Development of a Negligible Zero-Drift NDIR Analyzer for Measuring NH3 Emitted from an Urban Household Solid Waste Incinerator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Apparatus
2.2. Experimental Procedure
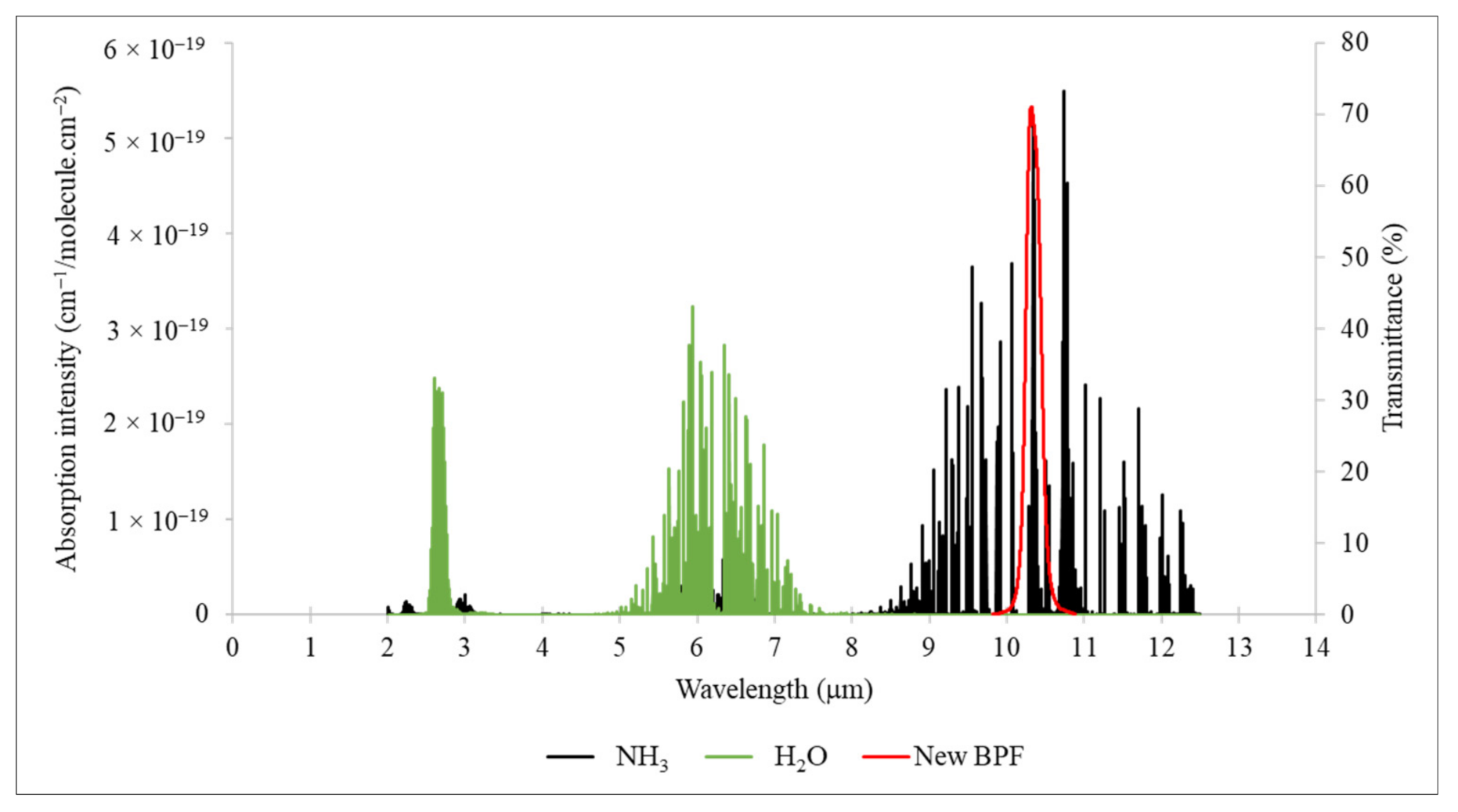
2.2.1. Development of a Least-Interfering BPF for NH3 NDIR
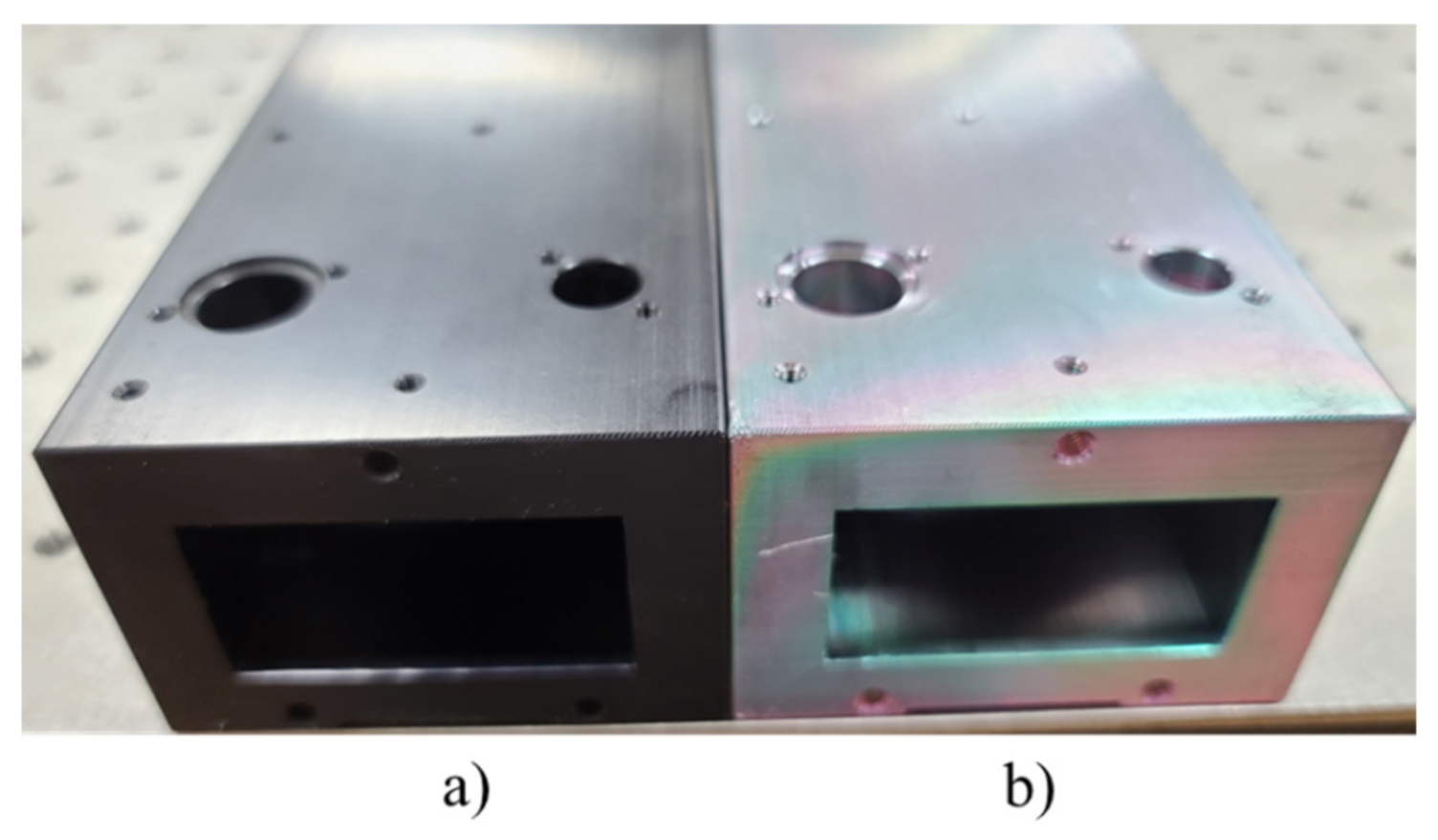
2.2.2. Investigating Coating Materials for the Gas Chamber of the NH3 NDIR Analyzer
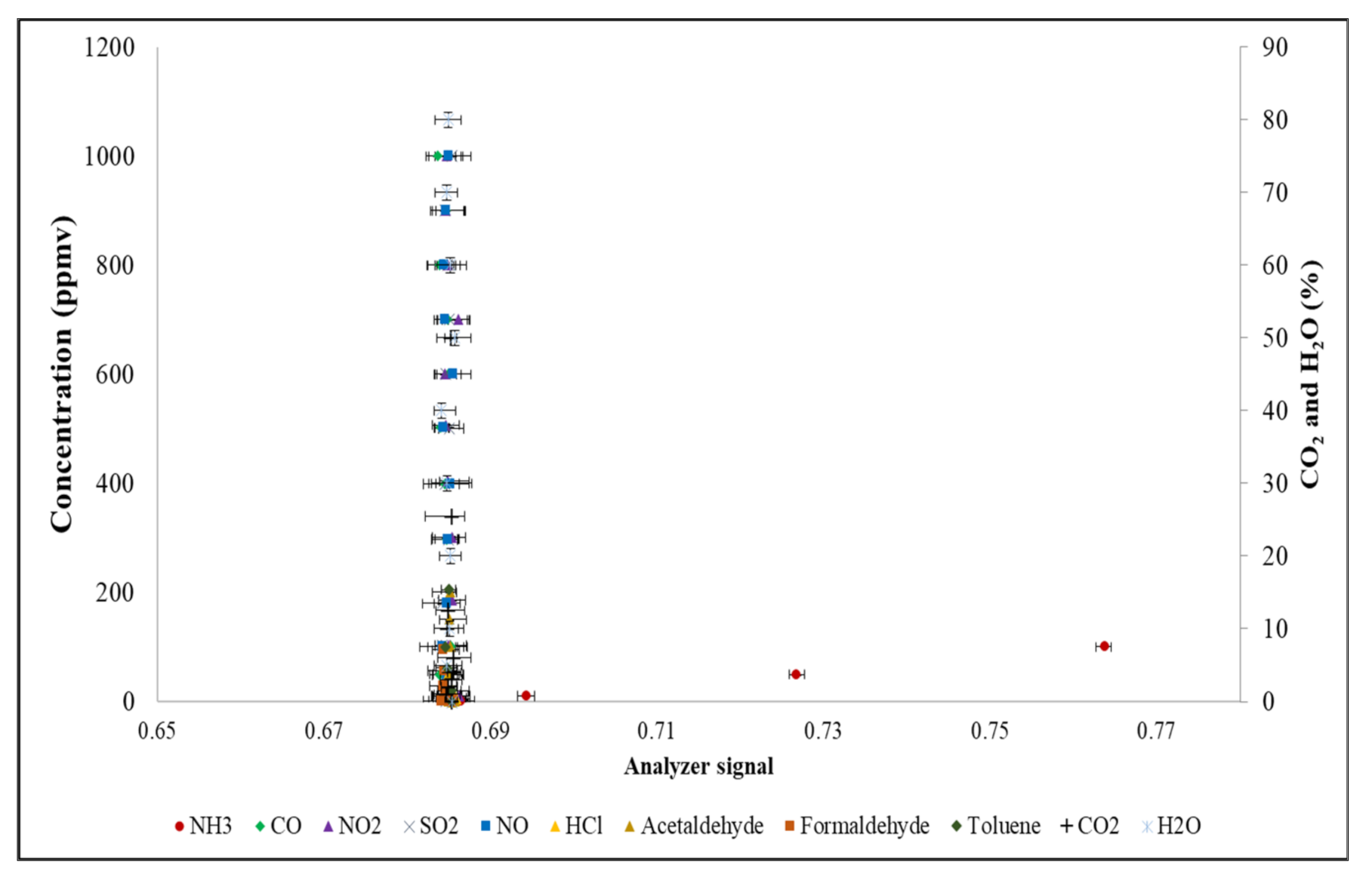
2.2.3. Development of an Alternative Method to Reduce Zero-Drift of the NH3 NDIR Analyzer
Investigating the Optimal Beam Reducer
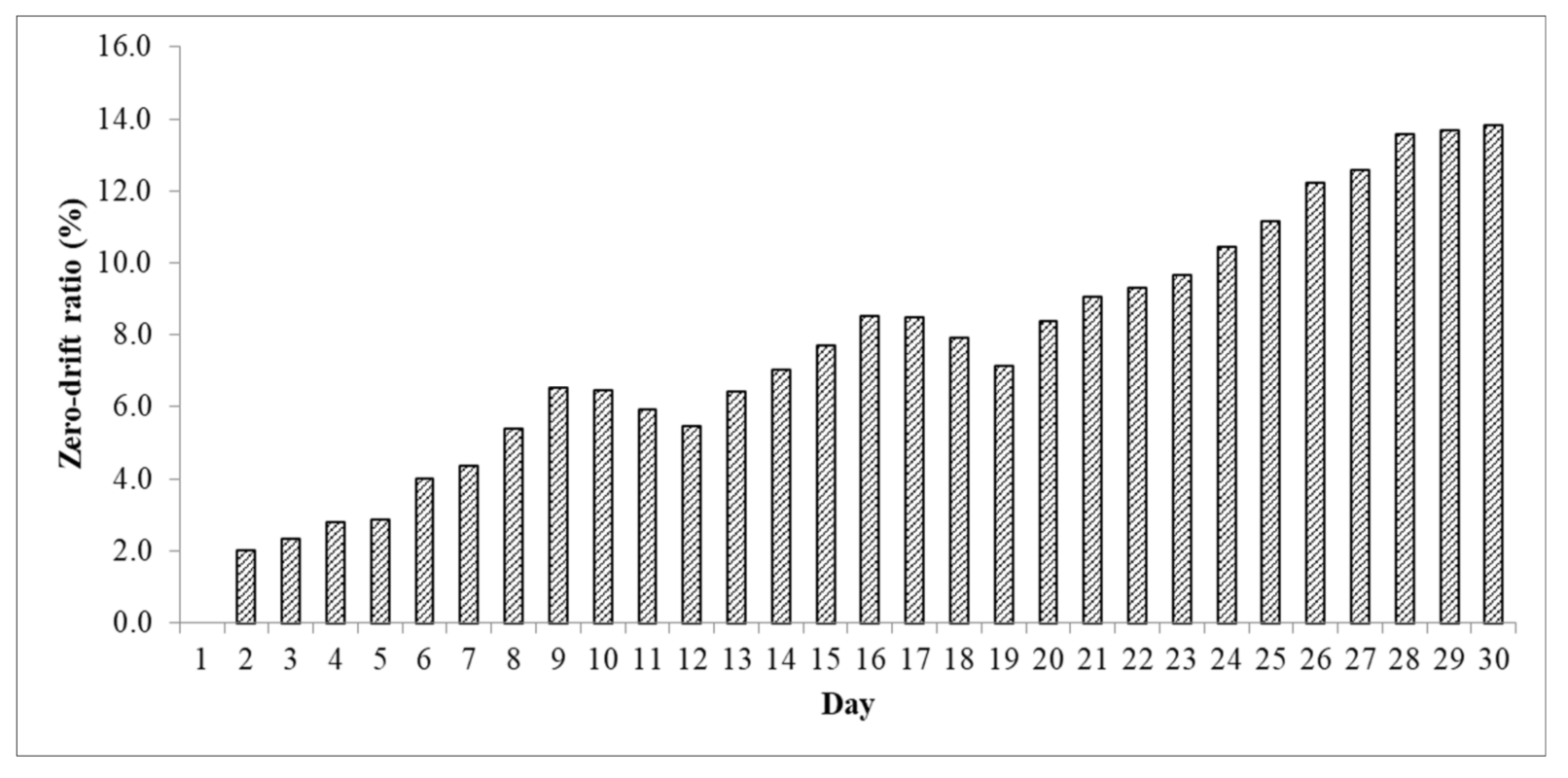
Effect of IR Source and Detector Aging on Analyzer Zero-Drift
3. Results and Discussion
3.1. Development of a Least-Interfering BPF for NH3 NDIR
3.2. Investigating Coating Materials for the NH3 NDIR Analyzer Gas Chamber
3.3. Development of an Alternative Method to Reduce Zero-Drift in the NH3 NDIR Analyzer
3.3.1. Investigating an Optimal Beam Reducer
3.3.2. Effect of IR Source and Detector Aging on Zero-Drift of the Analyzer
3.4. Limitations of the Analyzer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US National Library of Medicine PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 20 May 2021).
- Pohanish, R.P. Sittig’s Handbook of Toxic and Hazardous Chemicals and Carcinogens. Available online: https://www.elsevier.com/books/sittigs-handbook-of-toxic-and-hazardous-chemicals-and-carcinogens/pohanish/978-1-4377-7869-4 (accessed on 20 May 2021).
- Ezimand, K.; Kakroodi, A.A. Prediction and spatio—Temporal analysis of ozone concentration in a metropolitan area. Ecol. Indic. 2019, 103, 589–598. [Google Scholar] [CrossRef]
- Guo, H.; Chen, K.; Wang, P.; Hu, J.; Ying, Q.; Gao, A.; Zhang, H. Simulation of summer ozone and its sensitivity to emission changes in China. Atmos. Pollut. Res. 2019, 10, 1543–1552. [Google Scholar] [CrossRef]
- Han, Y.-J.; Kim, H.-W.; Cho, S.-H.; Kim, P.-R.; Kim, W.-J. Metallic elements in PM2.5 in different functional areas of Korea: Concentrations and source identification. Atmos. Res. 2015, 153, 416–428. [Google Scholar] [CrossRef]
- Jeon, W.-B.; Lee, S.-H.; Lee, H.; Park, C.; Kim, D.-H.; Park, S.-Y. A study on high ozone formation mechanism associated with change of NOx/VOCs ratio at a rural area in the Korean Peninsula. Atmos. Environ. 2014, 89, 10–21. [Google Scholar] [CrossRef]
- Kim, B.-U.; Kim, O.; Kim, H.C.; Kim, S. Influence of fossil-fuel power plant emissions on the surface fine particulate matter in the Seoul Capital Area, South Korea. J. Air Waste Manag. Assoc. 2016, 66, 863–873. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Shen, T.; Feng, X. Prediction of hourly ground-level PM2.5 concentrations 3 days in advance using neural networks with satellite data in eastern China. Atmos. Pollut. Res. 2017, 8, 1005–1015. [Google Scholar] [CrossRef]
- Zhong, Z.; Zheng, J.; Zhu, M.; Huang, Z.; Zhang, Z.; Jia, G.; Wang, X.; Bian, Y.; Wang, Y.; Li, N. Recent developments of anthropogenic air pollutant emission inventories in Guangdong province, China. Sci. Total Environ. 2018, 627, 1080–1092. [Google Scholar] [CrossRef]
- Behera, S.N.; Sharma, M.; Aneja, V.P.; Balasubramanian, R. Ammonia in the atmosphere: A review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ. Sci. Pollut. Res. 2013, 20, 8092–8131. [Google Scholar] [CrossRef]
- US EPA. Managing Air Quality—Emissions Inventories. Available online: https://www.epa.gov/air-quality-management-process/managing-air-quality-emissions-inventories (accessed on 20 May 2021).
- US EPA. Control and Pollution Prevention Options for Ammonia Emissions. Available online: https://www.osti.gov/biblio/104094-control-pollution-prevention-options-ammonia-emissions (accessed on 20 May 2021).
- IPCC. Good Practice Guidance and Uncertainty Management in National Greenhouse Gas. Inventories. Available online: https://www.ipcc-nggip.iges.or.jp/public/gp/english/ (accessed on 20 May 2021).
- Korea NIER. Integrated Pollution Prevention and Control. Reference Document on the Best Available Techniques for Municipal Waste Incineration. Available online: https://ecolibrary.me.go.kr/nier/#/search/detail/5254427 (accessed on 4 February 2019).
- US EPA. Development of Ammonia CEMS Performance Specifications. Available online: https://www.epa.gov/sites/production/files/2020-08/documents/04-nh3_cems.pdf (accessed on 20 May 2021).
- Worthington, B. 60 Years of Continuous Improvement in NDIR Gas Analyzers. In Proceedings of the 50th Annual ISA Analysis Division Symposium—50 Years of Analytical Solutions, Houston, TX, USA, 10–14 April 2005; pp. 95–107. [Google Scholar]
- Asadov, H.H.; Mirzabalayev, I.M.; Aliyev, D.Z.; Agayev, J.A.; Azimova, S.R.; Nabiyev, N.A.; Abdullayeva, S.N. Synthesis of corrected multi-wavelength spectrometers for atmospheric trace gases. Chin. Opt. Lett. 2009, 7, 361–363. [Google Scholar] [CrossRef]
- Liu, C.; Beirle, S.; Butler, T.; Liu, J.; Hoor, P.; Jöckel, P.; Pozzer, A.; Frankenberg, C.; Lawrence, M.G.; Lelieveld, J.; et al. Application of SCIAMACHY and MOPITT CO total column measurements to evaluate model results over biomass burning regions and Eastern China. Atmos. Chem. Phys. 2011, 11, 6083–6114. [Google Scholar] [CrossRef] [Green Version]
- Scholz, L.; Perez, A.O.; Knobelspies, S.; Wöllenstein, J.; Palzer, S. MID-IR Led-Based, Photoacoustic CO2sensor. In Proceedings of the Procedia Engineering, Chennai, India, 7–10 September 2015. [Google Scholar]
- Sun, Y.W.; Liu, C.; Chan, K.L.; Xie, P.H.; Liu, W.Q.; Zeng, Y.; Wang, S.M.; Huang, S.H.; Chen, J.; Wang, Y.P.; et al. Stack emission monitoring using non-dispersive infrared spectroscopy with an optimized nonlinear absorption cross interference correction algorithm. Atmos. Meas. Tech. 2013, 6, 1993–2005. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.Y.; Schell, M. Zero drift NDIR gas sensors. Sens. Rev. 2011. [Google Scholar] [CrossRef]
- Dinh, T.-V.; Choi, I.-Y.; Son, Y.-S.; Kim, J.-C. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Hussain, H.; Kim, J.; Yi, S. Characteristics and Temperature Compensation of Non-Dispersive Infrared (NDIR) Alcohol Gas Sensors According to Incident Light Intensity. Sensors 2018, 18, 2911. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.Y. Intrinsically Safe Improved Sensitivity NDIR Gas Sensor in a Can. U.S. Patent 8,158,946, 17 April 2012. [Google Scholar]
- Wong, J.Y. Super-Miniaturized NDIR Gas Sensor. U.S. Patent 8,097,856, 17 January 2012. [Google Scholar]
- Wong, J.Y. Differential Temperature Source NDIR Gas Sensing Methodology. U.S. Patent 8,866,085, 21 October 2014. [Google Scholar]
- HITRAN. High.-Resolution Transmission Molecular Absorption Database. Available online: https://hitran.org/ (accessed on 1 December 2017).
- Hwang, K.-L.; Choi, S.-M.; Kim, M.-K.; Heo, J.-B.; Zoh, K.-D. Emission of greenhouse gases from waste incineration in Korea. J. Environ. Manag. 2017, 196, 710–718. [Google Scholar] [CrossRef]
- Maitre, A. Municipal waste incinerators: Air and biological monitoring of workers for exposure to particles, metals, and organic compounds. Occup. Environ. Med. 2003, 60, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Dinh, T.-V.; Kim, D.-J.; Ahn, J.-W.; Choi, I.-Y.; Lee, J.-Y.; Son, Y.-S.; Kim, J.-C. A Potential Approach to Compensate the Gas Interference for the Analysis of NO by a Non-dispersive Infrared Technique. Anal. Chem. 2020, 92, 12152–12159. [Google Scholar] [CrossRef]
- Dinh, T.-V.; Ahn, J.-W.; Choi, I.-Y.; Kim, J.-C. A Novel Bandpass Filter for the Analysis of Carbon Monoxide Using a Non-Dispersive Infrared Technique. Atmosphere 2018, 9, 495. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Xin, H.; Li, H.; Dong, H. Assessment of Tubing Type on Ammonia Gas Adsorption. Appl. Eng. Agric. 2012, 28, 265–269. [Google Scholar] [CrossRef]
- Shah, S.B.; Westerman, P.W.; Arogo, J. Measuring Ammonia Concentrations and Emissions from Agricultural Land and Liquid Surfaces: A Review. J. Air Waste Manag. Assoc. 2006, 56, 945–960. [Google Scholar] [CrossRef] [Green Version]
- Vaittinen, O.; Metsälä, M.; Persijn, S.; Vainio, M.; Halonen, L. Adsorption of ammonia on treated stainless steel and polymer surfaces. Appl. Phys. B 2014, 115, 185–196. [Google Scholar] [CrossRef]
- Jones, R.C. Performance of Detectors for Visible and Infrared Radiation. Adv. Electron. Electron. Phys. 1953, 5, 1–96. [Google Scholar] [CrossRef]
- Gonz´alez, F.J. Noise measurements on optical detectors. Rev. Mex. FI´SICA 2006, 52, 550–554. [Google Scholar]
- Lang, S.B.; Shaw, S.A.; Rice, L.H.; Timmerhaus, K.D. Pyroelectric Thermometer for Use at Low Temperatures. Rev. Sci. Instrum. 1969, 40, 274–284. [Google Scholar] [CrossRef]
- Rogalski, A. Infrared Detectors, 2nd ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2011; ISBN 978-1-4200-7671-4. [Google Scholar]
- Korea Environment Corporation Cleansys Data. Available online: https://cleansys.or.kr/index.do (accessed on 1 December 2020).
- Korea MOE. National Emission Standard for an Incineration Plant. Available online: https://www.law.go.kr/LSW/lsBylInfoPLinkR.do?lsiSeq=206530&lsNm=%EB%8C%80%EA%B8%B0%ED%99%98%EA%B2%BD%EB%B3%B4%EC%A0%84%EB%B2%95+%EC%8B%9C%ED%96%89%EA%B7%9C%EC%B9%99&bylNo=0008&bylBrNo=00&bylCls=BE&bylEfYd=20190101&bylEfYdYn=Y (accessed on 4 February 2019).
- Jahnke, J.A. Continuous Emission Monitoring, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; ISBN 0-471-29227-3. [Google Scholar]
- Granite, E.J.; King, W.P.; Stanko, D.C.; Pennline, H.W. Implications of mercury interactions with band-gap semiconductor oxides. Main Gr. Chem. 2008, 7, 227–237. [Google Scholar] [CrossRef]
- McLarnon, C.R.; Steen, D. Combined SO2, NOx, PM, and Hg Removal from Coal Fired Boilers. In Proceedings of the EPRI-DOE-EPA Combined Utility Air Pollutant Control Symposium: The Mega Symposium-Particulates and Air Toxics, Washington, DC, USA, 19–22 May 2003. [Google Scholar]
- ES 01303.1. Ammonia in Flue Gas.—UV/VIS Spectrometry—Indophenol Method. Available online: https://www.law.go.kr/LSW//flDownload.do?flSeq=92976017 (accessed on 4 February 2019).
- Korotcenkov, G. Integrated Analytical Systems. In Handbook of Gas. Sensor Materials; Springer New York: New York, NY, USA, 2013; ISBN 978-1-4614-7164-6. [Google Scholar]
- Fonollosa, J.; Solórzano, A.; Jiménez-Soto, J.M.; Oller-Moreno, S.; Marco, S. Gas Sensor Array for Reliable Fire Detection. Procedia Eng. 2016, 168, 444–447. [Google Scholar] [CrossRef]
- Xing, Y.; Urasinska-Wojcik, B.; Gardner, J.W. Plasmonic Enhanced CMOS Non-Dispersive Infrared Gas Sensor for Acetone and Ammonia Detection. In Proceedings of the I2MTC 2018—2018 IEEE International Instrumentation and Measurement Technology Conference: Discovering New Horizons in Instrumentation and Measurement, Houston, TX, USA, 14–17 May 2018. [Google Scholar]
- Chan, K.L.; Ning, Z.; Westerdahl, D.; Wong, K.C.; Sun, Y.W.; Hartl, A.; Wenig, M.O. Dispersive infrared spectroscopy measurements of atmospheric CO2 using a Fabry-Pérot interferometer sensor. Sci. Total Environ. 2014, 472, 27–35. [Google Scholar] [CrossRef]
- Mayrwöger, J.; Reichl, W.; Krutzer, C.; Jakoby, B. Gas monitoring with a Fabry-Perot based bolometer: Cross-sensitivity to water vapor. Procedia Eng. 2010, 5, 1220–1223. [Google Scholar] [CrossRef]
- Sun, Y.W.; Zeng, Y.; Liu, W.Q.; Xie, P.H.; Chan, K.L.; Li, X.X.; Wang, S.M.; Huang, S.H. Cross-interference correction and simultaneous multi-gas analysis based on infrared absorption. Chin. Phys. B 2012, 21, 090701. [Google Scholar] [CrossRef]






| No. | Model | Company | Type | Range | Technique |
|---|---|---|---|---|---|
| 1 | Serinus 44 | Ecotech Group, Victoria, Australia | Extractive | 0–20 ppm | Chemiluminescence |
| 2 | NH3 analyzer | Chromatotec Group, Val-de-Virvée, France | Extractive | 0–100 ppm | UV spectroscopy |
| 3 | Orion™ 2110XP | Thermo Fisher Scientific, Waltham, MA, USA | Extractive | 0–10 ppm | Ammonia electrode |
| 4 | LAS300XD | Environmental S.A., Paris, France | In situ | 0–500 ppm | TDLS |
| 5 | MIR 9000H | Environmental S.A., Pari, France | Extractive | - | Gas Filters Correlation technology at 180 °C |
| 6 | ZSS | Fuji Electric Co., Ltd., Tokyo, Japan | In situ | 0–15, 5000 ppm | NDIR |
| 7 | MEXA-ONE-QL-NX | Horiba Ltd., Kyoto, Japan | Extractive | 0–50, 0–2000 ppm | Quantum Cascade Laser Infrared (QCL-IR) Spectroscopy |
| 8 | Innova 1314i | LumaSense Technologies, Inc., Santa Clara, CA, USA | Extractive | 0–300 ppm | Photoacoustic Spectroscopy |
| Mixture | NH3 (ppmv) | HCl (ppmv) | CO (ppmv) | NO (ppmv) | NO2 (ppmv) | CO2 (%) | SO2 (ppmv) | Toluene (ppmv) | Formaldehyde (ppmv) | Acetaldehyde (ppmv) | H2O (%RH) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 10 | 1.0 | 0.1 | 0.1 | 0.1 | 10 |
| Case 2 | 1.0 | 5.0 | 25 | 20 | 2.5 | 15 | 25 | 0.5 | 0.5 | 0.5 | 20 |
| Case 3 | 1.0 | 10 | 50 | 45 | 5.0 | 20 | 50 | 1.0 | 1.0 | 1.0 | 50 |
| Case 4 | 5.0 | 1.0 | 1.0 | 1.0 | 1.0 | 10 | 1.0 | 0.1 | 0.1 | 0.1 | 10 |
| Case 5 | 5.0 | 5.0 | 25 | 20 | 2.5 | 15 | 25 | 0.5 | 0.5 | 0.5 | 20 |
| Case 6 | 5.0 | 10 | 50 | 45 | 5.0 | 20 | 50 | 1.0 | 1.0 | 1.0 | 50 |
| Case 7 | 10 | 1.0 | 1.0 | 1.0 | 1.0 | 10 | 1.0 | 0.1 | 0.1 | 0.1 | 10 |
| Case 8 | 10 | 5.0 | 25 | 20 | 2.5 | 15 | 25 | 0.5 | 0.5 | 0.5 | 20 |
| Case 9 | 10 | 10 | 50 | 45 | 5.0 | 20 | 50 | 1.0 | 1.0 | 1.0 | 50 |
| No. | Current Study | Previous Study | ||
|---|---|---|---|---|
| CWL (μm) | HBW (nm) | CWL (μm) | HBW (nm) | |
| 1 | 10.39 | 190 | 2.25 [45] | - |
| 2 | 3.03 [45] | - | ||
| 3 | 5.70 [45] | - | ||
| 4 | 9.7 [46] | - | ||
| 5 | 10.6 [47] | 180 | ||
| No. | Aluminum-Anodized Chamber | Aluminum-Silcotek® Coated Chamber |
|---|---|---|
| 1 | 369 | 68.0 |
| 2 | 401 | 66.0 |
| 3 | 420 | 63.0 |
| Mean (second) | 397 | 66.0 |
| SD (second) | 25.8 | 2.52 |
| RSD (%) | 6.50 | 3.83 |
| No. | Beam Reducer | Linear Function | Quadratic Function |
|---|---|---|---|
| 1 | 20% | y = −0.0002x + 1.3875 r2 = 0.9186 | y = 2 × 10−6x2 − 0.0003x + 1.3885 r2 = 0.9572 |
| 2 | 30% | y = −3 × 10−6x + 0.7385 r2 = 0.0026 | y = −3 × 10−6x2 + 0.0002x + 0.737 r2 = 0.8763 |
| 3 | 40% | y = 0.0003x + 0.3652 r2 = 0.9704 | y = 2 × 10−6x2 + 0.0001x + 0.3666 r2 = 0.9997 |
| 4 | 50% | y = 0.0003x + 0.3236 r2 = 0.9932 | y = 1 × 10−6x2 + 0.0002x + 0.3243 r2 = 0.9998 |
| 5 | 60% | y = 0.0001x + 0.2205 r2 = 1 | y = 1 × 10−8x2 + 0.0001x + 0.2205 r2 = 1 |
| 6 | 70% | y = 0.0001x + 0.1505 r2 = 0.9997 | y = −4 × 10−8x2 + 0.0001x + 0.1505 r2 = 0.9998 |
| 7 | 80% | y = 4 × 10−5x + 0.0632 r2 = 0.9963 | y = −9 × 10−8x2 + 5 × 10−5x + 0.0632 r2 = 0.9998 |
| 8 | 90% | y = −5 × 10−5x + 0.0327 r2 = 0.5555 | y = 2 × 10−6x2 − 0.0002x + 0.0337 r2 = 0.9652 |
| Mixture | NDIR Analyzer | Indophenol Method | RPD (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min (ppmv) | Max (ppmv) | Mean (ppmv) | RSD (%) | Min (ppmv) | Max (ppmv) | Mean (ppmv) | RSD (%) | ||
| Case 1 | 0.95 | 1.09 | 1.00 | 4.97 | 0.98 | 0.99 | 0.98 | 0.59 | 1.84 |
| Case 2 | 0.94 | 1.09 | 1.00 | 4.92 | 0.98 | 1.02 | 1.00 | 2.09 | 0.19 |
| Case 3 | 0.97 | 1.00 | 0.99 | 1.15 | 0.99 | 1.02 | 1.01 | 1.71 | 2.40 |
| Case 4 | 4.92 | 5.04 | 4.95 | 0.82 | 4.95 | 5.05 | 5.00 | 1.00 | 1.00 |
| Case 5 | 4.92 | 5.04 | 4.96 | 1.09 | 4.95 | 5.00 | 4.97 | 0.58 | 0.11 |
| Case 6 | 4.92 | 5.04 | 4.96 | 1.12 | 4.95 | 5.00 | 4.98 | 0.58 | 0.47 |
| Case 7 | 9.91 | 10.1 | 9.96 | 0.69 | 9.95 | 10.1 | 10.0 | 0.50 | 0.39 |
| Case 8 | 9.92 | 10.0 | 9.94 | 0.28 | 9.95 | 10.0 | 9.97 | 0.29 | 0.30 |
| Case 9 | 9.86 | 10.1 | 9.94 | 0.82 | 9.91 | 9.95 | 9.94 | 0.23 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinh, T.-V.; Choi, I.-Y.; Park, B.-G.; Lee, J.-H.; Kim, I.-Y.; Gil, H.-N.; Lee, S.-W.; Kim, J.-C. Development of a Negligible Zero-Drift NDIR Analyzer for Measuring NH3 Emitted from an Urban Household Solid Waste Incinerator. Atmosphere 2021, 12, 858. https://doi.org/10.3390/atmos12070858
Dinh T-V, Choi I-Y, Park B-G, Lee J-H, Kim I-Y, Gil H-N, Lee S-W, Kim J-C. Development of a Negligible Zero-Drift NDIR Analyzer for Measuring NH3 Emitted from an Urban Household Solid Waste Incinerator. Atmosphere. 2021; 12(7):858. https://doi.org/10.3390/atmos12070858
Chicago/Turabian StyleDinh, Trieu-Vuong, In-Young Choi, Byeong-Gyu Park, Jee-Hyun Lee, In-Young Kim, Han-Nui Gil, Sang-Woo Lee, and Jo-Chun Kim. 2021. "Development of a Negligible Zero-Drift NDIR Analyzer for Measuring NH3 Emitted from an Urban Household Solid Waste Incinerator" Atmosphere 12, no. 7: 858. https://doi.org/10.3390/atmos12070858
APA StyleDinh, T.-V., Choi, I.-Y., Park, B.-G., Lee, J.-H., Kim, I.-Y., Gil, H.-N., Lee, S.-W., & Kim, J.-C. (2021). Development of a Negligible Zero-Drift NDIR Analyzer for Measuring NH3 Emitted from an Urban Household Solid Waste Incinerator. Atmosphere, 12(7), 858. https://doi.org/10.3390/atmos12070858








