Microbiological Aerosol, Particulate Matter Concentrations and Antibiotic Resistant Staphylococcus spp. in the Premises of Poland’s Oldest Agricultural School
Abstract
:1. Introduction
2. Materials and Methods
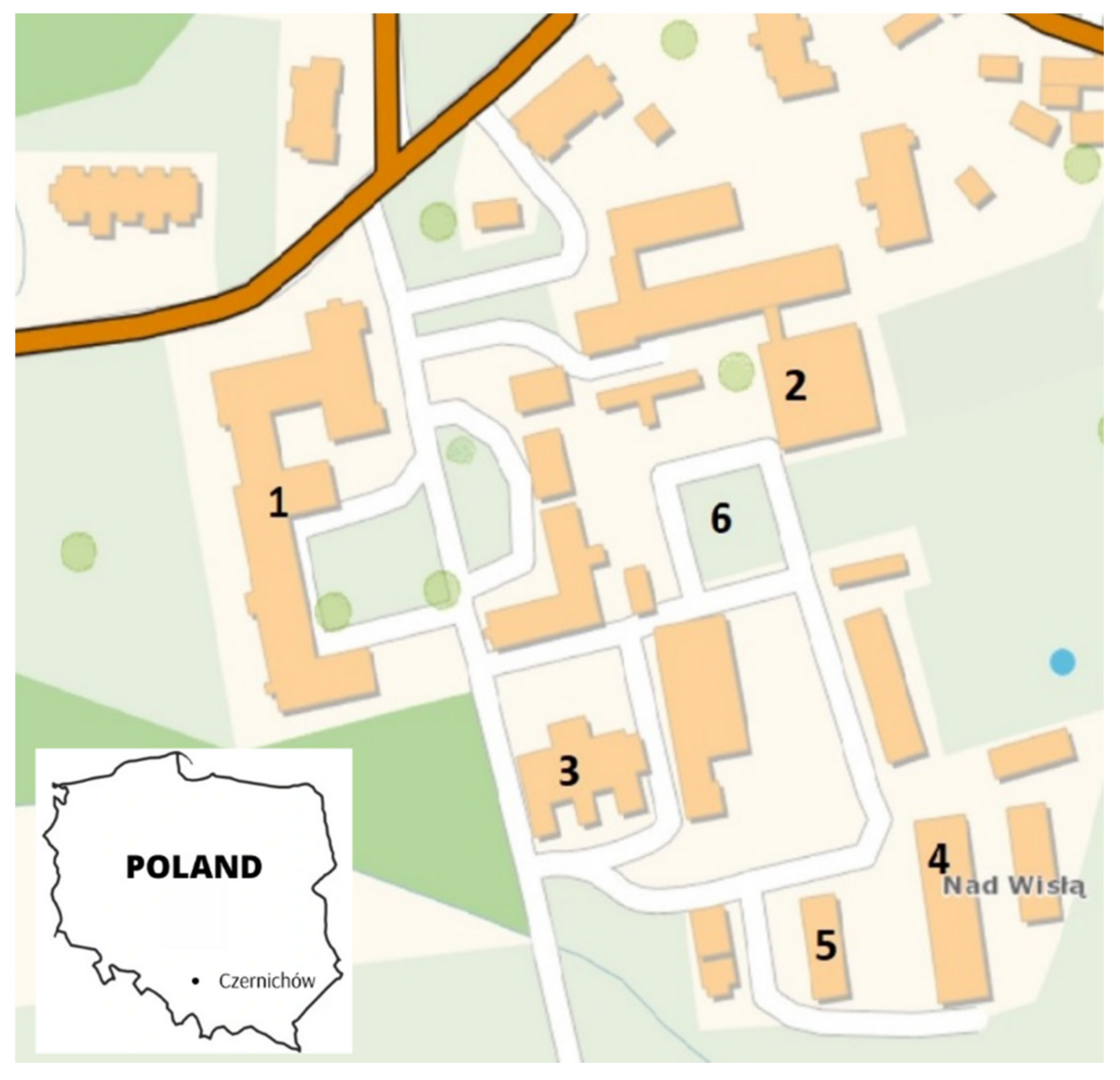
2.1. Study Site and Study Design
2.2. Bioaerosol Measurements
2.3. Airborne Dust Concentration and Physical Parameter Measurements
2.4. Species Identification of Staphylococcus spp.
2.5. Antimicrobial Resistance Testing
2.6. Data Interpretation and Statistical Analysis
3. Results and Discussion
3.1. Microclimatic Parameters
3.2. Particulate Matter Concentrations
3.3. Bioaerosol Concentration
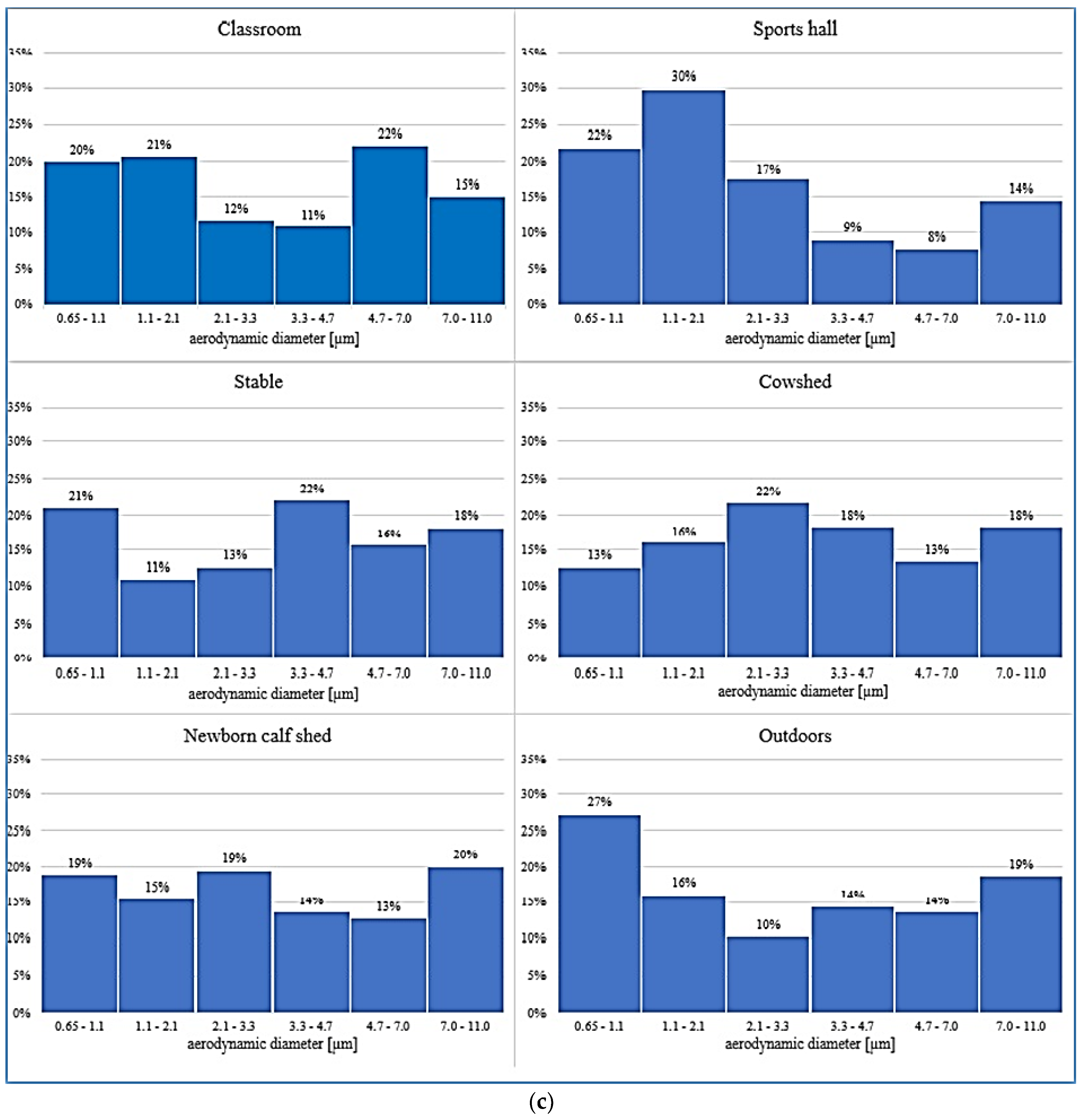
3.4. Bioaerosols vs. Particulate Matter Levels
3.5. Species Identification and Antimicrobial Resistance of Staphylococcus spp.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chien, Y.-C.; Chen, C.-J.; Lin, T.-H.; Chen, S.-H.; Chien, Y.-C. Characteristics of microbial aerosols released from chicken and swine feces. J. Air Waste Manag. 2011, 61, 882–889. [Google Scholar] [CrossRef]
- Directive 2000/54/EC of the European Parliament and of the Council of 18 September 2000 on the Protection of Workers from Risks Related to Exposure to Biological Agents at Work (Seventh Individual Directive within the Meaning of Article 16 of Directive 89/391/EEC); European Parliament: Brussels, Belgium, 2000.
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol Health Effects and Exposure Assessment: Progress and Prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [PubMed] [Green Version]
- Dutkiewicz, J.; Cisak, E.; Sroka, J.; Wójcik-Fatla, A.; Zając, V. Biological agents as occupational hazards—Selected issues. Ann. Agric. Environ. Med. 2011, 18, 286–293. [Google Scholar] [PubMed]
- Ropek, D.; Frączek, K. Microbiological air quality in livestock farm buildings. Environ. Med. 2016, 19, 16–22. [Google Scholar]
- Pavia, D.; Agnew, J.E.; Clarke, S.W. Inhaled aerosols: Deposition and clearance. In Progress Radiopharmacy; Cox, P.H., Mather, S.J., Sampson, C.B., Lazarus, C.R., Eds.; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1986; pp. 579–589. [Google Scholar]
- Szulc, J.; Otlewska, A.; Okrasa, M.; Majchrzycka, K.; Sulyok, M.; Gutarowska, B. Microbiological contamination at workplaces in a combined heat and power (CHP) station processing plant biomass. Int. J. Environ. Res. Public Health 2017, 14, 99. [Google Scholar] [CrossRef] [Green Version]
- Szulc, J.; Okrasa, M.; Dybka-Stępień, K.; Sulyok, M.; Nowak, A.; Otlewska, A.; Szponar, B.; Majchrzycka, K. Assessment of Microbiological Indoor Air quality in cattle breeding farms. Aerosol Air Qual. Res. 2020, 20, 1353–1373. [Google Scholar] [CrossRef] [Green Version]
- Plewa-Tutaj, K.; Pietras-Szewczyk, M.; Lonc, E. Attempt to Estimate Spatial Distribution of Microbial Air Contamination on the Territory and in Proximity of a Selected Poultry Farm. Environ. Prot. 2014, 36, 21–28. [Google Scholar]
- Madsen, A.M.; Kurdi, I.; Feld, L.; Tendal, K. Airborne MRSA and total Staphylococcus aureus as associated with particles of different sizes on pig farms. Ann. Work Expo. Health 2018, 62, 966–997. [Google Scholar] [CrossRef] [Green Version]
- Clauß, M. Emission of Bioaerosols from Livestock Facilities: Methods and Results from Available Bioaerosol Investigations in and Around Agricultural Livestock Farming; Thünen Working Paper, No. 138a; Johann Heinrich von Thünen-Institut: Braunschweig, Germany, 2020. [Google Scholar] [CrossRef]
- Evans, C. Bioaerosol Exposures from Three Utah Cattle Operations. Graduate Theses & Non-Theses.112. 2017. Available online: http://digitalcommons.mtech.edu/grad_rsch/112 (accessed on 20 May 2021).
- Dave, P.; Mahendra, R.; Pal, M. Etiologic significance of Aspergillus terreus in primary cutaneous mycosis of an agricultural worker. Mol. Microbiol. Res. 2015, 5, 1–4. [Google Scholar] [CrossRef]
- Owen, M.K.; Ensor, D.S.; Sparks, L.E. Airborne particle sizes and sources found in indoor air. Atmos. Environ. 1992, 26A, 2149–2162. [Google Scholar] [CrossRef]
- Bulski, K.; Frączek, K.; Cendrowska, A.; Chmiel, M.J. Bacteriological Air Quality at Animal Veterinary Practice. Annu. Set Environ. Prot. 2019, 21, 841–945. [Google Scholar]
- Harkawy, A.; Górny, R.L.; Ogierman, L.; Wlazło, A.; Ławniczek-Wałczyk, A.; Niesler, A. Bioaerosol assessment in naturally ventilated historical library building with restricted personnel access. Ann. Agric. Environ. Med. 2011, 18, 323–329. [Google Scholar]
- Kim, E.; Kim, H.-J.; Yang, S.-M.; Kim, C.-G.; Choo, D.-W.; Kim, H.-Y. Rapid Identification of Staphylococcus Species Isolated from Food Samples by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. J. Microbiol. Biotechnol. 2019, 29, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Wanecka, A.; Król, J.; Twardoń, J.; Mrowiec, J.; Korzeniowska-Kowal, A.; Wzorek, A. Efficacy of MALDI-TOF mass spectrometry as well as genotypic and phenotypic methods in identification of staphylococci other than Staphylococcus aureus isolated from intramammary infections in dairy cows in Poland. J. Vet. Diagn. Investig. 2019, 31, 523–530. [Google Scholar] [CrossRef]
- Dubois, D.; Leyssene, D.; Chacornac, J.P.; Kostrzewa, M.; Schmit, P.O.; Talon, R.; Bonnet, R.; Delmas, J. Identification of a Variety of Staphylococcus Species by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2010, 48, 941–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustyńska, D.; Pośniak, M. Harmful Factors in the Working Environment—Limit Values. Interdepartmental Commission for Maximum Admissible Concentrations and Intensities for Agents Harmful to Health in the Working Environment; CIOP-PIB: Warsaw, Poland, 2016. (In Polish) [Google Scholar]
- Prędecka, S.; Kosut, S. Analysis of microbiological hazards in the indoor air of the selected rooms of the Main School of Fire Service. Zesz. Nauk. SGSP/Szk. Główna Służ. Pożar. 2017, 62, 105–125. [Google Scholar]
- Tan, Z.; Zhang, Y. A Review of Effects and Control Methods of Particulate Matter in Animal Indoor Environments. J. Air Waste Manag. 2004, 54, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Cyrys, J.; Pitz, M.; Bischof, W.; Wichmann, H.-E.; Heinrich, J. Relationship between indoor and outdoor levels of fine particle mass, particle number concentrations and black smoke under different ventilation conditions. J. Expo. Sci. Environ. Epidemiol. 2004, 14, 275–283. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 17 July 2021).
- Traczyk, P.; Gruszecka-Kosowska, A. The Condition of Air Pollution in Kraków, Poland, in 2005–2020, with Health Risk Assessment. Int. J. Environ. Res. Public Health 2020, 17, 6063. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Ambient Air Pollution Database May 2016; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Regulation of the Minister of Family, Labor and Social Policy of June 12, 2018 on the Maximum Permissible Concentrations of Harmful Factors in the Working Environment (Item 1286); Chancellery of the Prime Minister: Warsaw, Poland, 2018.
- Islam, A.; Ikeguchi, A.; Naide, T. Concentration of aerosol numbers and airborne bacteria, and temperature and relative humidity, and their interrelationships in a tie˗stall dairy barn. Animals 2019, 9, 1023. [Google Scholar] [CrossRef] [Green Version]
- Cambra-Lopez, M.; Aarnink, A.J.A.; Zhao, Y.; Calvet, S.; Torres, A.G. Airborne particulate matter from livestock production systems: A review of an air pollution problem. Environ. Pollut. 2010, 158, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Takai, H.; Pedersen, S.; Johnsen, J.O.; Metz, J.H.M.; Groot Koerkamp, P.W.G.; Uenk, G.H.; Phillips, V.R.; Holden, M.R.; Sneath, R.W.; Short, J.L.; et al. Concentrations and emissions of airborne dust in livestock building in Northern Europe. J. Agric. Eng. Res. 1998, 70, 59–77. [Google Scholar] [CrossRef] [Green Version]
- Winkel, A.; Mosquera, J.; Groot Koerkamp, P.W.G.; Ogink, N.W.M.; Aarnink, A.J.A. Emissions of particulate matter from animal houses in the Netherlands. Atmos. Environ. 2015, 111, 202–212. [Google Scholar] [CrossRef]
- Brągoszewska, E.; Pastuszka, J.S. Influence of meteorological factors on the level and characteristics of culturable bacteria in the air in Gliwice, Upper Silesia (Poland). Aerobiologia 2018, 34, 241–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, W.-K.; Kang, J.-H. Exposure levels of airborne bacteria and fungi in Korean swine and poultry sheds. Arch. Environ. Occup. Health. 2005, 60, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Basińska, M.; Michałkiewicz, M. Variability of microbial air pollution and dust concentration inside and outside a selected school in Poznań. J. Ecol. Eng. 2016, 50, 17–25. [Google Scholar] [CrossRef]
- Bulski, K.; Korta-Pepłowska, M. Microbiological quality of indoor air at reptile store. Water Environ. Rural Areas 2017, 17, 27–35. [Google Scholar]
- Roussel, S.; Sudre, B.; Reboux, G.; Waser, M.; Buchele, G.; Vacheyrou, M.; Dalphin, J.C.; Millon, L.; Braun-Fahrländer, C.; von Mutius, E.; et al. Exposure to moulds and actinomycetes in Alpine farms: A nested environmental study of the PASTURE cohort. Environ. Res. 2011, 111, 744–750. [Google Scholar] [CrossRef]
- Reponen, T.A.; Gazenko, S.V.; Grinshpun, S.A.; Willeke, K.; Cole, E.C. Characteristic of airborne actinomycete spores. Appl. Environ. Microbiol. 1998, 64, 3807–3812. [Google Scholar] [CrossRef] [Green Version]
- Wlazło, A.; Górny, R.L.; Złotkowska, R.; Ławniczek, A.; Łudzeń-Izbińska, B.; Harkawy, A.S.; Anczyk, E. Workers’ exposure to selected biological agents in libraries of Upper Silesia. Occup. Med. 2008, 59, 159–170. [Google Scholar]
- Brągoszewska, E.; Mainka, A.; Pastuszka, J.S.; Lizończyk, K.; Desta, Y.G. Assessment of bacterial aerosol in a preschool, primary school and high school in Poland. Atmosphere 2018, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Clauß, M. Particle size distribution of airborne micro-organisms in the environment—A review. Landbauforsch. Appl. Agric. For. Res. 2015, 65, 77–100. [Google Scholar]
- Lee, S.-A.; Adhikari, A.; Grinshpun, S.A.; McKay, R.; Shukla, R.; Reponen, T. Personal exposure to airborne dust and microorganisms in agricultural environments. J. Occup. Environ. Hyg. 2006, 3, 118–130. [Google Scholar] [CrossRef]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dordet-Frisoni, E.; Dorchies, G.; De Araujo, C.; Talon, R.; Leroy, S. Genomic diversity in Staphylococcus xylosus. Appl. Environ. Microbiol. 2020, 73. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.-W.; Heo, S.; Ryu, S.; Blom, J.; Lee, J.H. Genomic insights into the virulence and salt tolerance of Staphylococcus equorum. Sci. Rep. 2017, 7, 5383. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.; Lee, J.H.; Jeong, D.W. Food-derived coagulase-negative Staphylococcus as starter cultures for fermented foods. Food Sci. Biotechnol. 2020, 29, 1023–1035. [Google Scholar] [CrossRef]
- Dakić, I.; Morrison, D.; Vuković, D.; Savić, B.; Shittu, A.; Jezek, P.; Hauschild, T.; Stepanović, S. Isolation and molecular characterization of Staphylococcus sciuri in the hospital environment. J. Clin. Microbiol. 2005, 43, 2782–2785. [Google Scholar] [CrossRef] [Green Version]
- Ćirković, I.; Hauschild, T.; Ježek, P.; Dimitrijević, V.; Vuković, D.; Stepanović, S. Identification and Antimicrobial Susceptibility Testing of Staphylococcus vitulinus by the BD Phoenix Automated Microbiology System. Curr. Microbiol. 2008, 57, 158–160. [Google Scholar] [CrossRef]
- Lienen, T.; Schnitt, A.; Hammerl, J.A.; Marino, S.F.; Maurischat, S.; Tenhagen, B.-A. Multidrug-resistant Staphylococcus cohnii and Staphylococcus urealyticus isolates from German dairy farms exhibit resistance to beta-lactam antibiotics and divergent penicillin-binding proteins. Sci. Rep. 2021, 11, 6075. [Google Scholar] [CrossRef]
- Ehlers, S.; Merrill, S.A. Staphylococcus saprophyticus; [Updated 28 June 2020]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482367/ (accessed on 20 May 2021).
- Żabicka, D.; Hryniewicz, W. Recommendations for the Selection of Tests for the Determination of Bacterial Susceptibility to Antibiotics and Chemotherapeutic Agents. Determination of the Susceptibility of Gram-Positive Cocci of the Staphylococcus Species; National Reference Center for Antimicrobial Susceptibility, National Medicines Institute: Warsaw, Poland, 2010. [Google Scholar]
- Otto, M. Coagulase-negative staphylococci as reservoirs of genes facilitating MRSA infection: Staphylococcal commensal species such as Staphylococcus epidermidis are being recognized as important sources of genes promoting MRSA colonization and virulence. BioEssays 2013, 35, 4–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petinaki, E.; Papagiannitsis, C. Resistance of Staphylococci to Macrolides-Lincosamides- Streptogramins B (MLSB): Epidemiology and Mechanisms of Resistance. In Staphylococcus aureus; Hemeg, H., Ozbak, H., Afrin, H., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Ross, J.I.; Eady, E.A.; Cove, J.H.; Cunliffe, W.J.; Baumberg, S.; Wootton, J.C. Inducible erythromycin resistance in staphylococci is encoded by a member of the ATP-binding transport super-gene family. Mol. Microbiol. 1990, 4, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Bagcigil, F.A.; Moodley, A.; Baptiste, K.E.; Jensen, V.F.; Guardabassi, L. Occurrence, species distribution, antimicrobial resistance and clonality of methicillin- and erythromycin-resistant staphylococci in the nasal cavity of domestic animals. Vet. Microbiol. 2007, 121, 307–315. [Google Scholar] [CrossRef] [PubMed]








| No. | Sampling Point | Description | Temp (°C)/Relative Humidity (%) | ||||
|---|---|---|---|---|---|---|---|
| Winter | Spring | Summer | Autumn | ||||
| 1 | Classroom (in the centre of the room, between tables) | A room where didactic classes take place. No air conditioning was present; natural ventilation only. C.a. 60 students stay there every day in groups of 15–20 persons. |  | 22.0a (±0.7)/44.8a (±1.1) | 22.6a (±0.5)/63.4b (±1.0) | 23.8b (±0.8)/54.6c (±1.2) | 21.8a (±0.5)/60.2d (±1.5) |
| 2 | Sports hall (in the middle of the hall) | Used on a daily basis for physical education of students and tournaments. Natural ventilation was only used based on opening windows and door. |  | 22.1a (±0.5)/52.8a (±1.4) | 21.8a (±0.5)/53.1a (±1.5) | 23.2b (±0.5)/53.6a (±1.0) | 20.0c (±0.5)/62.6b (±1.1) |
| 3 | Horse stable (in the aisle between the two rows of stalls) | Livestock room, which is a part of the “Kopytko” horse stable. A small building located in the almost central part of the land belonging to the school; c.a. 100 m away from other livestock facilities. The room keeps 12 horses that spend most of the day outside the facility. While the horses are away, the main entrance remains open to facilitate ventilation of the building. |  | 11.9a (±1.0)/53.6a (±2.1) | 14.3b (±0.6)/83.5b (±3.2) | 21.5c (±1.0)/57.0c (±1.1) | 9.9d (±1.0)/90.3d (±2.0) |
| 4 | Cowshed (in the aisle between the two rows of stalls) | The largest livestock enclosure in which a high number of adult dairy cows are housed 24 h a day (approximately 70 animals). It is not air-conditioned and, during warmer months, ventilation consists in opening the entrance gates located on opposite sides of the building. In the case of very high air temperatures, a fan located at the entrance to the barn is switched on. In addition, a lot of work is carried out in the room related to the maintenance of animals (milking, feeding and changing litter) and the main passage is filled with feed and straw for animals, which means that there is a high level of dust in the facility almost all the time. |  | 7.7a (±0.8)/73.6a (±2.3) | 17.0b (±0.9)/72.3a (±2.2) | 24.4c (±1.0)/46.1b (±1.2) | 12.9d (±1.0)/93.8c (±2.0) |
| 5 | Newborn calf shed (in the aisle between the two rows of boxes for calves) | A livestock room for keeping young cattle. It is quite a large building divided into two zones: the first is where the animal feed is stored and the second (the actual part) is where the calves are kept. Young animals are kept in separate boxes, 2–3 each. The floor of the boxes is lined with straw, which can be a source of dust and microorganisms that can be released into the air along with the movement of animals. Due to the young age of the animals, it is very rarely ventilated. |  | 8.9a (±1.2)/80.2a (±2.0) | 17.0b (±1.1)/78.5b (±1.3) | 20.5c (±1.0)/64.5c (±1.6) | 13.4d (±1.0)/92.5d (±2.0) |
| 6 | Outdoor background (by the bench frequented by students) | C. a. 20 m in front of the boarding house, c.a. 150 m from the livestock buildings. |  | 4.4a (±2.0)/86.6a (±1.5); avg. wind speed 1.0 km/h; precipitation 0 mm | 19.2b (±2.0)/69.6b (±1.0); avg. wind speed 1.0 km/h; precipitation 0 mm | 25.2c (±2.0)/49.7c (±1.0); avg. wind speed 2.4 km/h; precipitation 1.2 mm | 9.4d (±0.7)/98.1d (±2.1); avg. wind speed 5.0 km/h; precipitation 8.4 mm |
| Season | Sampling Point | |||||
|---|---|---|---|---|---|---|
| Classroom | Sports Hall | Stable | Cowshed | Newborn Calf Shed | Outdoor (Average Levels for the Region) | |
| PM10 | ||||||
| Winter | 0.076 (0.004) | 0.074 (0.006) | 0.162 (0.031) | 0.097 (0.006) | 0.220 (0.060) | 0.083 (0.023) (0.046) |
| Spring | 0.084 (0.009) | 0.088 (0.003) | 0.126 (0.004) | 0.151 (0.037) | 0.134 (0.008) | 0.078 (0.003) (0.028) |
| Summer | 0.062 (0.001) | 0.068 (0.004) | 0.067 (0.008) | 0.070 (0.044) | 0.128 (0.276) | 0.057 (0.003) (0.022) |
| Autumn | 0.170 (0.019) | 0.154 (0.003) | 0.182 (0.006) | 0.333 (0.295) | 0.387 (0.062) | 0.198 (0.006) (0.039) |
| PM4 | ||||||
| Winter | 0.073 (0.005) | 0.066 (0.002) | 0.113 (0.010) | 0.093 (0.004) | 0.132 (0.017) | 0.080 (0.002) |
| Spring | 0.080 (0.005) | 0.090 (0.004) | 0.124 (0.004) | 0.141 (0.009) | 0.128 (0.005) | 0.081 (0.006) |
| Summer | 0.062 (0.002) | 0.066 (0.002) | 0.065 (0.003) | 0.084 (0.015) | 0.082 (0.008) | 0.058 (0.012) |
| Autumn | 0.161 (0.023) | 0.152 (0.003) | 0.182 (0.005) | 0.263 (0.003) | 0.325 (0.059) | 0.203 (0.004) |
| PM2.5 | ||||||
| Winter | 0.070 (0.003) | 0.068 (0.002) | 0.099 (0.006) | 0.091 (0.002) | 0.111 (0.012) | 0.077 (0.076) (0.041) |
| Spring | 0.078 (0.004) | 0.091 (0.004) | 0.125 (0.011) | 0.134 (0.009) | 0.128 (0.005) | 0.078 (0.003) (0.017) |
| Summer | 0.061 (0.001) | 0.066 (0.003) | 0.064 (0.003) | 0.077 (0.006) | 0.076 (0.009) | 0.060 (0.005) (0.016) |
| Autumn | 0.156 (0.004) | 0.149 (0.003) | 0.177 (0.011) | 0.257 (0.026) | 0.321 (0.014) | 0.202 (0.013) (0.027) |
| PM1 | ||||||
| Winter | 0.065 (0.001) | 0.063 (0.003) | 0.084 (0.009) | 0.084 (0.002) | 0.092 (0.017) | 0.071 (0.001) |
| Spring | 0.078 (0.029) | 0.083 (0.002) | 0.114 (0.010) | 0.124 (0.010) | 0.118 (0.006) | 0.077 (0.005) |
| Summer | 0.060 (0.001) | 0.067 (0.003) | 0.063 (0.002) | 0.066 (0.10) | 0.068 (0.008) | 0.057 (0.003) |
| Autumn | 0.143 (0.003) | 0.147 (0.004) | 0.158 (0.004) | 0.200 (0.011) | 0.256 (0.022) | 0.222 (0.666) |
| Temp | RH | Bact. | Fungi | Act | Staph | PM10 | PM4 | PM2.5 | PM1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | - | |||||||||
| Relative humidity | −0.80 | - | ||||||||
| Bacteria | −0.43 | 0.20 | - | |||||||
| Fungi | −0.36 | 0.19 | 0.75 | - | ||||||
| Actinomycetes | −0.11 | 0.15 | 0.54 | 0.68 | - | |||||
| Staphylococci | −0.20 | 0.17 | 0.80 | 0.71 | 0.71 | - | ||||
| PM10 | −0.70 | 0.71 | 0.33 | 0.25 | 0.19 | 0.39 | - | |||
| PM4 | −0.62 | 0.69 | 0.17 | 0.14 | 0.14 | 0.31 | 0.95 | - | ||
| PM2.5 | −0.62 | 0.71 | 0.11 | 0.09 | 0.08 | 0.24 | 0.93 | 0.98 | - | |
| PM1 | −0.65 | 0.75 | 0.11 | 0.07 | 0.10 | 0.23 | 0.93 | 0.97 | 0.99 | - |
| Season | Sampling Site | |||||
|---|---|---|---|---|---|---|
| Classroom | Sports Hall | Stable | Cowshed | Newborn Calf Shed | Outdoor | |
| Total Bacteria | ||||||
| Winter | 4384 (2309) | 6572 (4066) | 15,383 (12,688) | 10,821 (2840) | 27,102 (6623) | 12,726 (2232) |
| Spring | 2523 (269) | 1783 (620) | 3954 (759) | 5803 (123) | 22,426 (8634) | 1925 (480) |
| Summer | 1543 (785) | 3614 (2108) | 4743 (3521) | 3746 (958) | 36,796 (17,463) | 951 (68) |
| Autumn | 4005 (889) | 1739 (581) | 1720 (488) | 4829 (1781) | 12,892 (3783) | 1220 (312) |
| Mold Fungi | ||||||
| Winter | 3366 (5024) | 774 (413) | 6219 (2224) | 5395 (3566) | 48,220 (7246) | 794 (311) |
| Spring | 1227 (630) | 664 (141) | 1885 (347) | 7310 (2009) | 7739 (2945) | 688 (102) |
| Summer | 610 (191) | 424 (42) | 1099 (217) | 3620 (3429) | 12,674 (10,426) | 725 (47) |
| Autumn | 297 (32) | 264 (172) | 868 (194) | 750 (80) | 3975 (123) | 688 (61) |
| Actinomycetes | ||||||
| Winter | 38 (4) | 205 (30) | 982 (526) | 3553 (233) | 18,430 (10,556) | 90 (51) |
| Spring | 1555 (577) | 346 (214) | 663 (593) | 8634 (5307) | 9817 (3005) | 292 (184) |
| Summer | 405 (114) | 876 (406) | 1205 (326) | 3208 (638) | 20,006 (8130) | 214 (124) |
| Autumn | 367 (137) | 106 (55) | 306 (212) | 738 (1002) | 871 (531) | 217 (121) |
| Staphylococci | ||||||
| Winter | 1602 (466) | 2676 (486) | 2564 (1072) | 7342 (395) | 15,402 (8134) | 346 (241) |
| Spring | 1571 (1462) | 900 (912) | 2890 (2716) | 7350 (4154) | 8899 (1580) | 38 (10) |
| Summer | 977 (213) | 916 (728) | 2026 (643) | 1669 (1682) | 19,770 (6953) | 130 (146) |
| Autumn | 3920 (2353) | 627 (220) | 632 (576) | 2285 (1160) | 15,854 (6233) | 327 (208) |
| Origin | Species | FOX | E | TE | CN | DA | SXT | CIP | No. of R |
|---|---|---|---|---|---|---|---|---|---|
| Classroom | Staphylococcus spp. | S | S | S | S | S | S | S | 0 |
| S. equorum | S | R | R | S | R | R | S | 4 | |
| S. equorum | S | S | S | S | S | S | S | 0 | |
| S. equorum | S | S | S | S | R | R | R | 3 | |
| S. equorum | S | R | R | S | S | S | S | 2 | |
| S. equorum | S | S | S | S | S | S | S | 0 | |
| S. sciuri | S | S | S | S | S | S | S | 0 | |
| Stable | S. succinus | S | S | S | S | S | S | S | 0 |
| S. xylosus | S | S | S | S | S | S | S | 0 | |
| S. succinus | S | R | S | S | S | S | S | 1 | |
| S. vitulinus | R | R | S | S | R | S | R | 4 | |
| S. equorum | S | S | S | S | S | S | S | 0 | |
| Cowshed | S. sciuri | S | S | S | R | R | S | S | 2 |
| S. succinus | S | S | S | S | S | S | R | 1 | |
| S. succinus | S | S | S | S | R | S | S | 1 | |
| S.succinus | S | S | S | S | S | S | S | 0 | |
| S. succinus | S | R | S | S | R | S | S | 2 | |
| S. succinus | S | R | S | S | S | S | S | 1 | |
| S. xylosus | S | S | R | S | S | S | S | 1 | |
| S. vitulinus | S | S | S | S | S | S | S | 0 | |
| Newborn calf shed | S. cohnii | S | R | S | S | S | S | S | 1 |
| S. equorum | S | R | S | S | R | S | S | 2 | |
| S. equorum | S | R | S | S | R | S | S | 2 | |
| S. equorum | S | S | S | S | R | S | S | 1 | |
| S. equorum | S | R | R | S | S | S | S | 2 | |
| S. equorum | S | S | S | S | S | S | S | 0 | |
| S. saprophyticus | S | S | S | S | S | S | S | 0 | |
| S. sciuri | S | S | S | S | S | S | S | 0 | |
| S. succinus | S | S | S | S | S | S | S | 0 | |
| S. succinus | S | S | S | S | S | S | S | 0 | |
| S. xylosus | S | S | S | S | S | S | S | 0 | |
| S. xylosus | S | R | R | S | R | S | R | 4 | |
| Outdoor | S. equorum | S | R | S | S | S | S | S | 1 |
| S. xylosus | S | R | S | S | R | S | S | 2 | |
| n/% of resistant strains | 1/2.94 | 13/38.23 | 5/14.7 | 1/2.94 | 11/32.4 | 2/5.88 | 4/11.8 | 19/55.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenart-Boroń, A.; Drab, D.; Chrobak, J. Microbiological Aerosol, Particulate Matter Concentrations and Antibiotic Resistant Staphylococcus spp. in the Premises of Poland’s Oldest Agricultural School. Atmosphere 2021, 12, 934. https://doi.org/10.3390/atmos12080934
Lenart-Boroń A, Drab D, Chrobak J. Microbiological Aerosol, Particulate Matter Concentrations and Antibiotic Resistant Staphylococcus spp. in the Premises of Poland’s Oldest Agricultural School. Atmosphere. 2021; 12(8):934. https://doi.org/10.3390/atmos12080934
Chicago/Turabian StyleLenart-Boroń, Anna, Dagmara Drab, and Justyna Chrobak. 2021. "Microbiological Aerosol, Particulate Matter Concentrations and Antibiotic Resistant Staphylococcus spp. in the Premises of Poland’s Oldest Agricultural School" Atmosphere 12, no. 8: 934. https://doi.org/10.3390/atmos12080934
APA StyleLenart-Boroń, A., Drab, D., & Chrobak, J. (2021). Microbiological Aerosol, Particulate Matter Concentrations and Antibiotic Resistant Staphylococcus spp. in the Premises of Poland’s Oldest Agricultural School. Atmosphere, 12(8), 934. https://doi.org/10.3390/atmos12080934







