Deposition of Aerosols onto Upper Ocean and Their Impacts on Marine Biota
Abstract
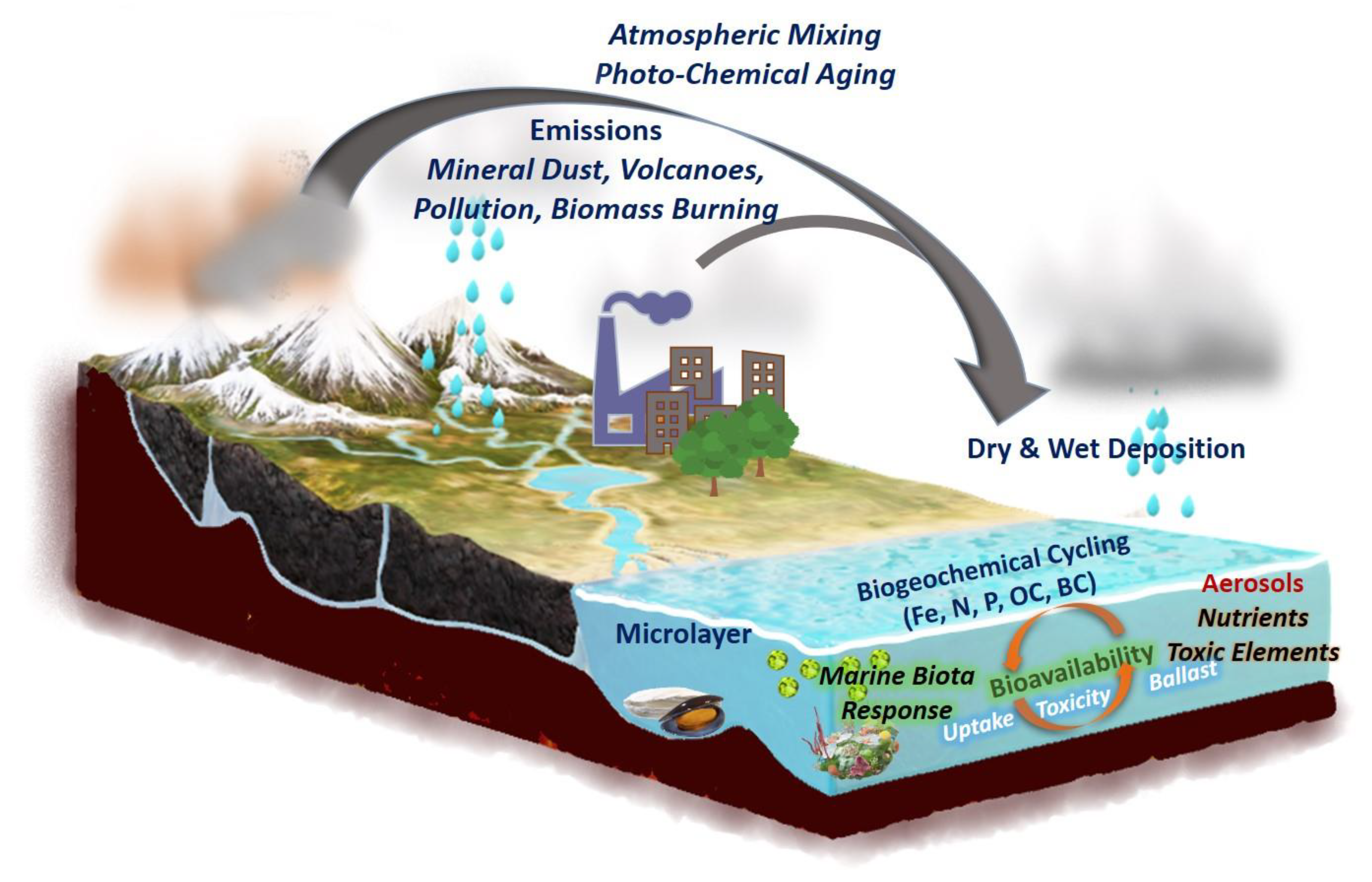
1. Introduction
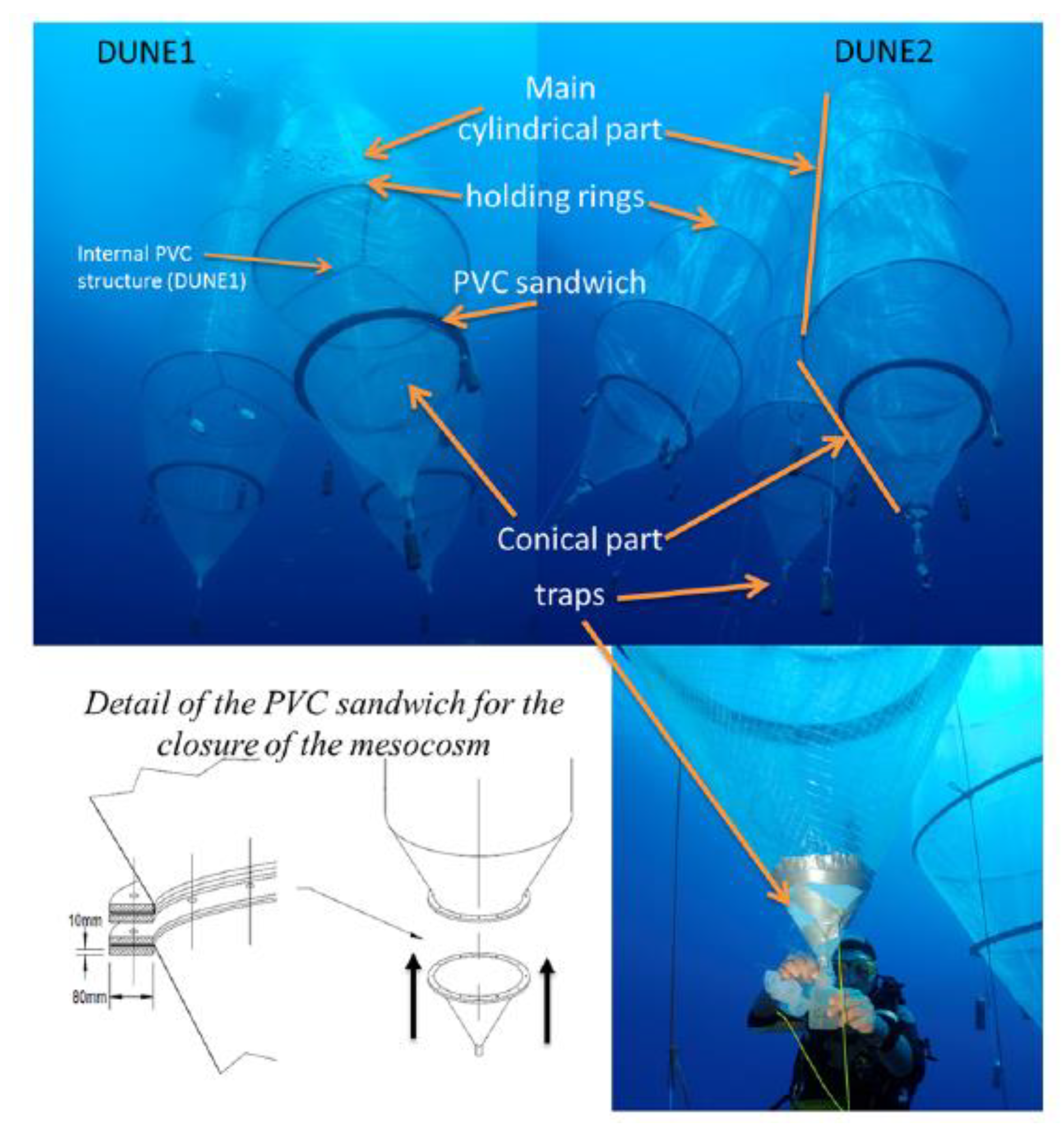
2. Mineral Dust Deposition onto the Upper Ocean
3. Deposition of Anthropogenic Aerosols to the Upper Ocean
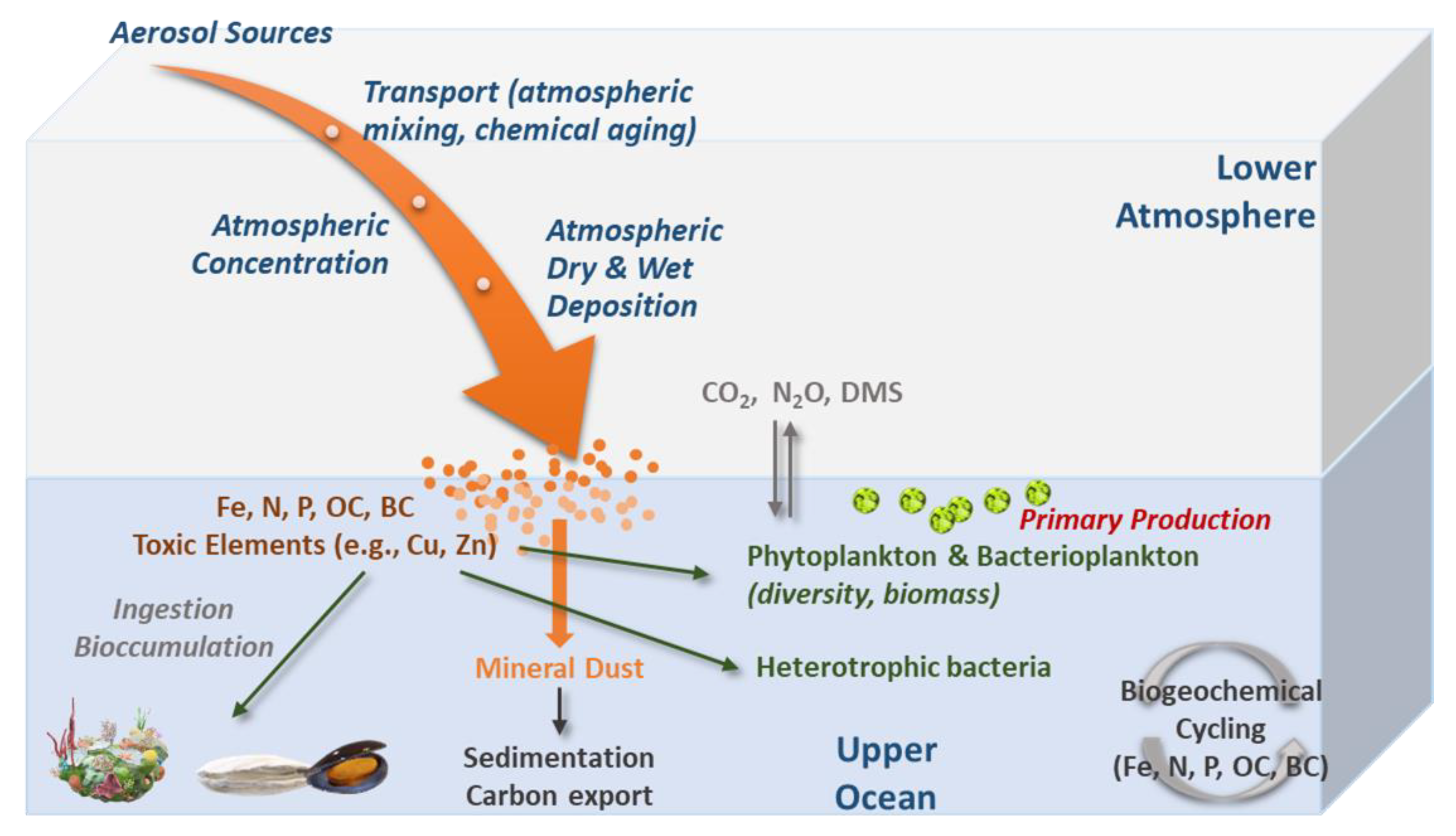
4. Effects of Atmospheric Deposition on Marine Biota
5. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pöschl, U. Atmospheric Aerosols: Composition, Transformation, Climate and Health Effects. Angew. Chem. Int. Ed. 2005, 44, 7520–7540. [Google Scholar] [CrossRef]
- Tomasi, C.; Lupi, A. Primary and Secondary Sources of Atmospheric Aerosol. In Atmospheric Aerosols; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 1–86. [Google Scholar]
- Heal, M.R.; Kumar, P.; Harrison, R.M. Particles, air quality, policy and health. Chem. Soc. Rev. 2012, 41, 6606. [Google Scholar] [CrossRef] [PubMed]
- Putaud, J.P.; Van Dingenen, R.; Alastuey, A.; Bauer, H.; Birmili, W.; Cyrys, J.; Flentje, H.; Fuzzi, S.; Gehrig, R.; Hansson, H.C.; et al. A European aerosol phenomenology—3: Physical and chemical characteristics of particulate matter from 60 rural, urban, and kerbside sites across Europe. Atmos. Environ. 2010, 44, 1308–1320. [Google Scholar] [CrossRef]
- Duarte, R.M.B.O.; Matos, J.T.V.; Paula, A.S.; Lopes, S.P.; Ribeiro, S.; Santos, J.F.; Patinha, C.; da Silva, E.F.; Soares, R.; Duarte, A.C. Tracing of aerosol sources in an urban environment using chemical, Sr isotope, and mineralogical characterization. Environ. Sci. Pollut. Res. 2017, 24, 11006–11016. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.C.; Duarte, R.M.B.O. Natural Organic Matter in Atmospheric Particles. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; Senesi, N., Xing, B., Huang, P.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 451–485. ISBN 9780470413005. [Google Scholar]
- Bikkina, P.; Sarma, V.V.S.S.; Kawamura, K.; Bikkina, S. Dry-deposition of inorganic and organic nitrogen aerosols to the Arabian Sea: Sources, transport and biogeochemical significance in surface waters. Mar. Chem. 2021, 231, 103938. [Google Scholar] [CrossRef]
- Almeida, G.P.; Bittencourt, A.T.; Evangelista, M.S.; Vieira-Filho, M.S.; Fornaro, A. Characterization of aerosol chemical composition from urban pollution in Brazil and its possible impacts on the aerosol hygroscopicity and size distribution. Atmos. Environ. 2019, 202, 149–159. [Google Scholar] [CrossRef]
- Saarikoski, S.; Reyes, F.; Vázquez, Y.; Tagle, M.; Timonen, H.; Aurela, M.; Carbone, S.; Worsnop, D.R.; Hillamo, R.; Oyola, P. Characterization of submicron aerosol chemical composition and sources in the coastal area of Central Chile. Atmos. Environ. 2019, 199, 391–401. [Google Scholar] [CrossRef]
- Emerson, E.W.; Katich, J.M.; Schwarz, J.P.; McMeeking, G.R.; Farmer, D.K. Direct Measurements of Dry and Wet Deposition of Black Carbon Over a Grassland. J. Geophys. Res. Atmos. 2018, 123, 12277–12290. [Google Scholar] [CrossRef]
- Emerson, E.W.; Hodshire, A.L.; DeBolt, H.M.; Bilsback, K.R.; Pierce, J.R.; McMeeking, G.R.; Farmer, D.K. Revisiting particle dry deposition and its role in radiative effect estimates. Proc. Natl. Acad. Sci. USA 2020, 117, 26076–26082. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, G.; Guieu, C.; Arneth, A.; Bellouin, N.; Bopp, L.; Boyd, P.W.; van der Gon, H.A.C.D.; Desboeufs, K.V.; Dulac, F.; Facchini, M.C.; et al. Ocean–Atmosphere Interactions of Particles. In Ocean-Atmosphere Interactions of Gases and Particles; Liss, P.C., Johnson, M.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 171–246. ISBN 9783642256431. [Google Scholar]
- Marañén, E.; Fernández, A.; Mouriño-Carballido, B.; MartÍnez-GarcÍa, S.; Teira, E.; Cermeño, P.; Chouciño, P.; Huete-Ortega, M.; Fernández, E.; Calvo-DÍaz, A.; et al. Degree of oligotrophy controls the response of microbial plankton to Saharan dust. Limnol. Oceanogr. 2010, 55, 2339–2352. [Google Scholar] [CrossRef]
- Paytan, A.; Mackey, K.R.M.; Chen, Y.; Lima, I.D.; Doney, S.C.; Mahowald, N.; Labiosa, R.; Post, A.F. Toxicity of atmospheric aerosols on marine phytoplankton. Proc. Natl. Acad. Sci. USA 2009, 106, 4601–4605. [Google Scholar] [CrossRef]
- Doney, S.C.; Mahowald, N.; Lima, I.; Feely, R.A.; Mackenzie, F.T.; Lamarque, J.F.; Rasch, P.J. Impact of anthropogenic atmospheric nitrogen and sulfur deposition on ocean acidification and the inorganic carbon system. Proc. Natl. Acad. Sci. USA 2007, 104, 14580–14585. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Niggemann, J.; Luo, L.; Dittmar, T.; Kao, S.J. Aerosols as a source of dissolved black carbon to the ocean. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Jurado, E.; Dachs, J.; Duarte, C.M.; Simó, R. Atmospheric deposition of organic and black carbon to the global oceans. Atmos. Environ. 2008, 42, 7931–7939. [Google Scholar] [CrossRef]
- Ginoux, P.; Prospero, J.M.; Gill, T.E.; Hsu, N.C.; Zhao, M. Global-scale attribution of anthropogenic and natural dust sources and their emission rates based on MODIS Deep Blue aerosol products. Rev. Geophys. 2012, 50, 3005. [Google Scholar] [CrossRef]
- Schulz, M.; Prospero, J.M.; Baker, A.R.; Dentener, F.; Ickes, L.; Liss, P.S.; Mahowald, N.M.; Nickovic, S.; García-Pando, C.P.; Rodríguez, S.; et al. Atmospheric Transport and Deposition of Mineral Dust to the Ocean: Implications for Research Needs. Environ. Sci. Technol. 2012, 46, 10390–10404. [Google Scholar] [CrossRef]
- Kienast, S.S.; Winckler, G.; Lippold, J.; Albani, S.; Mahowald, N.M. Tracing dust input to the global ocean using thorium isotopes in marine sediments: ThoroMap. Glob. Biogeochem. Cycles 2016, 30, 1526–1541. [Google Scholar] [CrossRef]
- Hayes, C.T.; Anderson, R.F.; Fleisher, M.Q.; Serno, S.; Winckler, G.; Gersonde, R. Quantifying lithogenic inputs to the North Pacific Ocean using the long-lived thorium isotopes. Earth Planet. Sci. Lett. 2013, 383, 16–25. [Google Scholar] [CrossRef]
- Hsieh, Y.T.; Henderson, G.M.; Thomas, A.L. Combining seawater 232Th and 230Th concentrations to determine dust fluxes to the surface ocean. Earth Planet. Sci. Lett. 2011, 312, 280–290. [Google Scholar] [CrossRef]
- Anderson, R.F.; Cheng, H.; Edwards, R.L.; Fleisher, M.Q.; Hayes, C.T.; Huang, K.F.; Kadko, D.; Lam, P.J.; Landing, W.M.; Lao, Y.; et al. How well can we quantify dust deposition to the ocean? Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374. [Google Scholar] [CrossRef]
- Hayes, C.T.; Fitzsimmons, J.N.; Boyle, E.A.; McGee, D.; Anderson, R.F.; Weisend, R.; Morton, P.L. Thorium isotopes tracing the iron cycle at the Hawaii Ocean Time-series Station ALOHA. Geochim. Cosmochim. Acta 2015, 169, 1–16. [Google Scholar] [CrossRef]
- Kadko, D.; Landing, W.M.; Shelley, R.U. A novel tracer technique to quantify the atmospheric flux of trace elements to remote ocean regions. J. Geophys. Res. Oceans 2015, 120, 848–858. [Google Scholar] [CrossRef]
- Kadko, D.; Prospero, J. Deposition of 7 Be to Bermuda and the regional ocean: Environmental factors affecting estimates of atmospheric flux to the ocean. J. Geophys. Res. 2011, 116, C02013. [Google Scholar] [CrossRef]
- Shelley, R.U.; Castrillejo, M.; Sanial, V.; Masqué, P.; Landing, W.M.; Van Beek, P.; Planquette, H.; Sarthou, G. Quantification of trace element atmospheric deposition fluxes to the Atlantic Ocean (>40 °N; GEOVIDE, GEOTRACES GA01) during spring 2014. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2017, 119, 34–49. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, N.; Huang, J.; Xu, X.; Zhang, H.; Zang, Z.; Huang, K.; Xu, X.; Wei, Y.; Guan, X.; et al. Quantifying contributions of natural and anthropogenic dust emission from different climatic regions. Atmos. Environ. 2018, 191, 94–104. [Google Scholar] [CrossRef]
- Guieu, C.; Loÿe-Pilot, M.D.; Benyahya, L.; Dufour, A. Spatial variability of atmospheric fluxes of metals (Al, Fe, Cd, Zn and Pb) and phosphorus over the whole Mediterranean from a one-year monitoring experiment: Biogeochemical implications. Mar. Chem. 2010, 120, 164–178. [Google Scholar] [CrossRef]
- Pulido-Villena, E.; Baudoux, A.-C.; Obernosterer, I.; Landa, M.; Caparros, J.; Catala, P.; Georges, C.; Harmand, J.; Guieu, C. Microbial food web dynamics in response to a Saharan dust event: Results from a mesocosm study in the oligotrophic Mediterranean Sea. Biogeosciences 2014, 11, 5607–5619. [Google Scholar] [CrossRef]
- Ridame, C.; Dekaezemacker, J.; Guieu, C.; Bonnet, S.; L’Helguen, S.; Malien, F. Contrasted Saharan dust events in LNLC environments: Impact on nutrient dynamics and primary production. Biogeosciences 2014, 11, 4783–4800. [Google Scholar] [CrossRef]
- Wuttig, K.; Wagener, T.; Bressac, M.; Dammshäuser, A.; Streu, P.; Guieu, C.; Croot, P.L. Impacts of dust deposition on dissolved trace metal concentrations (Mn, Al and Fe) during a mesocosm experiment. Biogeosciences 2013, 10, 2583–2600. [Google Scholar] [CrossRef]
- Middleton, N.J.; Goudie, A.S. Saharan dust: Sources and trajectories. Trans. Inst. Br. Geogr. 2001, 26, 165–181. [Google Scholar] [CrossRef]
- Jickells, T.D.; An, Z.S.; Andersen, K.K.; Baker, A.R.; Bergametti, C.; Brooks, N.; Cao, J.J.; Boyd, P.W.; Duce, R.A.; Hunter, K.A.; et al. Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 2005, 308, 67–71. [Google Scholar] [CrossRef]
- Guerzoni, S.; Chester, R.; Dulac, F.; Herut, B.; Loÿe-Pilot, M.D.; Measures, C.; Migon, C.; Molinaroli, E.; Moulin, C.; Rossini, P.; et al. The role of atmospheric deposition in the biogeochemistry of the Mediterranean Sea. In Proceedings of the Progress in Oceanography; Pergamon: Oxford, UK, 1999; Volume 44, pp. 147–190. [Google Scholar]
- Abouchami, W.; Näthe, K.; Kumar, A.; Galer, S.J.G.; Jochum, K.P.; Williams, E.; Horbe, A.M.C.; Rosa, J.W.C.; Balsam, W.; Adams, D.; et al. Geochemical and isotopic characterization of the bodélé depression dust source and implications for transatlantic dust transport to the Amazon basin. Earth Planet. Sci. Lett. 2013, 380, 112–123. [Google Scholar] [CrossRef]
- Schuerger, A.C.; Smith, D.J.; Griffin, D.W.; Jaffe, D.A.; Wawrik, B.; Burrows, S.M.; Christner, B.C.; Gonzalez-Martin, C.; Lipp, E.K.; Schmale, D.G.; et al. Science questions and knowledge gaps to study microbial transport and survival in Asian and African dust plumes reaching North America. Aerobiologia 2018, 34, 425–435. [Google Scholar] [CrossRef]
- Hunter, H.; Cervone, G. Analysing the influence of African dust storms on the prevalence of coral disease in the Caribbean Sea using remote sensing and association rule data mining. Int. J. Remote Sens. 2017, 38, 1494–1521. [Google Scholar] [CrossRef]
- Chuvochina, M.S.; Marie, D.; Chevaillier, S.; Petit, J.-R.; Normand, P.; Alekhina, I.A.; Bulat, S.A. Community Variability of Bacteria in Alpine Snow (Mont Blanc) Containing Saharan Dust Deposition and Their Snow Colonisation Potential. Microbes Environ. 2011, 26, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gu, J.; Wang, X. The impact of Sahara dust on air quality and public health in European countries. Atmos. Environ. 2020, 241, 117771. [Google Scholar] [CrossRef]
- Franchy, G.; Ojeda, A.; López-Cancio, J.; Hernández-León, S. Plankton community response to Saharan dust fertilization in subtropical waters off the Canary Islands. Biogeosciences Discuss. 2013, 10, 17275–17307. [Google Scholar] [CrossRef]
- Kandler, K.; Benker, N.; Bundke, U.; Cuevas, E.; Ebert, M.; Knippertz, P.; Rodríguez, S.; Schütz, L.; Weinbruch, S. Chemical composition and complex refractive index of Saharan Mineral Dust at Izaña, Tenerife (Spain) derived by electron microscopy. Atmos. Environ. 2007, 41, 8058–8074. [Google Scholar] [CrossRef]
- Kandler, K.; Schütz, L.; Jäckel, S.; Lieke, K.; Emmel, C.; Müller-Ebert, D.; Ebert, M.; Scheuvens, D.; Schladitz, A.; Šegvić, B.; et al. Ground-based off-line aerosol measurements at Praia, Cape Verde, during the Saharan Mineral Dust Experiment: Microphysical properties and mineralogy. Tellus Ser. B Chem. Phys. Meteorol. 2011, 63, 459–474. [Google Scholar] [CrossRef]
- Lieke, K.; Kandler, K.; Scheuvens, D.; Emmel, C.; Von Glahn, C.; Petzold, A.; Weinzierl, B.; Veira, A.; Ebert, M.; Weinbruch, S.; et al. Particle chemical properties in the vertical column based on aircraft observations in the vicinity of Cape Verde Islands. Tellus Ser. B Chem. Phys. Meteorol. 2011, 63, 497–511. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; di Lorenzo, F.; Elert, K. Mineralogy and physicochemical features of Saharan dust wet deposited in the Iberian Peninsula during an extreme red rain event. Atmos. Chem. Phys. 2018, 18, 10089–10122. [Google Scholar] [CrossRef]
- Martin, J.H.; Gordon, R.M.; Fitzwater, S.E. The case for iron. Limnol. Oceanogr. 1991, 36, 1793–1802. [Google Scholar] [CrossRef]
- Bressac, M.; Guieu, C. Post-depositional processes: What really happens to new atmospheric iron in the ocean’s surface? Glob. Biogeochem. Cycles 2013, 27, 859–870. [Google Scholar] [CrossRef]
- Guieu, C.; Dulac, F.; Ridame, C.; Pondaven, P. Introduction to project DUNE, a DUst experiment in a low Nutrient, low chlorophyll Ecosystem. Biogeosciences 2014, 11, 425–442. [Google Scholar] [CrossRef]
- Wagener, T.; Guieu, C.; Leblond, N. Effects of dust deposition on iron cycle in the surface Mediterranean Sea: Results from a mesocosm seeding experiment. Biogeosciences 2010, 7, 3769–3781. [Google Scholar] [CrossRef]
- Gross, A.; Goren, T.; Pio, C.; Cardoso, J.; Tirosh, O.; Todd, M.C.; Rosenfeld, D.; Weiner, T.; Custódio, D.; Angert, A. Variability in sources and concentrations of saharan dust phosphorus over the Atlantic Ocean. Environ. Sci. Technol. Lett. 2015, 2, 31–37. [Google Scholar] [CrossRef]
- Louis, J.; Bressac, M.; Pedrotti, M.L.; Guieu, C. Dissolved inorganic nitrogen and phosphorus dynamics in seawater following an artificial Saharan dust deposition event. Front. Mar. Sci. 2015, 2, 27. [Google Scholar] [CrossRef]
- van der Does, M.; Knippertz, P.; Zschenderlein, P.; Giles Harrison, R.; Stuut, J.B.W. The mysterious long-range transport of giant mineral dust particles. Sci. Adv. 2018, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Ishikawa, A.; Mastunaga, T.; Pointing, S.B.; Saito, Y.; Kasai, T.; Watanabe, K.; Aoki, K.; Horiuchi, A.; Lee, K.C.; et al. Atmospheric aerosol deposition influences marine microbial communities in oligotrophic surface waters of the western Pacific Ocean. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2016, 118, 37–45. [Google Scholar] [CrossRef]
- Baars, H.; Ansmann, A.; Althausen, D.; Engelmann, R.; Artaxo, P.; Pauliquevis, T.; Souza, R. Further evidence for significant smoke transport from Africa to Amazonia. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Amiri-Farahani, A.; Allen, J.R.; Neubauer, D.; Lohmann, U. Impact of Saharan dust on North Atlantic marine stratocumulus clouds: Importance of the semidirect effect. Atmos. Chem. Phys. 2017, 17, 6305–6322. [Google Scholar] [CrossRef]
- Karydis, V.A.; Tsimpidi, A.P.; Bacer, S.; Pozzer, A.; Nenes, A.; Lelieveld, J. Global impact of mineral dust on cloud droplet number concentration. Atmos. Chem. Phys. 2017, 17, 5601–5621. [Google Scholar] [CrossRef]
- Luo, C.; Wang, W.; Sheng, L.; Zhou, Y.; Hu, Z.; Qu, W.; Li, X.; Hai, S. Influence of polluted dust on chlorophyll-a concentration and particulate organic carbon in the subarctic North Pacific Ocean based on satellite observation and the WRF-Chem simulation. Atmos. Res. 2020, 236, 104812. [Google Scholar] [CrossRef]
- Bressac, M.; Guieu, C.; Doxaran, D.; Bourrin, F.; Obolensky, G.; Grisoni, J.-M. A mesocosm experiment coupled with optical measurements to assess the fate and sinking of atmospheric particles in clear oligotrophic waters. Geo. Mar. Lett. 2012, 32, 153–164. [Google Scholar] [CrossRef]
- Desboeufs, K.; Leblond, N.; Wagener, T.; Bon Nguyen, E.; Guieu, C. Chemical fate and settling of mineral dust in surface seawater after atmospheric deposition observed from dust seeding experiments in large mesocosms. Biogeosciences 2014, 11, 5581–5594. [Google Scholar] [CrossRef]
- Bressac, M.; Guieu, C.; Doxaran, D.; Bourrin, F.; Desboeufs, K.; Leblond, N.; Ridame, C. Quantification of the lithogenic carbon pump following a simulated dust-deposition event in large mesocosms. Biogeosciences 2014, 11, 1007–1020. [Google Scholar] [CrossRef]
- Prospero, J.M.; Landing, W.M.; Schulz, M. African dust deposition to Florida: Temporal and spatial variability and comparisons to models. J. Geophys. Res. Atmos. 2010, 115. [Google Scholar] [CrossRef]
- Prospero, J.M.; Mayol-Bracero, O.L. Understanding the transport and impact of African dust on the Caribbean Basin. Bull. Am. Meteorol. Soc. 2013, 94, 1329–1337. [Google Scholar] [CrossRef]
- Philip, S.; Martin, R.V.; Snider, G.; Weagle, C.L.; Van Donkelaar, A.; Brauer, M.; Henze, D.K.; Klimont, Z.; Venkataraman, C.; Guttikunda, S.K.; et al. Anthropogenic fugitive, combustion and industrial dust is a significant, underrepresented fine particulate matter source in global atmospheric models. Environ. Res. Lett. 2017, 12, 044018. [Google Scholar] [CrossRef]
- Fu, J.; Wang, B.; Chen, Y.; Ma, Q. The influence of continental air masses on the aerosols and nutrients deposition over the western North Pacific. Atmos. Environ. 2018, 172, 1–11. [Google Scholar] [CrossRef]
- Kim, I.N.; Lee, K.; Gruber, N.; Karl, D.M.; Bullister, J.L.; Yang, S.; Kim, T.W. Increasing anthropogenic nitrogen in the North Pacific Ocean. Science 2014, 346, 1102–1106. [Google Scholar] [CrossRef]
- Jickells, T.D.; Buitenhuis, E.; Altieri, K.; Baker, A.R.; Capone, D.; Duce, R.A.; Dentener, F.; Fennel, K.; Kanakidou, M.; LaRoche, J.; et al. A reevaluation of the magnitude and impacts of anthropogenic atmospheric nitrogen inputs on the ocean. Glob. Biogeochem. Cycles 2017, 31, 289–305. [Google Scholar] [CrossRef]
- Penuelas, J.; Janssens, I.A.; Ciais, P.; Obersteiner, M.; Sardans, J. Anthropogenic global shifts in biospheric N and P concentrations and ratios and their impacts on biodiversity, ecosystem productivity, food security, and human health. Glob. Chang. Biol. 2020, 26, 1962–1985. [Google Scholar] [CrossRef] [PubMed]
- Duce, R.A.; LaRoche, J.; Altieri, K.; Arrigo, K.R.; Baker, A.R.; Capone, D.G.; Cornell, S.; Dentener, F.; Galloway, J.; Ganeshram, R.S.; et al. Impacts of atmospheric anthropogenic nitrogen on the open ocean. Science 2008, 320, 893–897. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Lin, Q.; Yuan, Q.; Liu, L.; Zhang, J.; Zhang, Y.; Shao, L.; Niu, H.; Yang, S.; et al. Iron solubility in fine particles associated with secondary acidic aerosols in east China. Environ. Pollut. 2020, 264, 114769. [Google Scholar] [CrossRef] [PubMed]
- López-García, P.; Gelado-Caballero, M.D.; Patey, M.D.; Hernández-Brito, J.J. Atmospheric fluxes of soluble nutrients and Fe: More than three years of wet and dry deposition measurements at Gran Canaria (Canary Islands). Atmos. Environ. 2021, 246, 118090. [Google Scholar] [CrossRef]
- Marcotte, A.R.; Anbar, A.D.; Majestic, B.J.; Herckes, P. Mineral Dust and Iron Solubility: Effects of Composition, Particle Size, and Surface Area. Atmosphere 2020, 11, 533. [Google Scholar] [CrossRef]
- Conway, T.M.; Hamilton, D.S.; Shelley, R.U.; Aguilar-Islas, A.M.; Landing, W.M.; Mahowald, N.M.; John, S.G. Tracing and constraining anthropogenic aerosol iron fluxes to the North Atlantic Ocean using iron isotopes. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-González, P.; Hawco, N.J.; Bundy, R.M.; Virginia Armbrust, E.; Follows, M.J.; Cael, B.B.; White, A.E.; Ferrón, S.; Karl, D.M.; John, S.G. Anthropogenic Asian aerosols provide Fe to the North Pacific Ocean. Proc. Natl. Acad. Sci. USA 2020, 117, 27862–27868. [Google Scholar] [CrossRef]
- Mahowald, N.; Jickells, T.D.; Baker, A.R.; Artaxo, P.; Benitez-Nelson, C.R.; Bergametti, G.; Bond, T.C.; Chen, Y.; Cohen, D.D.; Herut, B.; et al. Global distribution of atmospheric phosphorus sources, concentrations and deposition rates, and anthropogenic impacts. Glob. Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef]
- Zhao, H.; Yuan, M.; Strokal, M.; Wu, H.C.; Liu, X.; Murk, A.; Kroeze, C.; Osinga, R. Impacts of nitrogen pollution on corals in the context of global climate change and potential strategies to conserve coral reefs. Sci. Total Environ. 2021, 774, 145017. [Google Scholar] [CrossRef]
- Yu, L.; Ma, X.; Gao, H.; Zong, H.; Yao, X.; Lin, Z.; Zhang, Z.; Zhang, C.; Yao, X.; Zhang, Z. Distribution and source identification of nitrogen and phosphorus in aerosols in the Qinhuangdao coast, north China. Atmos. Environ. 2020, 234, 117475. [Google Scholar] [CrossRef]
- Yoshizue, M.; Iwamoto, Y.; Adachi, K.; Kato, S.; Sun, S.; Miura, K.; Uematsu, M. Individual particle analysis of marine aerosols collected during the North–South transect cruise in the Pacific Ocean and its marginal seas. J. Oceanogr. 2019, 75, 513–524. [Google Scholar] [CrossRef]
- Witkowska, A.; Lewandowska, A.; Falkowska, L.M. Parallel measurements of organic and elemental carbon dry (PM1, PM2.5) and wet (rain, snow, mixed) deposition into the Baltic Sea. Mar. Pollut. Bull. 2016, 104, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Decesari, S.; Facchini, M.C.; Matta, E.; Mircea, M.; Fuzzi, S.; Chughtai, A.R.; Smith, D.M. Water soluble organic compounds formed by oxidation of soot. Atmos. Environ. 2002, 36, 1827–1832. [Google Scholar] [CrossRef]
- Mead, R.N.; Mullaugh, K.M.; Brooks Avery, G.; Kieber, R.J.; Willey, J.D.; Podgorski, D.C. Insights into dissolved organic matter complexity in rainwater from continental and coastal storms by ultrahigh resolution Fourier transform ion cyclotron resonance mass spectrometry. Atmos. Chem. Phys. 2013, 13, 4829–4838. [Google Scholar] [CrossRef]
- Mari, X.; Guinot, B.; Van Thuoc, C.; Brune, J.; Lefebvre, J.; Ram, P.; Sriram, A.; Raimbault, P.; Dittmar, T.; Niggemann, J. Biogeochemical Impacts of a Black Carbon Wet Deposition Event in Halong Bay, Vietnam. Front. Mar. Sci. 2019, 6, 1–18. [Google Scholar] [CrossRef]
- Doherty, S.J.; Warren, S.G.; Grenfell, T.C.; Clarke, A.D.; Brandt, R.E. Light-absorbing impurities in Arctic snow. Atmos. Chem. Phys. 2010, 10, 11647–11680. [Google Scholar] [CrossRef]
- Fang, Z.; Yang, W.; Chen, M.; Zheng, M.; Hu, W. Abundance and sinking of particulate black carbon in the western Arctic and Subarctic Oceans. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stubbins, A.; Hood, E.; Raymond, P.A.; Aiken, G.R.; Sleighter, R.L.; Hernes, P.J.; Butman, D.; Hatcher, P.G.; Striegl, R.G.; Schuster, P.; et al. Anthropogenic aerosols as a source of ancient dissolved organic matter in glaciers. Nat. Geosci. 2012, 5, 198–201. [Google Scholar] [CrossRef]
- Fishwick, M.P.; Ussher, S.J.; Sedwick, P.N.; Lohan, M.C.; Worsfold, P.J.; Buck, K.N.; Church, T.M. Impact of surface ocean conditions and aerosol provenance on the dissolution of aerosol manganese, cobalt, nickel and lead in seawater. Mar. Chem. 2018, 198, 28–43. [Google Scholar] [CrossRef]
- Pinhassi, J.; Gómez-Consarnau, L.; Alonso-Sáez, L.; Sala, M.; Vidal, M.; Pedrós-Alió, C.; Gasol, J. Seasonal changes in bacterioplankton nutrient limitation and their effects on bacterial community composition in the NW Mediterranean Sea. Aquat. Microb. Ecol. 2006, 44, 241–252. [Google Scholar] [CrossRef]
- Gallisai, R.; Peters, F.; Volpe, G.; Basart, S.; Baldasano, J.M. Saharan Dust Deposition May Affect Phytoplankton Growth in the Mediterranean Sea at Ecological Time Scales. PLoS ONE 2014, 9, e110762. [Google Scholar] [CrossRef]
- Bristow, L.A.; Mohr, W.; Ahmerkamp, S.; Kuypers, M.M.M. Nutrients that limit growth in the ocean. Curr. Biol. 2017, 27, R474–R478. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.R.; Kelly, S.D.; Biswas, K.F.; Witt, M.; Jickells, T.D. Atmospheric deposition of nutrients to the Atlantic Ocean. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Mahowald, N.M.; Baker, A.R.; Bergametti, G.; Brooks, N.; Duce, R.A.; Jickells, T.D.; Kubilay, N.; Prospero, J.M.; Tegen, I. Atmospheric global dust cycle and iron inputs to the ocean. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Marty, J.C.; Chiavérini, J.; Pizay, M.D.; Avril, B. Seasonal and interannual dynamics of nutrients and phytoplankton pigments in the western Mediterranean Sea at the DYFAMED time-series station (1991–1999). Deep-Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 1965–1985. [Google Scholar] [CrossRef]
- Pulido-Villena, E.; Rérolle, V.; Guieu, C. Transient fertilizing effect of dust in P-deficient LNLC surface ocean. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef]
- Bosc, E.; Bricaud, A.; Antoine, D. Seasonal and interannual variability in algal biomass and primary production in the Mediterranean Sea, as derived from 4 years of SeaWiFS observations. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- López-Sandoval, D.C.; Fernández, A.; Marañón, E. Dissolved and particulate primary production along a longitudinal gradient in the Mediterranean Sea. Biogeosciences 2011, 8, 815–825. [Google Scholar] [CrossRef]
- Markaki, Z.; Loÿe-Pilot, M.D.; Violaki, K.; Benyahya, L.; Mihalopoulos, N. Variability of atmospheric deposition of dissolved nitrogen and phosphorus in the Mediterranean and possible link to the anomalous seawater N/P ratio. Mar. Chem. 2010, 120, 187–194. [Google Scholar] [CrossRef]
- Marín-Beltrán, I.; Logue, J.B.; Andersson, A.F.; Peters, F. Atmospheric Deposition Impact on Bacterial Community Composition in the NW Mediterranean. Front. Microbiol. 2019, 10, 858. [Google Scholar] [CrossRef] [PubMed]
- Echeveste, P.; Croot, P.; von Dassow, P. Differences in the sensitivity to Cu and ligand production of coastal vs offshore strains of Emiliania huxleyi. Sci. Total Environ. 2018, 625, 1673–1680. [Google Scholar] [CrossRef]
- Yang, T.; Chen, Y.; Zhou, S.; Li, H. Impacts of aerosol copper on marine phytoplankton: A review. Atmosphere 2019, 10, 1. [Google Scholar] [CrossRef]
- Chien, C.-T.; Mackey, K.R.M.; Dutkiewicz, S.; Mahowald, N.M.; Prospero, J.M.; Paytan, A. Effects of African dust deposition on phytoplankton in the western tropical Atlantic Ocean off Barbados. Glob. Biogeochem. Cycles 2016, 30, 716–734. [Google Scholar] [CrossRef]
- Park, G.H.; Lee, S.E.; Kim, Y.-I.; Kim, D.; Lee, K.; Kang, J.; Kim, Y.H.; Kim, H.; Park, S.; Kim, T.W. Atmospheric deposition of anthropogenic inorganic nitrogen in airborne particles and precipitation in the East Sea in the northwestern Pacific Ocean. Sci. Total Environ. 2019, 681, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Dachs, J.; Llabrés, M.; Alonso-Laita, P.; Gasol, J.M.; Tovar-Sánchez, A.; Sañudo-Wilhemy, S.; Agustí, S. Aerosol inputs enhance new production in the subtropical northeast Atlantic. J. Geophys. Res. Biogeosciences 2006, 111. [Google Scholar] [CrossRef]
- Liao, W.-H.; Yang, S.-C.; Ho, T.-Y. Trace metal composition of size-fractionated plankton in the Western Philippine Sea: The impact of anthropogenic aerosol deposition. Limnol. Oceanogr. 2017, 62, 2243–2259. [Google Scholar] [CrossRef]
- Liu, L.L.; Hsieh, C.Y.; Kuo, M.Y.; Chen, C.; Shau, Y.H.; Lui, H.K.; Yuan, C.S.; Chen, C.T.A. Evidence for Fossil Fuel PM1 Accumulation in Marine Biota. Environ. Sci. Technol. 2020, 54, 4068–4078. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Lu, W.; Hong, Y.; Chen, J.; Dong, S.; Huang, Q. Long-term wet precipitation of PM2.5 disturbed the gut microbiome and inhibited the growth of marine medaka Oryzias melastigma. Sci. Total Environ. 2021, 755, 142512. [Google Scholar] [CrossRef]
- Lachs, L.; Oñate-Casado, J. Fisheries and Tourism: Social, Economic, and Ecological Trade-offs in Coral Reef Systems. In YOUMARES 9—The Oceans: Our Research, Our Future; Springer: Berling/Heidelberg, Germany, 2020; pp. 243–260. [Google Scholar]
- Okin, G.S.; Baker, A.R.; Tegen, I.; Mahowald, N.M.; Dentener, F.J.; Duce, R.A.; Galloway, J.N.; Hunter, K.; Kanakidou, M.; Kubilay, N.; et al. Impacts of atmospheric nutrient deposition on marine productivity: Roles of nitrogen, phosphorus, and iron. Glob. Biogeochem. Cycles 2011, 25. [Google Scholar] [CrossRef]
- Paris, R.; Desboeufs, K.V. Effect of atmospheric organic complexation on iron-bearing dust solubility. Atmos. Chem. Phys. 2013, 13, 4895–4905. [Google Scholar] [CrossRef]
- Baker, A.R.; Croot, P.L. Atmospheric and marine controls on aerosol iron solubility in seawater. Mar. Chem. 2010, 120, 4–13. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura, A.; Simões, E.F.C.; Almeida, A.S.; Martins, R.; Duarte, A.C.; Loureiro, S.; Duarte, R.M.B.O. Deposition of Aerosols onto Upper Ocean and Their Impacts on Marine Biota. Atmosphere 2021, 12, 684. https://doi.org/10.3390/atmos12060684
Ventura A, Simões EFC, Almeida AS, Martins R, Duarte AC, Loureiro S, Duarte RMBO. Deposition of Aerosols onto Upper Ocean and Their Impacts on Marine Biota. Atmosphere. 2021; 12(6):684. https://doi.org/10.3390/atmos12060684
Chicago/Turabian StyleVentura, Andreia, Eliana F. C. Simões, Antoine S. Almeida, Roberto Martins, Armando C. Duarte, Susana Loureiro, and Regina M. B. O. Duarte. 2021. "Deposition of Aerosols onto Upper Ocean and Their Impacts on Marine Biota" Atmosphere 12, no. 6: 684. https://doi.org/10.3390/atmos12060684
APA StyleVentura, A., Simões, E. F. C., Almeida, A. S., Martins, R., Duarte, A. C., Loureiro, S., & Duarte, R. M. B. O. (2021). Deposition of Aerosols onto Upper Ocean and Their Impacts on Marine Biota. Atmosphere, 12(6), 684. https://doi.org/10.3390/atmos12060684








