Effect of Metal Oxides and Smelting Dust on SO2 Conversion to SO3
Abstract
1. Introduction
2. Experiment and Method
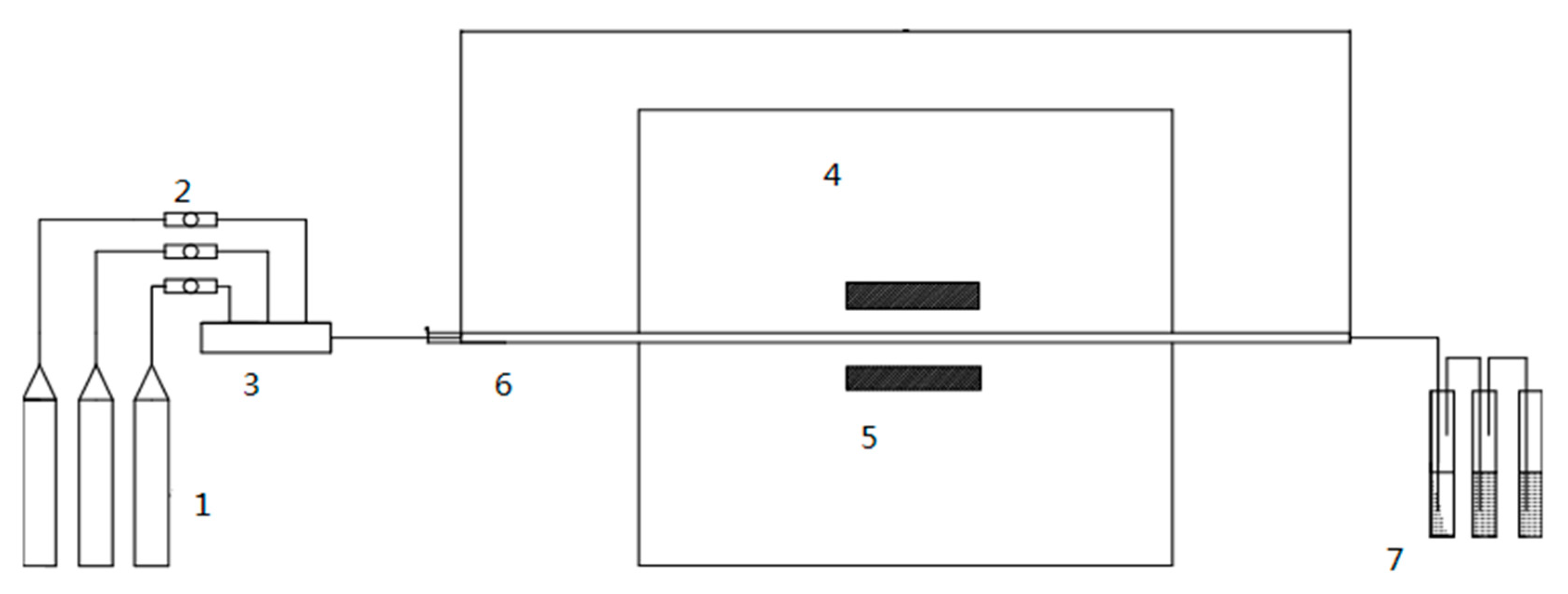
2.1. Experiment Setup
2.2. Test Cases
2.3. Materials
2.4. Experiment
3. Result and Discussion
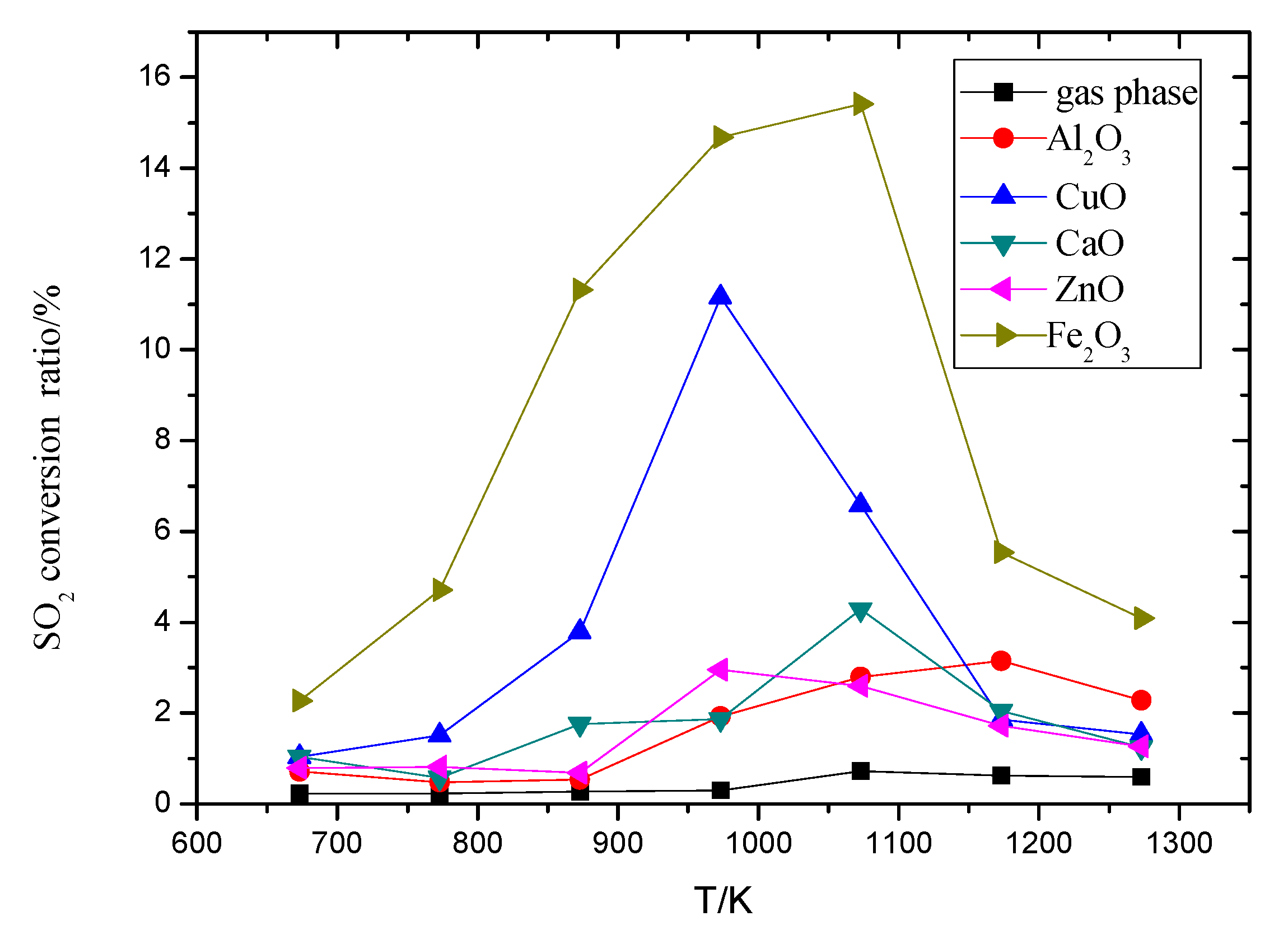
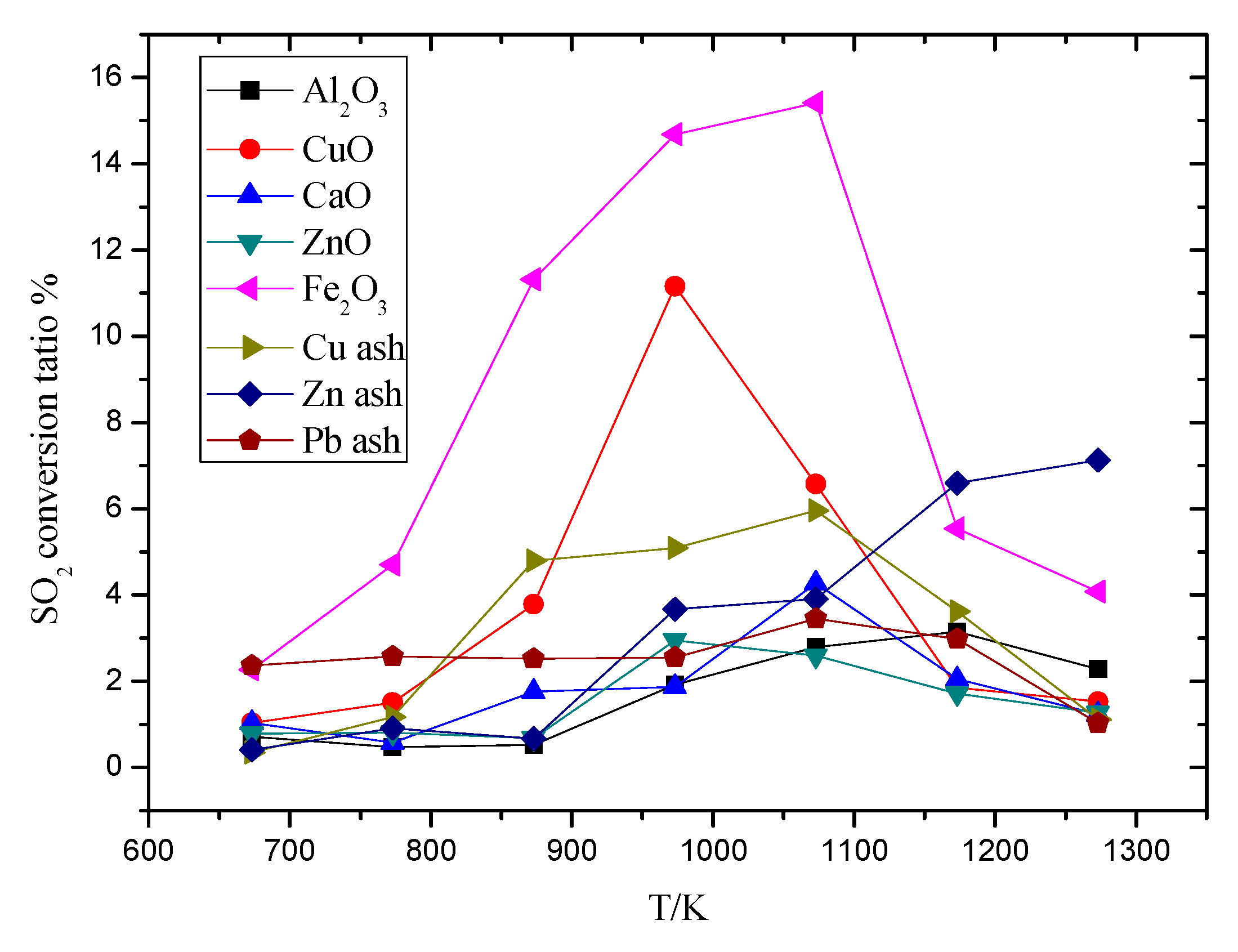
3.1. The Influence on Temperature to SO2 Conversion Ratio
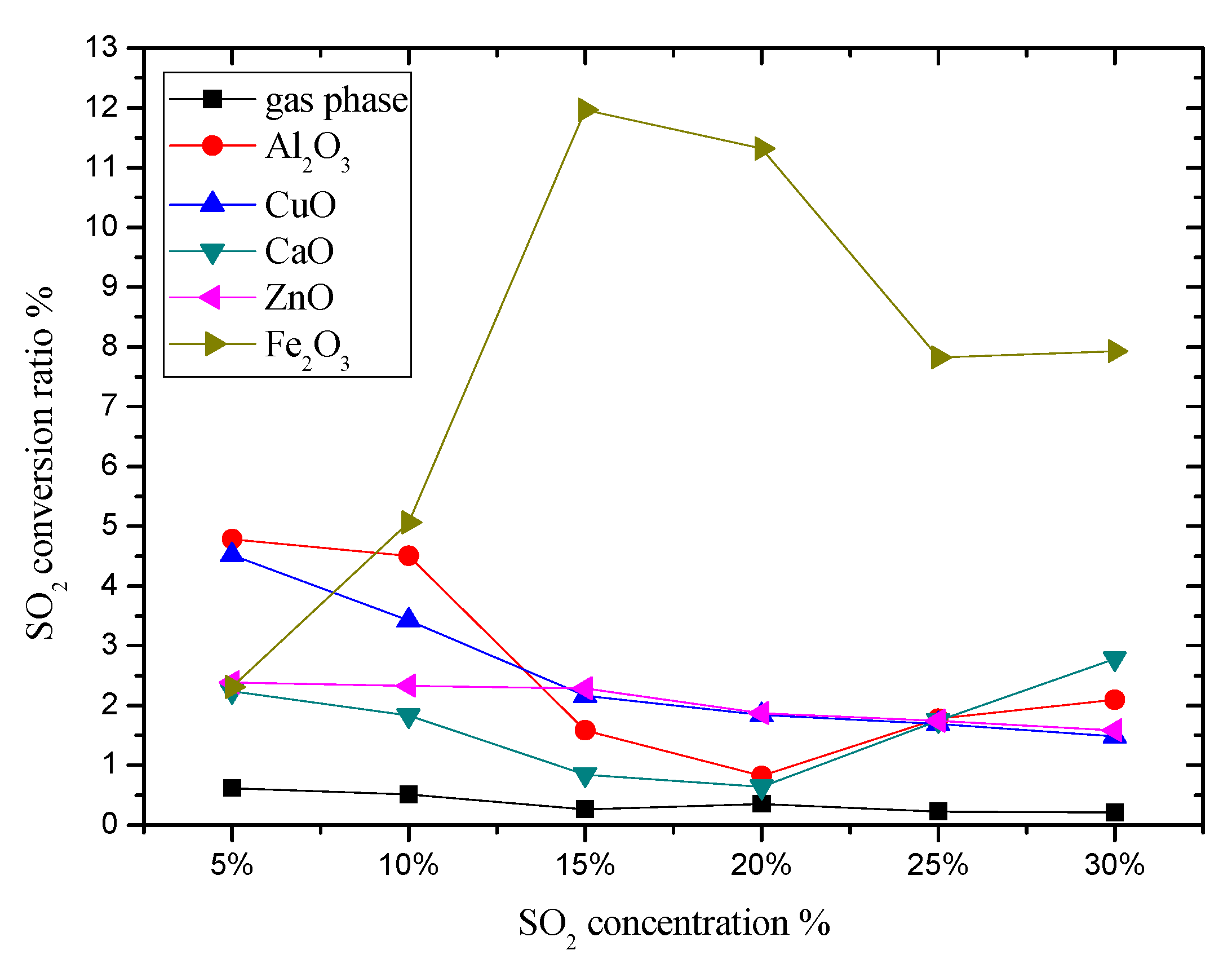
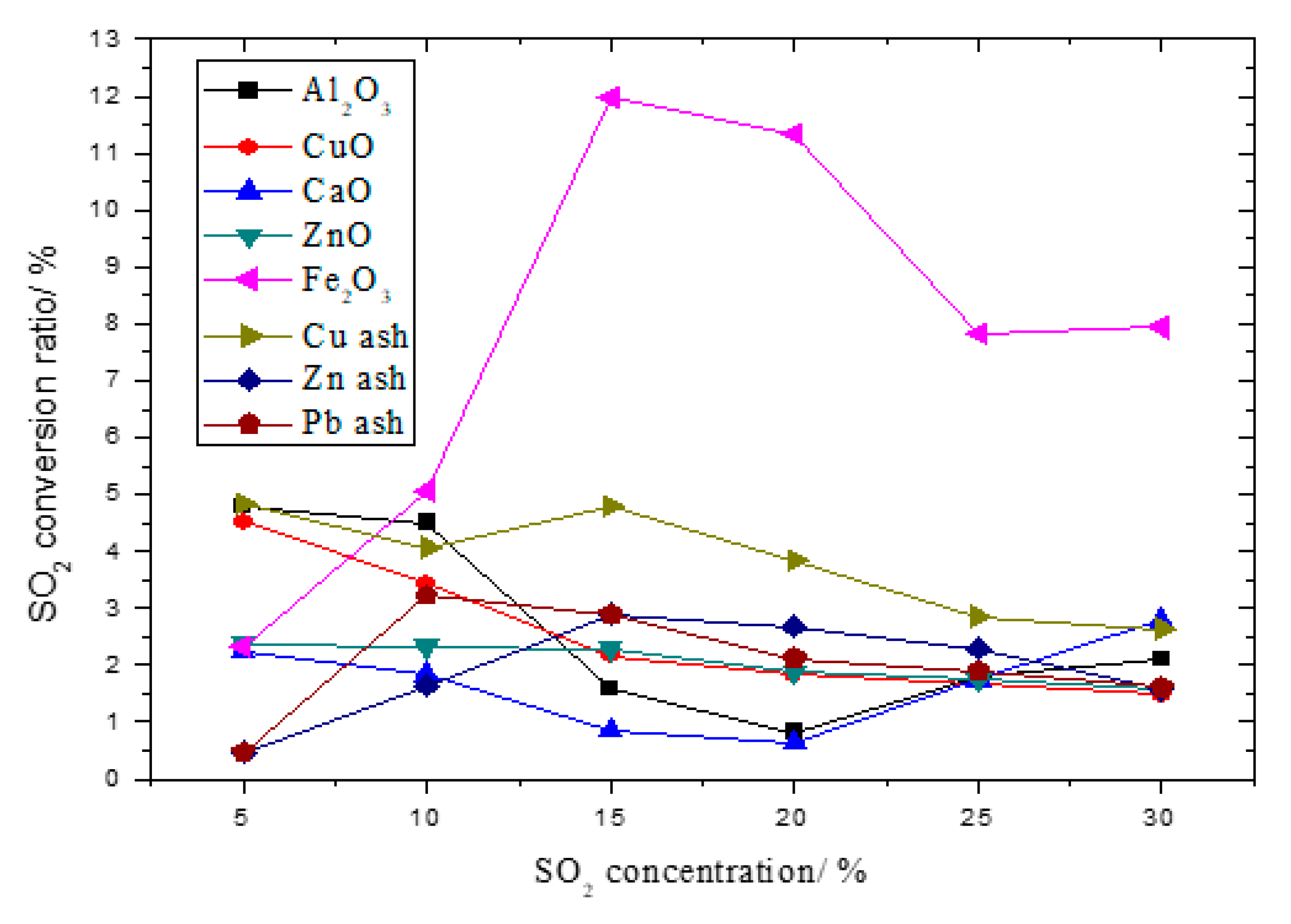
3.2. The Influence on Initial SO2 Concentration to SO2 Conversion Rate
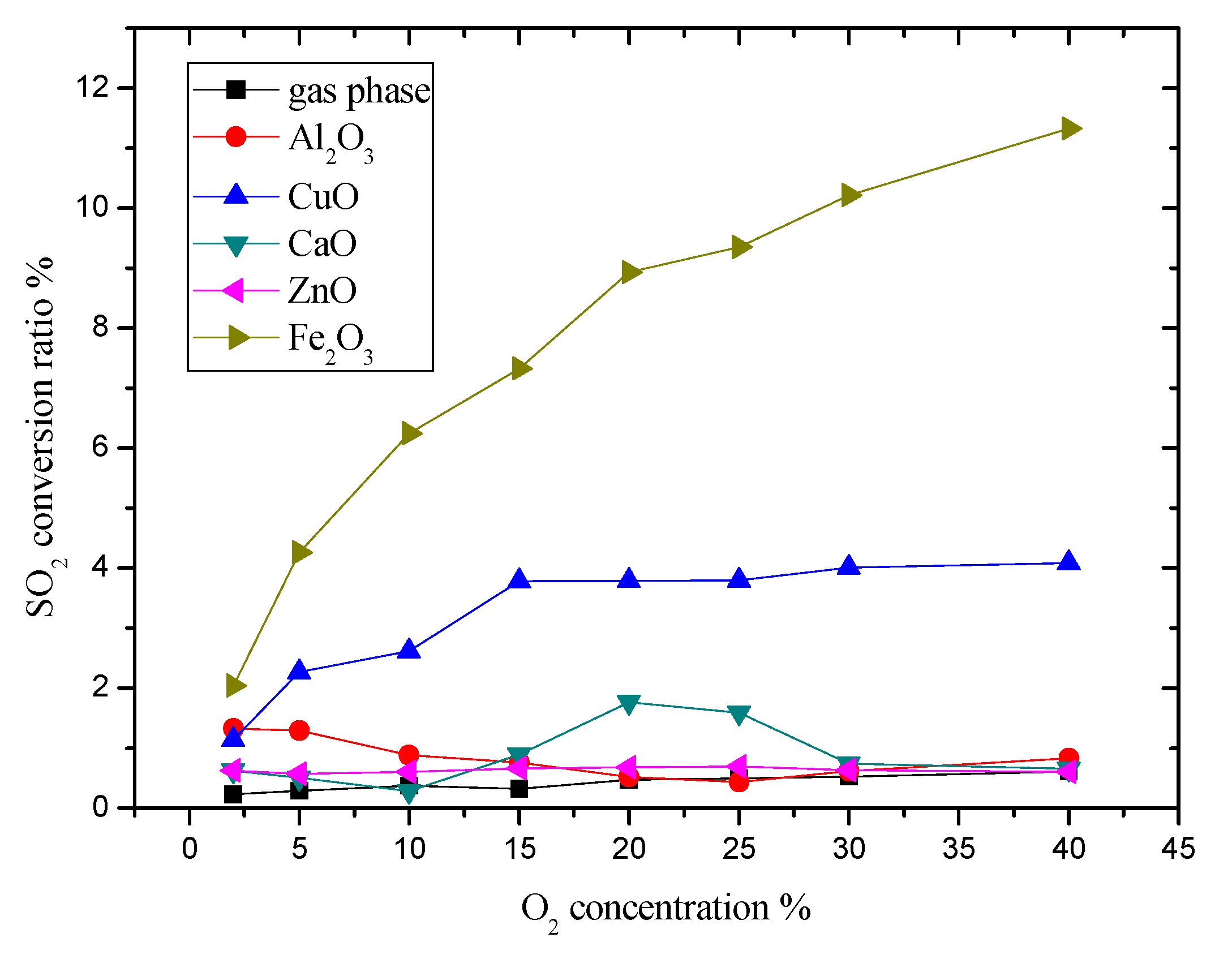
3.3. The Influence on Initial O2 Concentration to SO2 Conversion Rate
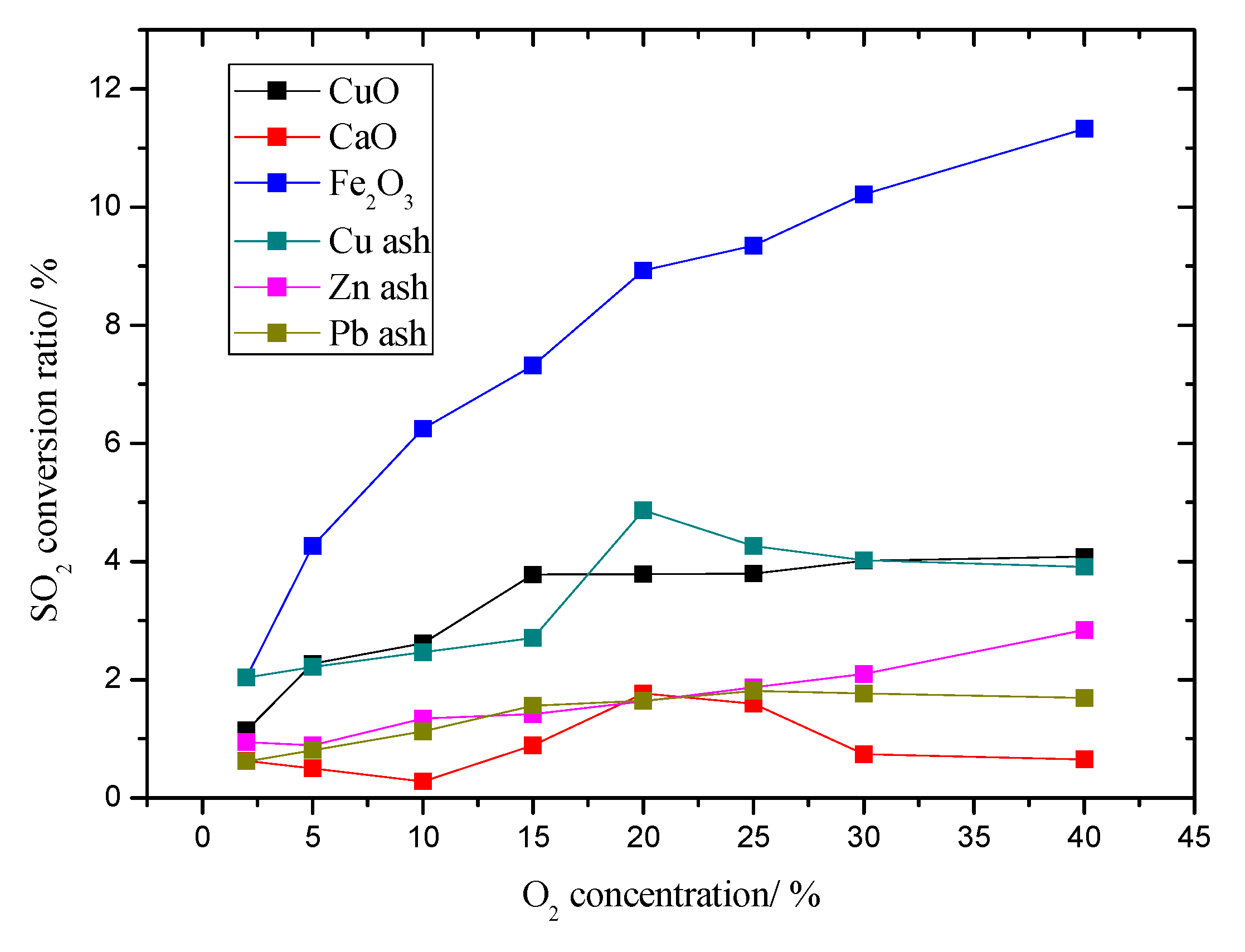
3.4. The Influence on Flue Dust to SO2 Conversion Rate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleig, D.; Alzueta, M.U.; Normann, F.; Abian, M.; Andersson, K.; Johnsson, F. Measurement and modeling of sulfur trioxide formation in a flow reactor under post-flame conditions. Combust. Flame 2013, 160, 1142–1151. [Google Scholar] [CrossRef]
- Alzueta, M.U.; Bilbao, R.; Glarborg, P. Inhibition and sensitization of fuel oxidation by SO2. Combust. Flame 2001, 217, 2234–2251. [Google Scholar] [CrossRef]
- Hindiyarti, L.; Glarborg, P. Reactions of SO3 with the O/H radical pool under combustion conditions. Phys. Chem. A 2007, 111, 3984–3991. [Google Scholar] [CrossRef]
- Jørgensen, T.L.; Livbjerg, H.; Glarborg, P. Homogeneous and heterogeneously catalyzed oxidation of SO2. Chem. Eng. Sci. 2007, 62, 4496–4499. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Miller, C.A.; Erickson, C.; Jambhekar, R. Emissions of sulfur trioxide from coal-fired power plants. Air Waste Manag. 2004, 54, 750–762. [Google Scholar] [CrossRef]
- Scheffknecht, G.; Al-Makhadmeh, L.; Schnell, U.; Maier, J. Oxy-fuel coal combustion-a review of the current state-of-the art. Int. J. Greenh. Gas. Control. 2011, 5, S16–S35. [Google Scholar] [CrossRef]
- Cheng, J.; Zhou, J.; Liu, J.; Zhou, Z.; Cen, K. Sulfur removal at high temperature during coal combustion in furnaces: A review. Prog. Energy Combust. Sci. 2003, 29, 381–405. [Google Scholar] [CrossRef]
- Duana, L.; Duana, Y.; Sarbassovb, Y.; Li, Y.; Anthony, E.J. SO3 formation under oxy-CFB combustion conditions. Int. J. Greenh. Gas. Control. 2015, 43, 172–178. [Google Scholar] [CrossRef]
- Masters, S.G.; Chrissanthopoulos, A.; Eriksen, K.M.; Boghosian, S.; Fehrmann, R. Catalytic Activity and Deactivation of SO2 Oxidation Catalysts in Simulated Power Plant Flue Gases. J. Catal. 1997, 166, 16–24. [Google Scholar] [CrossRef]
- Lehmusto, J.; Vainio, E.; Lauren, T. The Effect of Deposit Temperature on the Catalytic SO2-to-SO3 Conversion in a Copper Flash Smelting Heat Recovery Boiler. Metall. Mater. Trans. 2017, 49, 434–439. [Google Scholar] [CrossRef]
- Sun, P.S.; Ning, P.; Song, W.B. Liquid-phase catalytic oxidation of smelting-gases containing SO2 in low concentration. J. Clean. Prod. 1998, 6, 323–327. [Google Scholar]
- Belo, L.P.; Elliott, L.K.; Stanger, R.J.; Spörll, R.; Shah, K.V.; Maire, J.; Wall, T.F. High-temperature conversion of SO2 to SO3: Homogeneous Experiments and Catalytic Effect of Fly Ash from Air and Oxy-fuel Firing. Energy Fuels 2014, 28, 7243–7251. [Google Scholar] [CrossRef]
- Ebara, R.; Tanaka, F.; Kawasaki, M. Sulfuric acid dew point corrosion in waste heat boiler tube for copper smelting furnace. Eng. Fail. Anal. 2013, 33, 29–36. [Google Scholar] [CrossRef]
- Croiset, E.; Thambimuthu, K.V. NOx and SO2 emissions from O2/CO2 recycle coal combustion. Fuel 2001, 80, 2117–2121. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Method-8.2009. Determination of Sulfuric Acid and Sulfur Dioxide Emissions from Combination Boilers, Recovery Furnaces, and Thermal Oxidizers–Isokinetic Method(S); EPA: Washington, DC, USA, 2009.
- Chughtai, A.R.; Brooks, M.E.; Smith, D.M. Effect of Metal Oxides and Black Carbon (Soot) on SO2/O2/H2O Reaction Systems. Aerosol Sci. Technol. 1993, 19, 121–132. [Google Scholar] [CrossRef][Green Version]
- Bayless, D.J.; Jewmaidang, J.; Tanneer, S.; Birru, R. Kinetics of low-temperature homogeneous SO3 formation for use in flue gas conditioning for improved electrostatic precipitator performance. Proc. Combust. Inst. 2000, 28, 2499–2505. [Google Scholar] [CrossRef]
- Song, Q.; Shibamori, Y.; Sadakata, M.; Koshi, M. Research on Homogeneous Oxidation of NO and SO2 in Flue Gas by Chain Reactions. Energy Fuels 2003, 17, 1549–1553. [Google Scholar] [CrossRef]
- Shi, Y.; Fan, M. Reaction kinetics for the catalytic oxidation of sulfur dioxide with microscale and nanoscale iron oxides. Ind. Eng. Chem. Res. 2007, 46, 80–86. [Google Scholar] [CrossRef]
- Xu, S.R.; Shuai, Q.; Cheng, J.H.; Wang, X.G. Catalytic Performance of SO2 by Colloidal Gold Supported on Nano γ-Fe2O3. Adv. Mater. Res. 2010, 178, 65–70. [Google Scholar] [CrossRef]
- Macken, C.; Hodnett, B.K. Testing of the CuO/Al2O3 catalyst–sorbent in extended operation for the simultaneous removal of NOx and SO2 from flue gases. Ind. Eng. Chem. Res. 2000, 39, 3868–3874. [Google Scholar] [CrossRef]
- Galloway, B.D.; MacDonald, R.A. Characterization of sulfur products on CaO at high temperature for air and oxy-combustion. Int. J. Coal Geol. 2016, 167, 1–9. [Google Scholar] [CrossRef]
- Ginosar, D.M.; Rollins, H.W.; Petkovic, L.M.; Burch, K.C.; Rush, M.J. High-temperature sulfuric acid decomposition over complex metal oxide catalysts. Int. J. Hydrog. Energy 2009, 34, 4065–4073. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Thomey, D.; de Oliveira, L.; Roeb, M.; Sattler, C.; Tsongidis, N.; Sakellariou, K. Oxide particles as combined heat storage medium and sulphur trioxide decomposition catalysts for solar hydrogen production through sulphur cycles. Int. J. Hydrog. Energy 2018, 12, 1–5. [Google Scholar] [CrossRef]
- Fu, H.; Wang, X.; Wu, H.; Yin, Y.; Chen, J. Heterogeneous Uptake and Oxidation of SO2 on Iron Oxides. J. Phys. Chem. C 2007, 111, 6077–6085. [Google Scholar] [CrossRef]
- Banerjee, A.M.; Shirole, A.R.; Pai, M.R.; Tripathia, A.K.; Bharadwaja, S.R.; Dasa, D.; Sinha, P.K. Catalytic activities of Fe2O3 and chromium doped Fe2O3 for sulfuric acid decomposition reaction in an integrated boiler, preheater, and catalytic decomposer. Appl. Catal. B Environ. 2012, 127, 36–46. [Google Scholar] [CrossRef]
- Xiao, H.; Dong, L.; Ning, X. Heterogeneous Catalytic Mechanism of SO2 Oxidation with Fe2O3. Proc. Csee 2016, 36, 5866–5872. [Google Scholar]
- Bart, H.J.; Ning, P.; Sun, P.; Song, W. Chemisorptive catalytic oxidation process for SO2 from smelting waste gases by Fe(II). Sep. Technol. 1996, 6, 253–260. [Google Scholar] [CrossRef]
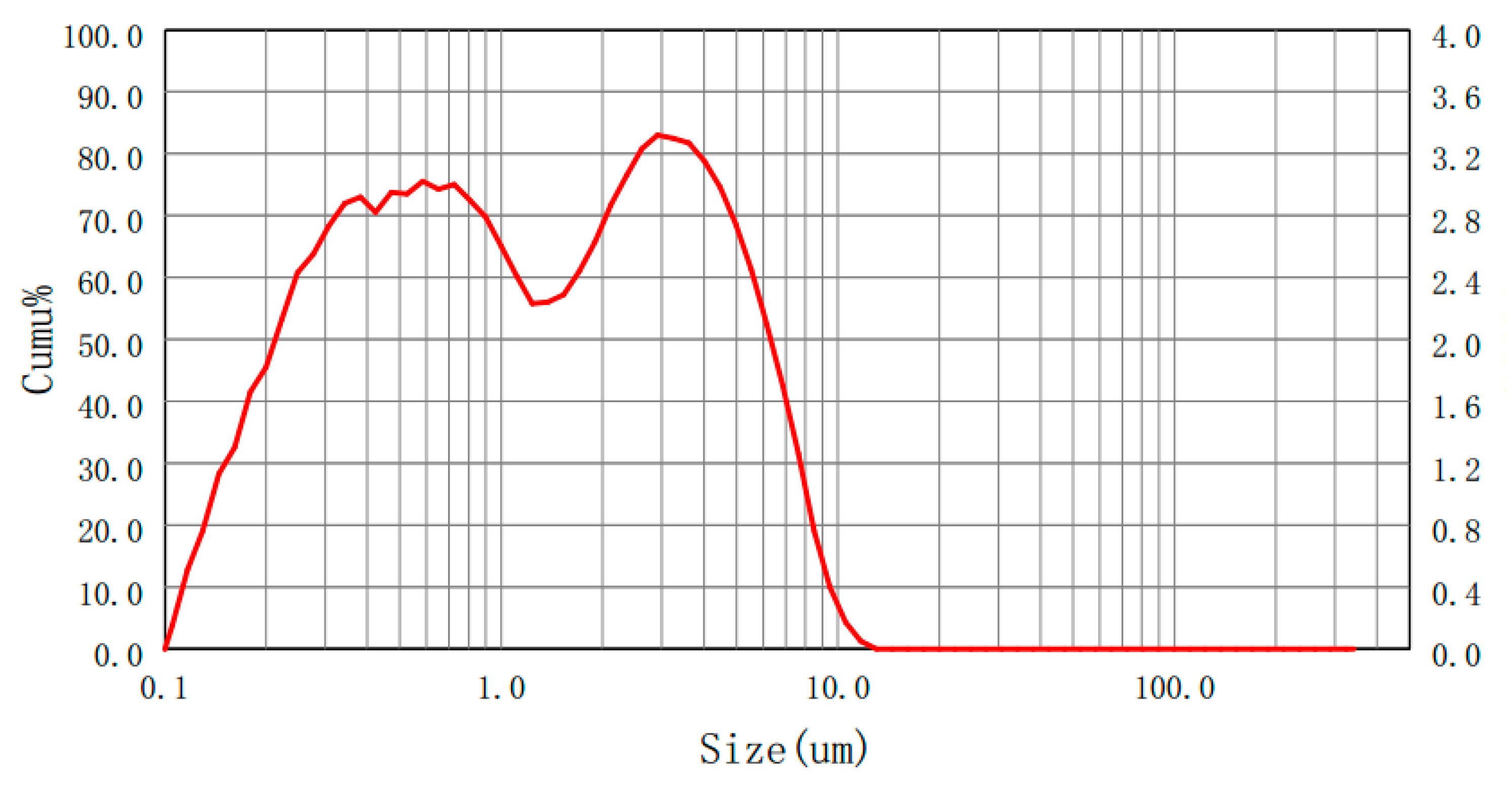
| Metal Oxides | Median Diameter (um) | Specific Surface Area (m2/kg) | 97%Granularity (um) | Purity |
|---|---|---|---|---|
| Fe2O3 | 1.105 | 3580 | <7.051 | 99.0% |
| CuO | 6.190 | 452.2 | <19.5 | 99.0% |
| Al2O3 | 77.20 | 38.12 | <196.2 | 99.0% |
| CaO | 55.82 | 139.7 | <187.7 | 99.0% |
| ZnO | 17.27 | 698 | <112.4 | 99.0% |
| Dust | CuO | PbO | ZnO | Al2O3 | MgO | Fe2O3 | CaO | CdO | Median Diameter (um) | Specific Surface Area (m2/kg) | 97% Granularity (um) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| zinc | 9.26 | 12.28 | 15.46 | 3.88 | 0.81 | 5.03 | 0.53 | 15.06 | 343.6 | <41.06 | |
| copper | 1.58 | 2.57 | 55.16 | 0.98 | 10.27 | 0.92 | 5.504 | 1161 | <25.56 | ||
| lead | 0.24 | 37.7 | 0.31 | 0.09 | 22.86 | 2.294 | 1768 | <5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, Q.; Yang, H.; Wu, Y.; Chen, J.; Hu, S. Effect of Metal Oxides and Smelting Dust on SO2 Conversion to SO3. Atmosphere 2021, 12, 734. https://doi.org/10.3390/atmos12060734
Liu H, Zhang Q, Yang H, Wu Y, Chen J, Hu S. Effect of Metal Oxides and Smelting Dust on SO2 Conversion to SO3. Atmosphere. 2021; 12(6):734. https://doi.org/10.3390/atmos12060734
Chicago/Turabian StyleLiu, Haipeng, Qin Zhang, Hongying Yang, Yanan Wu, Jiacheng Chen, and Shen Hu. 2021. "Effect of Metal Oxides and Smelting Dust on SO2 Conversion to SO3" Atmosphere 12, no. 6: 734. https://doi.org/10.3390/atmos12060734
APA StyleLiu, H., Zhang, Q., Yang, H., Wu, Y., Chen, J., & Hu, S. (2021). Effect of Metal Oxides and Smelting Dust on SO2 Conversion to SO3. Atmosphere, 12(6), 734. https://doi.org/10.3390/atmos12060734





