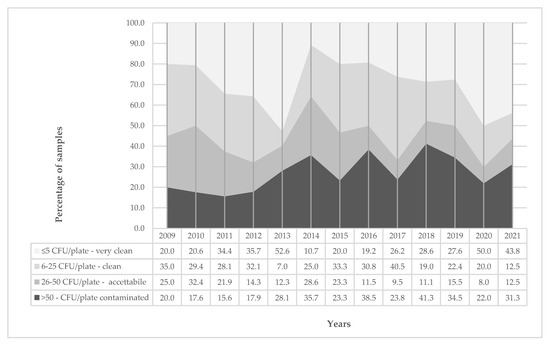
Figure 2.
Distribution (%) of surface samples by level of contamination over years (2009–2021).
As reported in Table 5, in which the contaminated surfaces (>50 CFU/plate) have been stratified by ICU and analyzed along six subsequent two-year periods (from 2009 to 2020), no significant decreasing trends in the percentage of contaminated sample have been observed.

Table 5.
Temporal trend of contaminated surface samples in each ICU.
To analyze which of the investigated surfaces is more contaminated, the results of the surface samples have been stratified by level of contamination. Figure 3 shows the distribution (%) of samples by surfaces and level of contamination. The cumulative results do not add up to 100% since the samples belonging to the “very clean” category have not been reported, to better show the differences among surfaces. The points connected with the line show the average number of CFU/plate collected in each surface. Over-beds (mean: 49.8 CFU/plate, SD 36.5) and cardiac monitors (mean: 41.7 CFU/plate, SD 36.7) show the highest average level of contamination. Over-beds result contaminated (>50 CFU/plate) in 51/87 samples (58.6%), above all next to electric sockets.
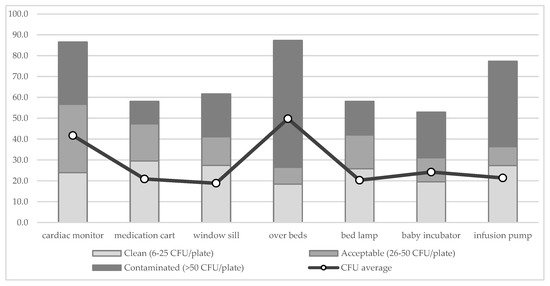
Figure 3.
Distribution (%) of samples by surfaces and level of contamination. The points connected with the line show the mean number of CFU/plate of each surface.
The last analysis refers to the samples over the threshold level that could increase the risk for HAI (500 CFU/100 cm2) [18]. Overall, 34 of 482 samples (7.1%) exceeded 500 CFU/100 cm2. The percentage distribution of over threshold surfaces varies among ICUs: it is 10% in ICU 1, 9.3% in ICU 5 and 6.3% in ICU 4. ICU 2 and ICU 3 show a lower percentage of 1.7 and 1.6%, respectively. It must be underlined while in ICU 4 most of threshold levels (71.4%) occurred before 2015, in ICU 1 100% of them occurred after 2015.
Figure 4 describes the distribution of samples > 500 CFU/100 cm2 by surfaces and ICUs. It is interesting to observe that most of these surfaces are those next to the beds, and commonly touched by patients and healthcare workers.
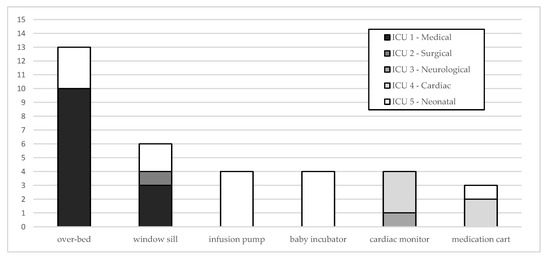
Figure 4.
Distribution of samples > 500 CFU/100 cm2 by surfaces and ICUs.
Finally, microclimatic monitoring results are shown. Table 6 summarizes data related to temperature, relative humidity and air velocity recorded during samplings in each ICU. For each parameter mean values (min–max) and percentage of compliant samples are reported. In 11.9% of sampling only, the microclimatic parameters resulted as all being compliant with regulations.

Table 6.
Mean values of temperature, relative humidity, and air velocity and % of compliant samples by parameter and ICUs.
A large variability is observed in the percentage of compliant samples to the microclimatic parameters within and among ICUs. Regarding the temperature, in all cases of non-compliant results the registered values were higher than those recommended. Regarding the relative humidity and the air velocity, the non-compliant values were mainly lower than those minimum recommended.
ICU 1 shows the lowest compliance’ percentages for temperature (38.9%) and air velocity (35.2%).
4. Discussion and Conclusions
The investigated ICUs show different size (rooms), functional layout and assistance targets. The ICUs 2, 3 and 4 are all single multi-bed rooms, while ICU 1 includes single-bed and multi-bed rooms. ICU 5 has baby-incubators distributed in four rooms.
These characteristics are coherent with Italian regulations [25,26], following which the ICUs rooms can be single-bed or multiple-bed. In general, the guidelines support the option that single rooms are superior to multi-bed rooms in terms of patient safety [8,16,39,40,41,42], of privacy and sleeping quality [43]. In the literature there is not agreement about the protective effect of single-bed ICU rooms from nosocomial infection rate if compared to a multibed unit. Many studies consider the design features subordinate to other drivers of infection control, such as, for example, ICU management. The functional layout is mainly finalized to favor appropriate personnel behavior and its efficiency, rather than to contain the infection risk [11].
Another critical aspect is the ICUs’ size. In the Italian regulation [25,26] their sizes vary between newly built ICUs or restructured, or between single-bed or multiple-bed ICUs. For newly built ICUs the minimum surface area for a single room is 20 and 16 m2 per bed for a multiple-bed ICU, with a distance between beds of ≥ 2.5 m. For already existing ICUs, the minimum surface area for each single-bed room is 16 and 12 m2 per bed for a multi-bed ICU, except for Neurovascular ICUs in which the minimum surface is 9 m2 per bed. The importance of increasing the distance between beds is a basic recommendation in hospital environments mainly to reduce the spread of respiratory infections (e.g., tuberculosis), and it is currently the main preventive measure to counteract the spread of all indirectly transmitted infections, Covid-19 included, together with hand washing and the use of protective devices [44,45,46]. Other countries (e.g., USA, Australia) define different size standards, generally larger [27,28]. The role of design should be to reduce travel distances for staff, placing frequently needed spaces, equipment or materials as close as possible to the site of use. On this topic, we agree with Thompson et al. [39], who considers as efficient a unit small enough for care providers to be fully aware of all activities, yet large enough to permit safety and efficiency. It is not a matter of choosing a centralized or decentralized design, it is important that caregivers are allowed to observe patients from many points within the unit [8].
The investigated ICUs were all equipped with HVAC systems, since they are specific requirements reported in the Italian regulation [25,26]. In particular, the regulation indicates the following standards: a temperature between 20 and 24°, a relative humidity between 40 and 60% and at least six air changes/hour (ACH). A higher temperature range is recommended for Neurovascular ICUs (20–26°) and Neonatal ICUs (20–28°). The high efficiency air filtration is required in all ICUs, excluding Neonatal ICUs. Absolute filtration (≥99.95%) is required for isolation rooms only. The positive pressure is required in neonatal ICUs, while in other types of ICUs (resuscitation, cardiology, neurovascular) the pressure can be positive or negative (+ or −10 Pa), according to needs. During the current COVID-19 pandemic, for example, some authors recommended to place intensive care rooms under negative or even normal pressure to protect the staff and patients’ health [47], but it has been observed that this solution increased the risk of opportunistic infections in immunocompromised patients [48].
In a relevant number of samplings the investigated ICUs showed microclimatic parameters levels that were not compliant with the regulations reported above [25,26]. This situation could depend on several factors. First of all, the opening of doors inside the ICUs environments; in fact, during sampling campaigns the doors were frequently found open. This habit, also observed in other investigations [30,49,50,51], is deleterious, since it modifies the microclimatic conditions and it hinders the ICUs’ pressurization. A limit of this investigation is that the number of air changes/hour (ACH) and the air pressurization were not measured; therefore it is difficult to try to make robust conclusions about the role of building and HVAC characteristics on the concentrations of air sampled microbes. These parameters will be included in future investigations regarding these ICUs.
Secondly, the periodic maintenance of HVAC systems could be insufficient, since it generally occurs once a year in these environments. In particular, the air velocity resulted as compliant in 56.1% of samplings only, resulting lower than 0.05 m/sec in the others; this condition could be related to a shortage of the filters maintenance (e.g., filters saturation). This is another aspect to investigate in the future.
Coming back to the bacterial pollution described in this study, while a general decreasing trend of air contamination during the years is observed, the surfaces contamination does not show a significant improvement. In particular, ICU 4 and ICU 5 have shown an average air contamination > 200 CFU/m3, but a significant improvement has occurred in ICU 5 only. This Neonatal ICU is equipped with a decentralized HVAC system. The improvement could be related to a higher respect of a periodic filter’s maintenance. Contrariwise, in this ICU, as in the other ICUs investigated, the contamination of surfaces does not show a significant improvement. It could depend on the average acceptable level of CFU/plate observed in all ICUs. Regardless, 28% of samples were contaminated (>50 CFU/plate), supporting the need to review cleaning protocols and personnel’s behaviors. Actually, as already described in a previous study [52,53], the lack of knowledge is the major reason for non-adherence to procedures and education is an important factor to influence compliance with good practices. At the same time, an efficient service requires an adequate staff (e.g., nurses) in terms of competence and numerosity.
As reported in the literature [14,21], several frequently touched surfaces have shown a high level of contamination. Many of them exceeded the threshold level of infection risk, as indicated by Salgado et al. [18]; in particular over-bed surfaces, infusion pumps and baby incubators. While in ICU 4 they were reduced along the years, a particular attention should be given to ICU 1, since all these over-threshold samples have been collected in the last years (after 2015). This ICU also shows the lowest percentage of compliant samples in terms of microclimatic conditions. It means that it will be necessary to focus attention on this issue to increase patients’ and healthcare workers’ safety and comfort. At the same time it is important to reduce the number of people and their movements inside the ICUs’ environments, since the direct correlation between microbial pollution and number of people is well documented (e.g., operating rooms) [30,49,54,55]. Unfortunately, the occupational density during the samplings was not measured in this investigation and it is a limit of the study, since the number of occupants had certainly impacted the concentration of microbes in air samples significantly and could have helped to better understand the causes of the observed problems. This aspect will also be taken into account in the future investigations.
In conclusion, the care for patients admitted to ICUs is very demanding and consists of a complex of medical procedures, whose complexity depends on the underlying disease. A fundamental part of intensive care is prevention of infection. This study dealt with microbial contamination in different types of ICUs, monitoring both air and surfaces contamination, but also considering their environmental characteristics and type of activity. Several criticalities have been observed that allowed us to identify priorities and areas with major intervention needs.
Author Contributions
Conceptualization, D.D.; methodology, D.D., M.F.; software, D.D., L.A.; validation, D.D., M.F.; formal analysis, D.D.; investigation, M.F.; data curation, M.F., L.A.; writing—original draft preparation, D.D., L.A.; writing—review and editing, D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- D’Alessandro, D.; Fara, G.M. Hospital environments and epidemiology of healthcare-associated infections. In Indoor Air Quality in Healthcare Facilities; SpringerBriefs in Public Health; Capolongo, S., Settimo, G., Gola, M., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 41–52. [Google Scholar]
- Valentin, A.; Ferdinande, P.; ESICM Working Group on Quality Improvement. Recommendations on basic requirements for intensive care units: Structural and organizational aspects. Intensive Care Med. 2011, 37, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L. Guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am. J. Infect. Control 2007, 35, S65–S164. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Tseng, L.; Yang, L.S. Microbial air contamination in an intensive care unit. Int. J. Public Health Sci. 2015, 4, 145–151. [Google Scholar] [CrossRef]
- Verderber, S.; Gray, S.; Suresh-Kumar, S.; Kercz, D.; Parshuram, C. Intensive Care Unit Built Environments: A Comprehensive Literature Review (2005–2020). Health Environ. Res. Des. J. 2021, 1–48. [Google Scholar] [CrossRef]
- Bracco, D.; Dubois, M.J.; Bouali, R.; Eggimann, P. Single rooms may help to prevent nosocomial bloodstream infection and cross-transmission of methicillin-resistant Staphylococcus aureus in intensive care units. Intensive Care Med. 2007, 33, 836–840. [Google Scholar] [CrossRef]
- Bloemendaal, A.L.; Fluit, A.C.; Jansen, W.M.; Vriens, M.R.; Ferry, T.; Argaud, L.; Amorim, J.M.; Resende, A.C.; Pascual, A.; Lopez-Cerero, L.; et al. Acquisition and cross-transmission of Staphylococcus aureus in European intensive care units. Infect. Control. Hosp. Epidemiol. 2009, 30, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Teltsch, D.Y.; Hanley, J.; Loo, V.; Goldberg, P.; Gursahaney, A.; Buckeridge, D.L. Infection acquisition following intensive care unit room privatization. Arch. Intern. Med. 2011, 171, 32–38. [Google Scholar] [CrossRef]
- Cepeda, J.A.; Whitehouse, T.; Cooper, B.; Hails, J.; Jones, K.; Kwaku, F.; Taylor, L.; Hayman, S.; Cookson, B.; Shaw, S.; et al. Isolation of patients in single rooms or cohorts to reduce the spread of MRSA in intensive care units: Prospective two-centre study. Lancet 2005, 364, 295–304. [Google Scholar] [CrossRef]
- Stiller, A.; Schröder, C.; Gropmann, A.; Schwab, F.; Behnke, M.; Geffers, C.; Sunder, W.; Holzhausen, J.; Gastmeier, P. ICU ward design and nosocomial infection rates: A cross-sectional study in Germany. J. Hosp. Infect. 2017, 95, 71–75. [Google Scholar] [CrossRef]
- Ministerio de Sanidad y Política Social. Unidades de Cuidados Intensivos. Estanderas y Recomendaciones; Ministerio de Sanidad y Política Social: Madrid, Spain, 2010; NIPO EN LINEA: 840-10-098-6. [Google Scholar]
- Russotto, V.; Cortegiani, A.; Raineri, S.M.; Giarratano, A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J. Intensive Care 2015, 3, 54. [Google Scholar] [CrossRef]
- Gastmeier, P.; Loui, A.; Stamm-Balderjahn, S.; Hansen, S.; Zuschneid, I.; Sohr, D.; Behnke, M.; Obladen, M.; Vonberg, R.P.; Rüden, H. Outbreaks in neonatal intensive care units—They are not like others. Am. J. Infect. Control 2007, 35, 172–176. [Google Scholar] [CrossRef]
- Mora, M.; Mahnert, A.; Koskinen, K.; Pausan, M.R.; Oberauner-Wappis, L.; Krause, R.; Perras, A.K.; Gorkiewicz, G.; Berg, G.; Moissl-Eichinger, C. Microorganisms in Confined Habitats: Microbial Monitoring and Control of Intensive Care Units, Operating Rooms, Cleanrooms and the International Space Station. Front. Microbiol. 2016, 7, 1573. [Google Scholar] [CrossRef] [PubMed]
- Tringe, S.G.; Hugenholtz, P. A renaissance for the pioneering 16S rRNA gene. Curr. Opin. Microbiol. 2008, 11, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Passaretti, C.L.; Otter, J.A.; Reich, N.G.; Myers, J.; Shepard, J.; Ross, T.; Carroll, K.C.; Lipsett, P.; Perl, T.M. An evaluation of environmental decontamination with hydrogen peroxide vapor for reducing the risk of patient acquisition of multidrug-resistant organisms. Clin. Infect. Dis. 2013, 56, 27–35. [Google Scholar] [CrossRef]
- Salgado, C.D.; Sepkowitz, K.A.; John, J.F.; Cantey, J.R.; Attaway, H.H.; Freeman, K.D.; Sharpe, P.A.; Michels, H.T.; Schmidt, M.G. Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect. Control. Hosp. Epidemiol. 2013, 34, 479–486. [Google Scholar] [CrossRef]
- Handorean, A.; Robertson, C.E.; Harris, J.K.; Frank, D.; Hull, N.; Kotter, C.; Stevens, M.J.; Baumgardner, D.; Pace, N.R.; Hernandez, M. Microbial aerosol liberation from soiled textiles isolated during routine residuals handling in a modern health care setting. Microbiome 2015, 3, 72. [Google Scholar] [CrossRef]
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Pittet, D.; Allegranzi, B.; Sax, H.; Dharan, S.; Pessoa-Silva, C.L.; Donaldson, L.; Boyce, J.M.; WHO Global Patient Safety Challenge; World Alliance for Patient Safety. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect. Dis. 2006, 6, 641–652. [Google Scholar] [CrossRef]
- Finzi, G.; Mura, I.; Kob, K.; Sideli, C.; Lanzoni, L.; Mazzacane, S.; Odi, A.A.; Appicciafuoco, A.; Barchitta, M.; Bertinato, L.; et al. Linee Guida Sulla Valutazione del Processo di Sanificazione Ambientale Nelle Strutture Ospedaliere e Territoriali per Il Controllo Delle Infezioni Correlate All’assistenza (ICA); ANMDO: Bologna, Italy, 2018; Available online: https://www.anmdo.org/wp-content/uploads/2019/01/libro-uno-finzi-1.pdf (accessed on 8 July 2021).
- European Commission. EudraLex. The Rules Governing Medicinal Products in the European Union. Volume 4. EU Guidelines to Good Manifacturing Practice Medicinal Products for Human and Veterinary Use; European Commission: Brussels, Belgium, 2010. Available online: https://ec.europa.eu/health/sites/default/files/files/eudralex/vol-4/2011_intro_en.pdf (accessed on 12 July 2021).
- Saran, S.; Gurjar, M.; Baronia, A.; Sivapurapu, V.; Ghosh, P.S.; Raju, G.M.; Maurya, I. Heating, ventilation and air conditioning (HVAC) in intensive care unit. Crit Care. 2020, 24, 194. [Google Scholar] [CrossRef] [PubMed]
- Decree of the President of the Republic. Approvazione Dell’atto di Indirizzo e Coordinamento Alle Regioni e Alle Province Autonome di Trento e di Bolzano, in Materia di Requisiti Strutturali, Tecnologici ed Organizzativi Minimi per L’esercizio Delle Attivita’ Sanitarie da Parte Delle Strutture Pubbliche e Private. (GU Serie Generale n.42 del 20-02-1997—Suppl. Ordinario n. 37). 1997. Available online: https://www.gazzettaufficiale.it/eli/gu/1997/02/20/42/so/37/sg/pdf (accessed on 8 July 2021).
- Lazio region. Decree of the Commissioner ad Acta U0090 of 10 November 2010 (annex 1). Minimum Authorization Requirements for the Exercise of Health and Socio-Health Activities. Available online: http://www.regione.lazio.it/binary/rl_sanita/tbl_contenuti/Allegato_1_Decr_U0090_2010.pdf (accessed on 14 July 2021).
- College of Intensive Care Medicine of Australia and New Zealand. Minimum Standards for Intensive Care Units; CICM, 2011. Available online: https://www.cicm.org.au/CICM_Media/CICMSite/CICM-Website/Resources/Professional%20Documents/IC-1-Minimum-Standards-for-Intensive-Care-Units.pdf (accessed on 8 July 2021).
- Society of Critical Care Medicine. Guidelines for intensive care unit design. Guidelines/Practice Parameters Committee of the American College of Critical Care Medicine. Crit. Care Med. 1995, 23, 582–588. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention (CDC). Guidelines for Environmental Infection Control in Health-Care Facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC); U.S. Department of Health and Human Services: Atlanta, GA, USA, 2003. Available online: https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines-P.pdf (accessed on 8 July 2021).
- Pasquarella, C.; Vitali, P.; Saccani, E.; Manotti, P.; Boccuni, C.; Ugolotti, M.; Signorelli, C.; Mariotti, F.; Sansebastiano, G.E.; Albertini, R. Microbial air monitoring in operating theatres: Experience at the University Hospital of Parma. J. Hosp. Infect. 2012, 81, 50–57. [Google Scholar] [CrossRef]
- Vescia, N.; Brenier-Pinchart, M.P.; Osborn, J.F.; Cerquetani, F.; Cavarischia, R.; Grillot, R.; D’Alessandro, D. Field validation of a dusting cloth for mycological surveillance of surfaces. Am. J. Infect. Control 2011, 39, 156–158. [Google Scholar] [CrossRef]
- D’Alessandro, D.; Cerquetani, F.; Deriu, M.G.; Montagna, M.T.; Mura, I.; Napoli, C.; Vescia, N. Evaluation of fungal contamination in operating rooms using a dusting cloth pad: Comparison among different sampling methods. Am. J. Infect. Control 2013, 41, 658–660. [Google Scholar] [CrossRef]
- Rocha, A.C.; Baez, N.A.; Villaroel, E.V.; Quintero, G.M. Study of bioaerosols in surgical theatres and intensive care units from a public general hospital. JBM 2012, 2, 1–10. [Google Scholar] [CrossRef]
- EN (European Standard)—ISO (International Organization for Standardization). Clean Rooms and Associated Controlled Environments. Biocontamination Control. Part 1: General Principles and Methods; International Organization for Standardization: Geneva, Switzerland, 2004. [Google Scholar]
- Istituto Superiore per la Prevenzione e la Sicurezza del Lavoro (ISPESL). Linee Guida Sugli Standard di Sicurezza e di Igiene del Lavoro nel Reparto Operatorio; ISPESL: Roma, Italy, 2009. Available online: https://www.inail.it/cs/internet/docs/linee-guida-igiene-reparto-operatorio.pdf?section=attivita (accessed on 8 July 2021).
- CCLIn Sud-Ouest. Surveillance Microbiologique de L’environnement Dans les Établissements de Santé. Guide de Bonnes Pratiques. 2016. Available online: https://www.cpias-nouvelle-aquitaine.fr/wp-content/uploads/2015/08/Surv_microbio_environnement.pdf (accessed on 12 July 2021).
- Barca, S.; Caradonna, L.; Giaquinta, G.; Giovinazzo, R.; Guerrera, E.; Mameli, M.; Mansi, A.; Marena, G.; Mastromartino, T.; Sarto, D.; et al. Contaminazione Microbiologica Delle Superfici Negli Ambienti di Lavoro; INAIL, 2017. Available online: https://www.inail.it/cs/internet/docs/alg-pubbl-la-contaminazione-microbiologica-delle-superfici.pdf (accessed on 12 July 2021).
- European Commission. EudraLex. The Rules Governing Medicinal Products in the European Union. Volume 4. Goof Manifacturing Practice. Guidelines of Good Manifacturing Practice specific to Advanced Therapy Medicinal Products. 2017. Available online: https://ec.europa.eu/health/sites/default/files/files/eudralex/vol-4/2017_11_22_guidelines_gmp_for_atmps.pdf (accessed on 12 July 2021).
- Thompson, D.R.; Hamilton, D.K.; Cadenhead, C.D.; Swoboda, S.M.; Schwindel, S.M.; Anderson, D.C.; Schmitz, E.V.; St Andre, A.C.; Axon, D.C.; Harrell, J.W.; et al. Guidelines for intensive care unit design. Crit Care Med. 2012, 40, 1586–1600. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, H.; Mahmood, A.; Valente, M. Advantages and disadvantages of single versus multiple occupancy rooms in acute care environments: A review and analysis of the literature. Environ. Behav. 2005, 37, 760–786. [Google Scholar] [CrossRef]
- Harris, D.D.; Shepley, M.M.; White, R.D.; Kolberg, K.J.S.; Harrell, J.W. The impact of single family room design on patients and caregivers: Executive summary. J. Perinatol. 2006, 26, S38–S48. [Google Scholar] [CrossRef][Green Version]
- Chaudhury, H.; Mahmood, A.; Valente, M. Nurses’ perception of single-occupancy versus multioccupancy rooms in acute care environments: An exploratory comparative assessment. Appl. Nurs. Res. 2006, 19, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Gabor, J.Y.; Cooper, A.B.; Crombach, S.A.; Lee, B.; Kadikar, N.; Bettger, H.E.; Hanly, P.J. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am. J. Respir. Crit. Care Med. 2003, 167, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Appolloni, L.; D’Alessandro, D. Housing Spaces in Nine European Countries: A Comparison of Dimensional Requirements. Int. J. Environ. Res. Public Health 2021, 18, 4278. [Google Scholar] [CrossRef]
- Jones, N.R.; Qureshi, Z.U.; Temple, R.J.; Larwood, J.P.J.; Greenhalgh, T.; Bourouiba, L. Two metres or one: What is the evidence for physical distancing in covid-19. BMJ 2020, 370, m3223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Otter, J.A.; Price, J.R.; Cimpeanu, C.; Garcia, D.M.; Kinross, J.; Boshier, P.R.; Mason, S.; Bolt, F.; Holmes, A.H.; et al. Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. medRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Ichai, P.; Saliba, F.; Baune, P.; Daoud, A.; Coilly, A.; Samuel, D. Impact of negative air pressure in ICU rooms on the risk of polmunary aspergillosis in COVID-19 patients. Crit. Care 2020, 21, 538. [Google Scholar] [CrossRef]
- Agodi, A.; Auxilia, F.; Barchitta, M.; Cristina, M.L.; D’Alessandro, D.; Mura, I.; Nobile, M.; Pasquarella, C.; Italian Study Group of Hospital Hygiene. Operating theatre ventilation systems and microbial air contamination in total joint replacement surgery: Results of the GISIO-ISChIA study. J. Hosp. Infect. 2015, 90, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Balocco, C.; Petrone, G.; Cammarata, G. Assessing the effects of sliding doors on an operating theatre climate. Build. Simul. 2012, 5, 73–83. [Google Scholar] [CrossRef]
- Mears, S.C.; Blanding, R.; Belkoff, S.M. Door opening affects operating room pressure during joint arthroplasty. J. Orthoped. 2015, 38, 991–994. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.; Agodi, A.; Auxilia, F.; Brusaferro, S.; Calligaris, L.; Ferrante, M.; Montagna, M.T.; Mura, I.; Napoli, C.; Pasquarella, C.; et al. Prevention of healthcare associated infections: Medical and nursing students’ knowledge in Italy. Nurse Educ. Today 2014, 34, 191–195. [Google Scholar] [CrossRef]
- Masia, M.D.; Dettori, M.; Deriu, M.G.; Soddu, S.; Deriu, M.; Arghittu, A.; Azara, A.; Castiglia, P. Microbial monitoring as a tool for preventing infectious risk in the operating room: Results of 10 years activity. Atmosphere 2021, 12, 19. [Google Scholar] [CrossRef]
- Birgand, G.; Saliou, P.; Lucet, J.C. Influence of staff behavior on infectious risk in operating rooms: What is the evidence? Infect. Control. Hosp. Epidemiol. 2015, 36, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Pokrywka, M.; Byers, K. Traffic in the operating room: A review of factors influencing air flow and surgical wound contamination. Infect. Disord. Drug. Targets 2013, 13, 156–161. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).