Determining the Role of Acidity, Fate and Formation of IEPOX-Derived SOA in CMAQ
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Transport Model and Measurements
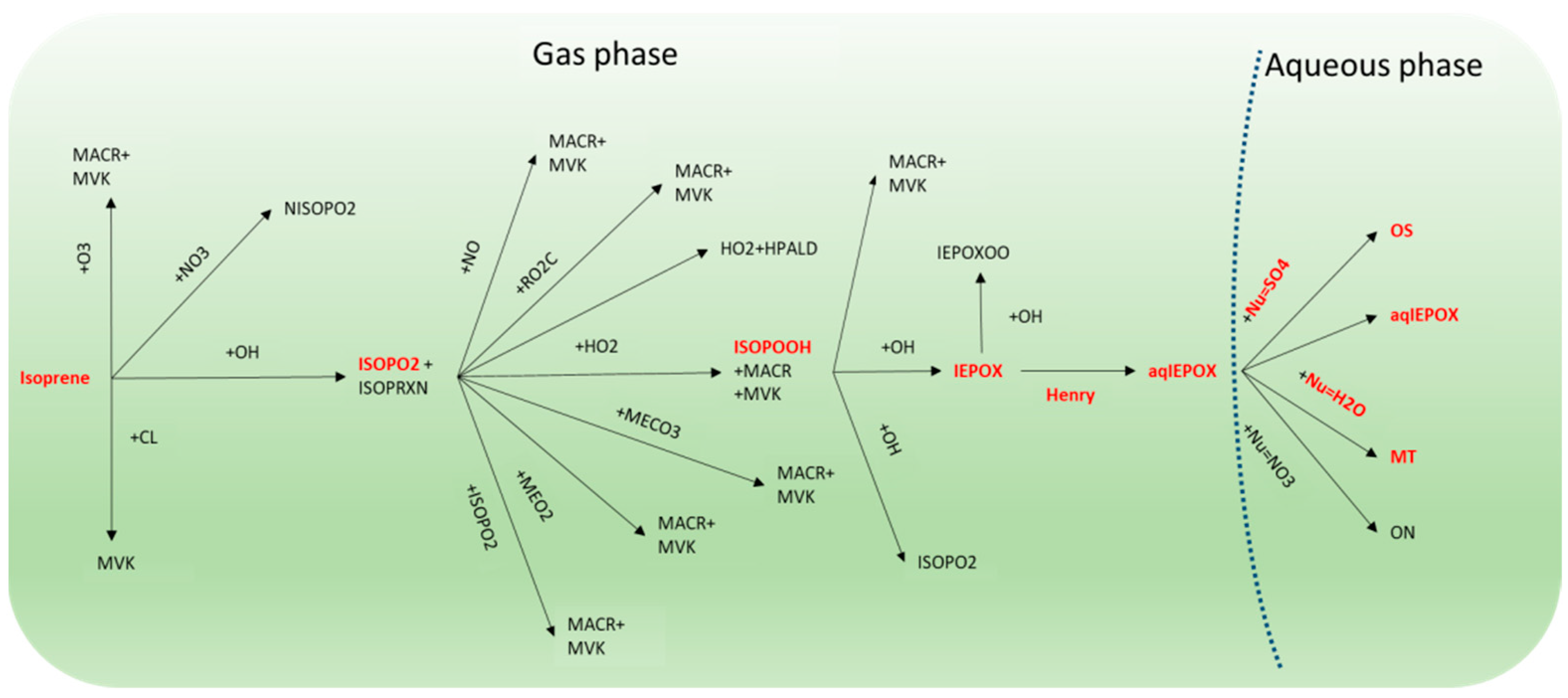
2.2. Chemical Mechanism
3. Results
3.1. Ozone, NOx and Sulfate
3.2. Henry’s Law Sensitivity Tests and Updates to the Simulations
3.2.1. Baseline Simulation
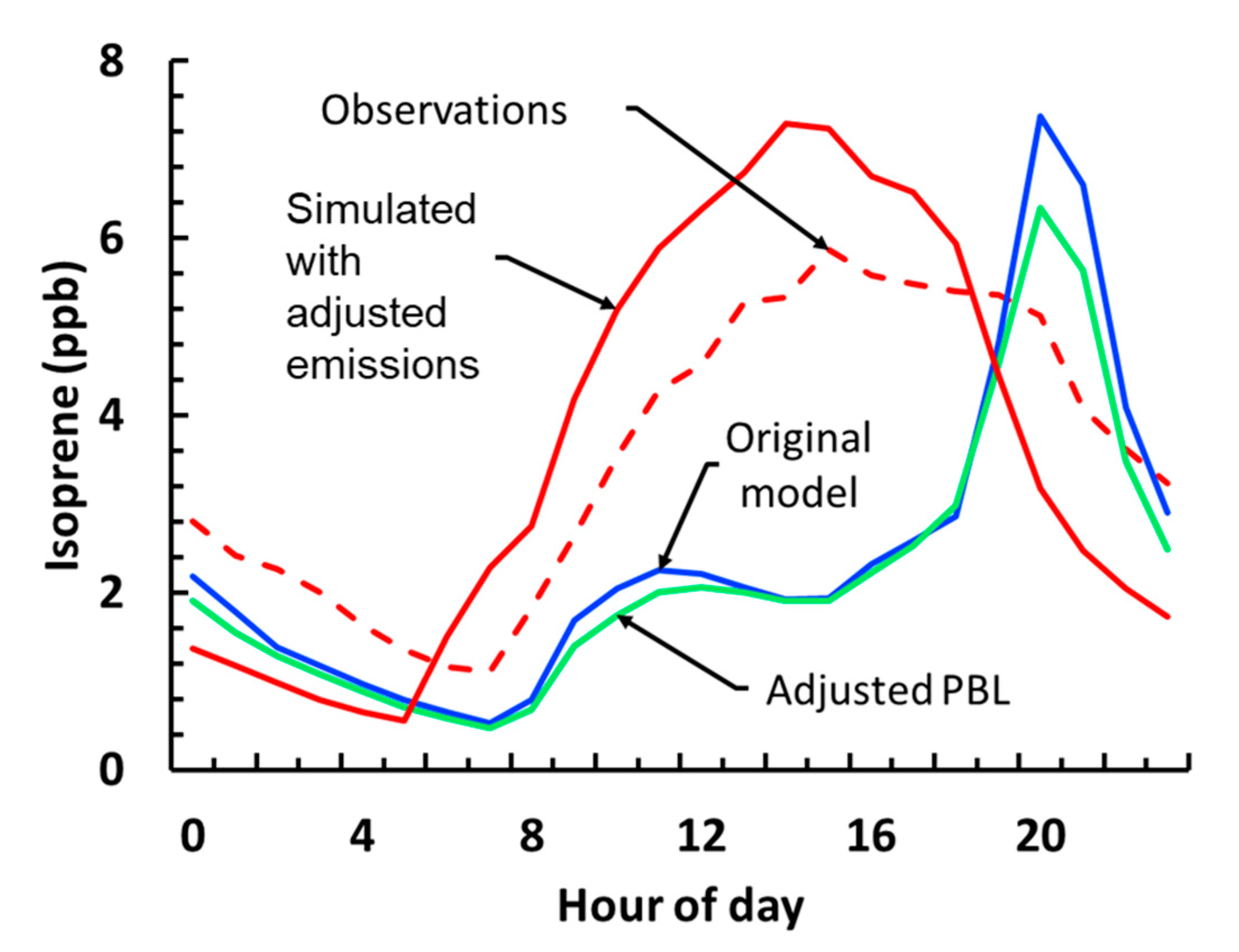
3.2.2. Planetary Boundary Layer Height (PBL) Prescription and Isoprene Emission Fitting (IEF)
3.2.3. Isoprene Epoxydiol (IEPOX) Deposition Correction (DEP)
3.2.4. Updated IEPOX Gas Phase Oxidation and Henry’s Law Sensitivity Tests

3.3. Comparing Aqueous SOA to Observations and Correlation with Sulfate
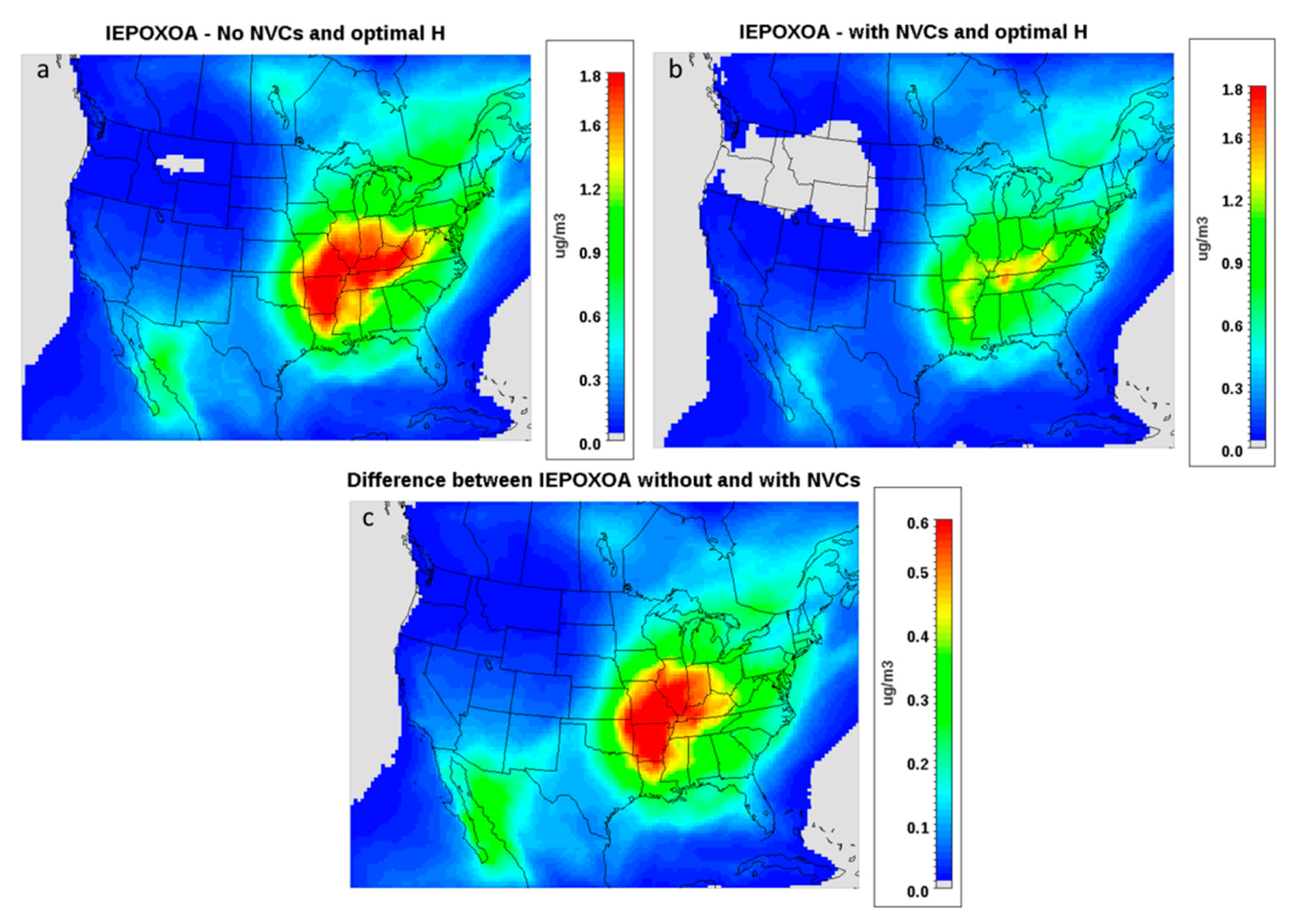
3.4. The Impact of Dust on IEPOX Organic Aerosol (OA)
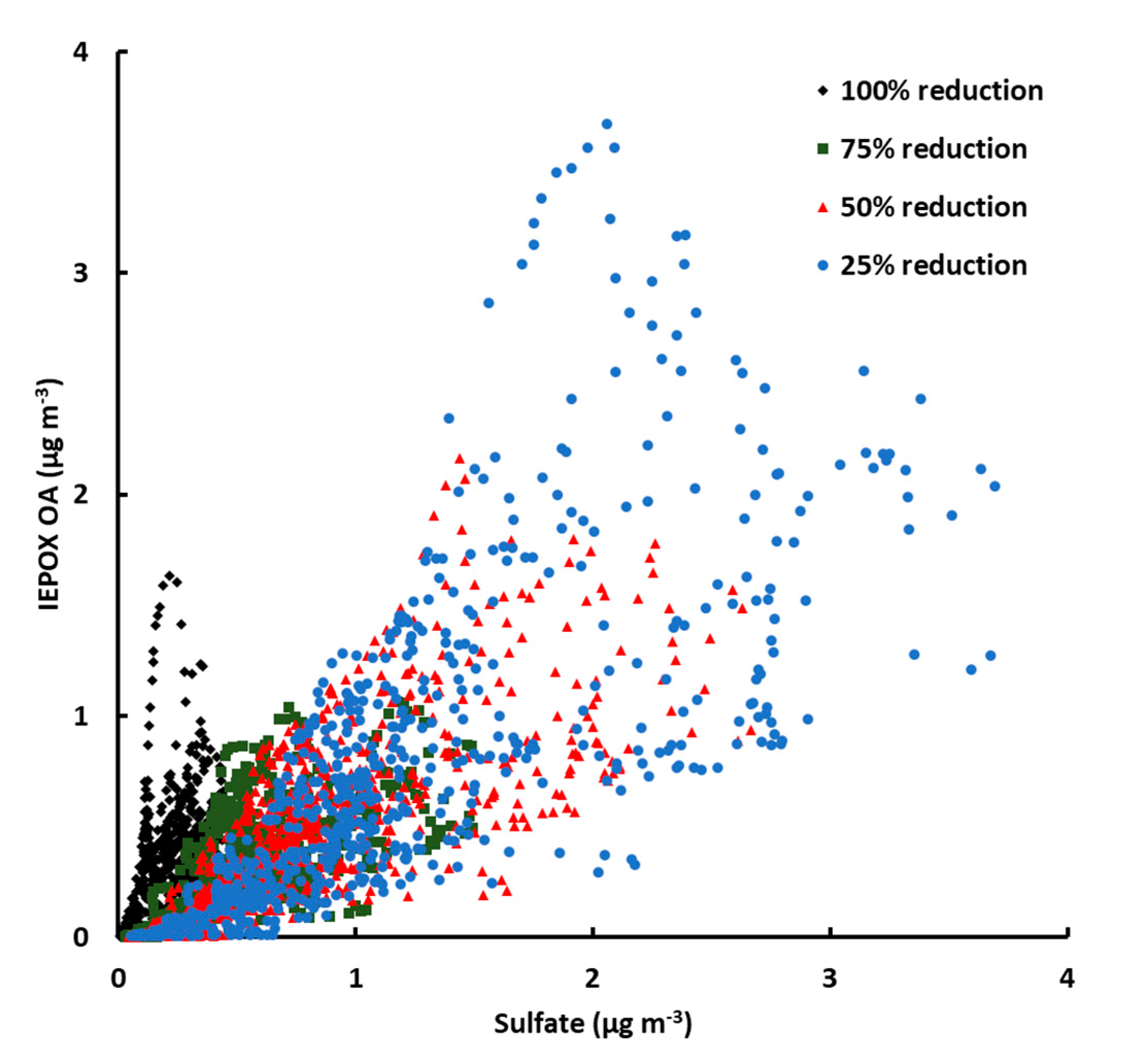
3.5. SO2 Reductions and the Future of IEPOX OA
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 2006, 6, 3181–3210. [Google Scholar] [CrossRef]
- Xu, L.; Guo, H.; Boyd, C.; Klein, M.; Bougiatioti, A.; Cerully, K.; Hite, J.; Isaacman-VanWertz, G.; Kreisberg, N.M.; Knote, C.; et al. Effects of anthropogenic emissions on aerosol formation from isoprene and monoterpenes in the southeastern United States. Proc. Natl. Acad. Sci. USA 2014, 112, 37–42. [Google Scholar] [CrossRef]
- Weber, R.J.; Amy, P.S.; Richard, E.P.; Armistead, R.; Bo, Y.; Mei, Z.; Joost, D.G.; Carsten, W.; Charles, B.; John, S.H.; et al. A study of secondary organic aerosol formation in the anthropogenic-influenced southeastern United States. J. Geophys. Res. 2007, 112, 13302. [Google Scholar] [CrossRef]
- Budisulistiorini, S.H.; Li, X.; Bairai, S.T.; Renfro, J.; Liu, Y.; Liu, Y.J.; McKinney, K.A.; Martin, S.T.; McNeill, V.F.; Pye, H.O.T.; et al. Examining the effects of anthropogenic emissions on isoprene-derived secondary organic aerosol formation during the 2013 Southern Oxidant and Aerosol Study (SOAS) at the Look Rock, Tennessee ground site. Atmos. Chem. Phys. 2015, 15, 8871–8888. [Google Scholar] [CrossRef]
- Budisulistiorini, S.H.; Nenes, A.; Carlton, A.G.; McNeill, V.F.; Pye, H.O.; Surratt, J.D. Simulating Aqueous-Phase Isoprene-Epoxydiol (IEPOX) Secondary Organic Aerosol Production During the 2013 Southern Oxidant and Aerosol Study (SOAS). Environ. Sci. Technol. 2017, 51, 5026–5034. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Petters, M.; Suda, S.; Guo, H.; Weber, R.; Carlton, A. Trends in particle-phase liquid water during the Southern Oxidant and Aerosol Study. Atmos. Chem. Phys. 2014, 14, 10911–10930. [Google Scholar] [CrossRef]
- Kleindienst, T.E.; Jaoui, M.; Lewandowski, M.; Offenberg, J.H.; Lewis, C.W.; Bhave, P.V.; Edney, E.O. Estimates of the contributions of biogenic and anthropogenic hydrocarbons to secondary organic aerosol at a southeastern US location. Atmos. Environ. 2007, 41, 8288–8300. [Google Scholar] [CrossRef]
- Pye, H.O.T.; Pinder, R.W.; Piletic, I.R.; Xie, Y.; Capps, S.L.; Lin, Y.-H.S.; Surratt, J.D.; Zhang, Z.; Gold, A.; Luecken, D.J.; et al. Epoxide Pathways Improve Model Predictions of Isoprene Markers and Reveal Key Role of Acidity in Aerosol Formation. Environ. Sci. Technol. 2013, 47, 11056–11064. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Zhang, H.; Pye, H.O.T.; Zhang, Z.; Marth, W.J.; Park, S.; Arashiro, M.; Cui, T.; Budisulistiorini, S.H.; Sexton, K.G.; et al. Epoxide as a precursor to secondary organic aerosol formation from isoprene photooxidation in the presence of nitrogen oxides. Proc. Natl. Acad. Sci. USA 2013, 110, 6718–6723. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Coggon, M.M.; Bates, K.H.; Zhang, X.; Schwantes, R.H.; Schilling, K.A.; Loza, C.L.; Flagan, R.C.; Wennberg, P.O.; Seinfeld, J.H. Organic aerosol formation from the reactive uptake of isoprene epoxydiols (IEPOX) onto non-acidified inorganic seeds. Atmos. Chem. Phys. 2014, 14, 3497–3510. [Google Scholar] [CrossRef]
- Offenberg, J.H.; Lewandowski, M.; Jaoui, M.; Kleindienst, T.E. Contributions of biogenic and anthropogenic hydrocarbons to secondary organic aerosol during 2006 in Research Triangle Park, NC. Aerosol Air Qual. Res. 2011, 11, 99–108. [Google Scholar] [CrossRef]
- Xu, L.; Middlebrook, A.M.; Liao, J.; de Gouw, J.; Guo, H.; Weber, R.J.; Nenes, A.; Lee, B.H.; Thornton, J.A.; Brock, C.; et al. Enhanced formation of Isoprene-derived Organic Aerosol in Power Plant Plumes during Southeast Nexus (SENEX). J. Geophys. Res. 2016, 121, 11137–11153. [Google Scholar] [CrossRef]
- Acosta, J.P. A Model Study of the Global Emissions of Terpenoid Volatile Organic Compounds from Terrestrial Vegetation in the Last Millennium. Master’s Thesis, Institute of Physics of Helsinki, Helsinki, Finland, 2013. [Google Scholar]
- Carlton, A.G.; Goldstein, A.; Jimenez, J.F. The Southern Oxidant and Aerosol Study (SOAS): Measuring and Modeling at the Interface of air Quality and Climate Change to Understand Biosphere-Atmosphere Interactions. Available online: http://climate.envsci.rutgers.edu/SOAS/SOAS_White_Paper_final.pdf (accessed on 31 May 2021).
- Guo, H.; Xu, L.; Bougiatioti, A.; Cerully, K.M.; Capps, S.L.; Hite, J.J.R.; Carlton, A.G.; Lee, S.-H.; Bergin, M.H.; Ng, N.L.; et al. Fine-particle water and pH in the southeastern United States. Atmos. Chem. Phys. 2015, 15, 5211–5228. [Google Scholar] [CrossRef]
- Weber, R.J.; Guo, H.; Russell, A.G.; Nenes, A. High aerosol acidity despite declining atmospheric sulfate concentrations over the past 15 years. Nat. Geosci. 2016, 9, 282–285. [Google Scholar] [CrossRef]
- Tilgner, A.; Schaefer, T.; Alexander, B.; Barth, M.; Collett, J.L., Jr.; Fahey, K.M.; Nenes, A.; Pye, H.O.T.; Herrmann, H.; McNeill, V.F. Acidity and the multiphase chemistry of atmospheric aqueous particles and clouds. Atmos. Chem. Phys. Discuss. 2021, 1–82, preprint, in review. [Google Scholar] [CrossRef]
- Vasilakos, P.; Russell, A.; Weber, R.; Nenes, A. Understanding nitrate formation in a world with less sulfate. Atmos. Chem. Phys. 2018, 18, 12765–12775. [Google Scholar] [CrossRef]
- Edgerton, E.S.; Hartsell, B.E.; Saylor, R.D.; Jansen, J.J.; Hansen, D.A.; Hidy, G.M. The Southeastern Aerosol Research and Characterization Study: Part II. Filter-based measurements of fine and coarse particulate matter mass and composition. J. Air Waste Manag. Assoc. 2005, 55, 1527–1542. [Google Scholar] [CrossRef]
- Edgerton, E.S.; Hartsell, B.E.; Saylor, R.D.; Jansen, J.J.; Hansen, D.A.; Hidy, G.M. The Southeastern Aerosol Research and Characterization Study, part 3: Continuous measurements of fine particulate matter mass and composition. J. Air Waste Manag. Assoc. 2006, 56, 1325–1341. [Google Scholar] [CrossRef]
- Marais, E.A.; Jacob, D.J.; Jimenez, J.L.; Campuzano-Jost, P.; Day, D.A.; Hu, W.; Krechmer, J.; Zhu, L.; Kim, P.S.; Miller, C.C. Aqueous-phase mechanism for secondary organic aerosol formation from isoprene: Application to the southeast United States and co-benefit of SO2 emission controls. Atmos. Chem. Phys. 2016, 16, 1603–1618. [Google Scholar] [CrossRef]
- Byun, D.W.; Schere, K.L. Review of the governing equations, computational algorithms, and other components of the Models-3 Community Multiscale Air Quality (CMAQ) Modeling System. Appl. Mech. Rev. 2006, 59, 51–77. [Google Scholar] [CrossRef]
- Fountoukis, C.; Nenes, A. ISORROPIA II: A computationally efficient thermodynamic equilibrium model for K+–Ca2+–Mg2+–NH4+–Na+–SO42−–NO3−–Cl−–H2O aerosols. Atmos. Chem. Phys. 2007, 7, 4639–4659. [Google Scholar] [CrossRef]
- Vukovich, J.; Pierce, T. The Implementation of BEIS3 within the SMOKE Modeling Framework. In Proceedings of the 11th International Emissions Inventory Conference, Atlanta, GA, USA, 15–18 April 2002. [Google Scholar]
- Allen, H.M.; Draper, D.C.; Ayres, B.R.; Ault, A.; Bondy, A.; Takahama, S.; Modini, R.L.; Baumann, K.; Edgerton, E.; Knote, C. Influence of crustal dust and sea spray supermicron particle concentrations and acidity on inorganic NO3− aerosol during the 2013 Southern Oxidant and Aerosol Study. Atmos. Chem. Phys. 2015, 15, 10669–10685. [Google Scholar] [CrossRef]
- Brophy, P.; Farmer, D. A switchable reagent ion high resolution time-of-flight chemical ionization mass spectrometer for real-time measurement of gas phase oxidized species: Characterization from the 2013 southern oxidant and aerosol study. Atmos. Meas. Tech. 2015, 8, 2945–2959. [Google Scholar] [CrossRef]
- Pye, H.O.T.; Murphy, B.N.; Xu, L.; Ng, N.L.; Carlton, A.G.; Guo, H.; Weber, R.; Vasilakos, P.; Appel, K.W.; Budisulistiorini, S.H.; et al. On the implications of aerosol liquid water and phase separation for organic aerosol mass. Atmos. Chem. Phys. 2017, 17, 343–369. [Google Scholar] [CrossRef]
- Lee, B.; D’Ambro, E.L.; Lopez-Hilfiker, F.D.; Schobesberger, S.; Mohr, C.; Zawadowicz, M.A.; Liu, J.M.; Shilling, J.E.; Hu, W.W.; Palm, B.B.; et al. Resolving Ambient Organic Aerosol Formation and Aging Pathways with Simultaneous Molecular Composition and Volatility Observations. ACS Earth Space Chem. 2020, 4, 391–402. [Google Scholar] [CrossRef]
- Travis, K.R.; Jacob, D.J.; Fisher, J.A.; Kim, P.S.; Marais, E.A.; Zhu, L.; Yu, K.; Miller, C.C.; Yantosca, R.M.; Sulprizio, M.P.; et al. Why do models overestimate surface ozone in the Southeast United States? Atmos. Chem. Phys. 2016, 16, 13561–13577. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-M.; Klein, P.M.; Xue, M. Evaluation of the updated YSU planetary boundary layer scheme within WRF for wind resource and air quality assessments. J. Geophys. Res. Atmos. 2013, 118, 10490–10505. [Google Scholar] [CrossRef]
- Paulot, F.; Crounse, J.D.; Kjaergaard, H.G.; Kurten, A.; St Clair, J.M.; Seinfeld, J.H.; Wennberg, P.O. Unexpected epoxide formation in the gas-phase photooxidation of isoprene. Science 2009, 325, 730–733. [Google Scholar] [CrossRef]
- Jacobs, M.I.; Darer, A.I.; Matthew, J. Elrod Rate Constants and Products of the OH Reaction with Isoprene-Derived Epoxides. Environ. Sci. Technol. 2013, 47, 12868–12876. [Google Scholar] [CrossRef]
- Eddingsaas, N.C.; VanderVelde, D.G.; Wennberg, P.O. Kinetics and products of the acid-catalyzed ring-opening of atmospherically relevant butyl epoxy alcohols. J. Phys. Chem. A 2010, 114, 8106–8113. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, T.; Wood, S.A.; Goss, K.-U.; Li, J.; Ying, Q.; Wania, F. Uncertain Henry’s law constants compromise equilibrium partitioning calculations of atmospheric oxidation products. Atmos. Chem. Phys. 2017, 17, 7529–7540. [Google Scholar] [CrossRef]
- Chan, M.N.; Surratt, J.D.; Claeys, M.; Edgerton, E.S.; Tanner, R.L.; Shaw, S.L.; Zheng, M.; Knipping, E.M.; Eddingsaas, N.C.; Wennberg, P.O.; et al. Characterization and quantification of isoprene-derived epoxydiols in ambient aerosol in the southeastern United States. Environ. Sci. Technol. 2010, 44, 4590–4596. [Google Scholar] [CrossRef]
- Earth System Research Laboratory Chemical Sciences Division. Available online: https://csl.noaa.gov/groups/csl7/measurements/2013senex/Ground/DataDownload/ (accessed on 31 May 2021).
- Surratt, J.D.; Chan, A.W.H.; Eddingsaas, N.C.; Chan, M.N.; Loza, C.L.; Kwan, A.J.; Hersey, S.P.; Flagan, R.C.; Wennberg, P.O.; Seinfeld, J.H. Reactive Intermediates Revealed in Secondary Organic Aerosol Formation from Isoprene. Proc. Natl. Acad. Sci. USA 2010, 107, 6640–6645. [Google Scholar] [CrossRef]
- Schmedding, R.; Rasool, Q.Z.; Zhang, Y.; Pye, H.O.T.; Zhang, H.; Chen, Y.; Surratt, J.D.; Lopez-Hilfiker, F.D.; Thornton, J.A.; Goldstein, A.H.; et al. Predicting secondary organic aerosol phase state and viscosity and its effect on multiphase chemistry in a regional-scale air quality model. Atmos. Chem. Phys. 2020, 20, 8201–8225. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Guo, H.; Zeng, L.; Verma, V.; Nenes, A.; Weber, R.J. Highly Acidic Ambient Particles, Soluble Metals, and Oxidative Potential: A Link between Sulfate and Aerosol Toxicity. Environ. Sci. Technol. 2017, 51, 2611–2620. [Google Scholar] [CrossRef]
- Guo, H.; Weber, R.J.; Nenes, A. High levels of ammonia do not raise fine particle pH sufficiently to yield nitrogen oxide-dominated sulfate production. Sci. Rep. 2017, 7, 12109. [Google Scholar] [CrossRef] [PubMed]
- Carlton, A.G.; Baker, K.R. Photochemical Modeling of the Ozark Isoprene Volcano: MEGAN, BEIS, and Their Impacts on Air Quality Predictions. Environ. Sci. Technol. 2011, 45, 4438–4445. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.L.; Hidy, G.M.; Tanenbaum, S.; Edgerton, E.S.; Hartsell, B.E. The Southeastern Aerosol Research and Characterization (SEARCH) study: Temporal trends in gas and PM concentrations and composition, 1999–2010. J. Air Waste Manag. Assoc. 2013, 63, 247–259. [Google Scholar] [CrossRef]
- Riva, M.; Chen, Y.; Zhang, Y.; Lei, Z.; Olson, N.; Boyer, H.C.; Narayan, S.; Yee, L.D.; Green, H.; Cui, T.; et al. Increasing Isoprene Epoxydiol-to-Inorganic Sulfate Aerosol (IEPOX:Sulfinorg) Ratio Results in Extensive Conversion of Inorganic Sulfate to Organosulfur Forms: Implications for Aerosol Physicochemical Properties. Environ. Sci. Technol. 2019, 53, 8682–8694. [Google Scholar] [CrossRef] [PubMed]






| Simulation Name | PBL Height | Isoprene Emissions | IEPOX Deposition Surrogate | IEPOX + OH− Oxidation Constant | H* Scaling Factor |
|---|---|---|---|---|---|
| BASELINE | default | default | VD_OP | Paulot | 1 |
| PBL | assimilated | default | VD_OP | Paulot | 1 |
| IEF | assimilated | adjusted | VD_OP | Paulot | 1 |
| DEP | assimilated | adjusted | HNO3 | Paulot | 1 |
| Oxidation | assimilated | adjusted | HNO3 | Jacobs | 1 |
| Henry 1 | assimilated | adjusted | HNO3 | Jacobs | 2.5 |
| Henry 2 | assimilated | adjusted | HNO3 | Jacobs | 5 |
| Henry 3 | assimilated | adjusted | HNO3 | Jacobs | 7 |
| Henry 4 | assimilated | adjusted | HNO3 | Jacobs | 9 |
| Henry 5 | assimilated | adjusted | HNO3 | Jacobs | 10 |
| Henry 6 | assimilated | adjusted | HNO3 | Jacobs | 100 |
| Regression Variable | Regression Coefficient in the Measurements (Xu et al. 2015) | Regression Coefficient for the Simulations |
|---|---|---|
| Sulfate | 0.424 | 0.527 |
| Water | −0.004 | 0.029 |
| H+ | 0.009 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilakos, P.; Hu, Y.; Russell, A.; Nenes, A. Determining the Role of Acidity, Fate and Formation of IEPOX-Derived SOA in CMAQ. Atmosphere 2021, 12, 707. https://doi.org/10.3390/atmos12060707
Vasilakos P, Hu Y, Russell A, Nenes A. Determining the Role of Acidity, Fate and Formation of IEPOX-Derived SOA in CMAQ. Atmosphere. 2021; 12(6):707. https://doi.org/10.3390/atmos12060707
Chicago/Turabian StyleVasilakos, Petros, Yongtao Hu, Armistead Russell, and Athanasios Nenes. 2021. "Determining the Role of Acidity, Fate and Formation of IEPOX-Derived SOA in CMAQ" Atmosphere 12, no. 6: 707. https://doi.org/10.3390/atmos12060707
APA StyleVasilakos, P., Hu, Y., Russell, A., & Nenes, A. (2021). Determining the Role of Acidity, Fate and Formation of IEPOX-Derived SOA in CMAQ. Atmosphere, 12(6), 707. https://doi.org/10.3390/atmos12060707






