Ambient Levels, Emission Sources and Health Effect of PM2.5-Bound Carbonaceous Particles and Polycyclic Aromatic Hydrocarbons in the City of Kuala Lumpur, Malaysia
Abstract
1. Introduction
2. Methodologies
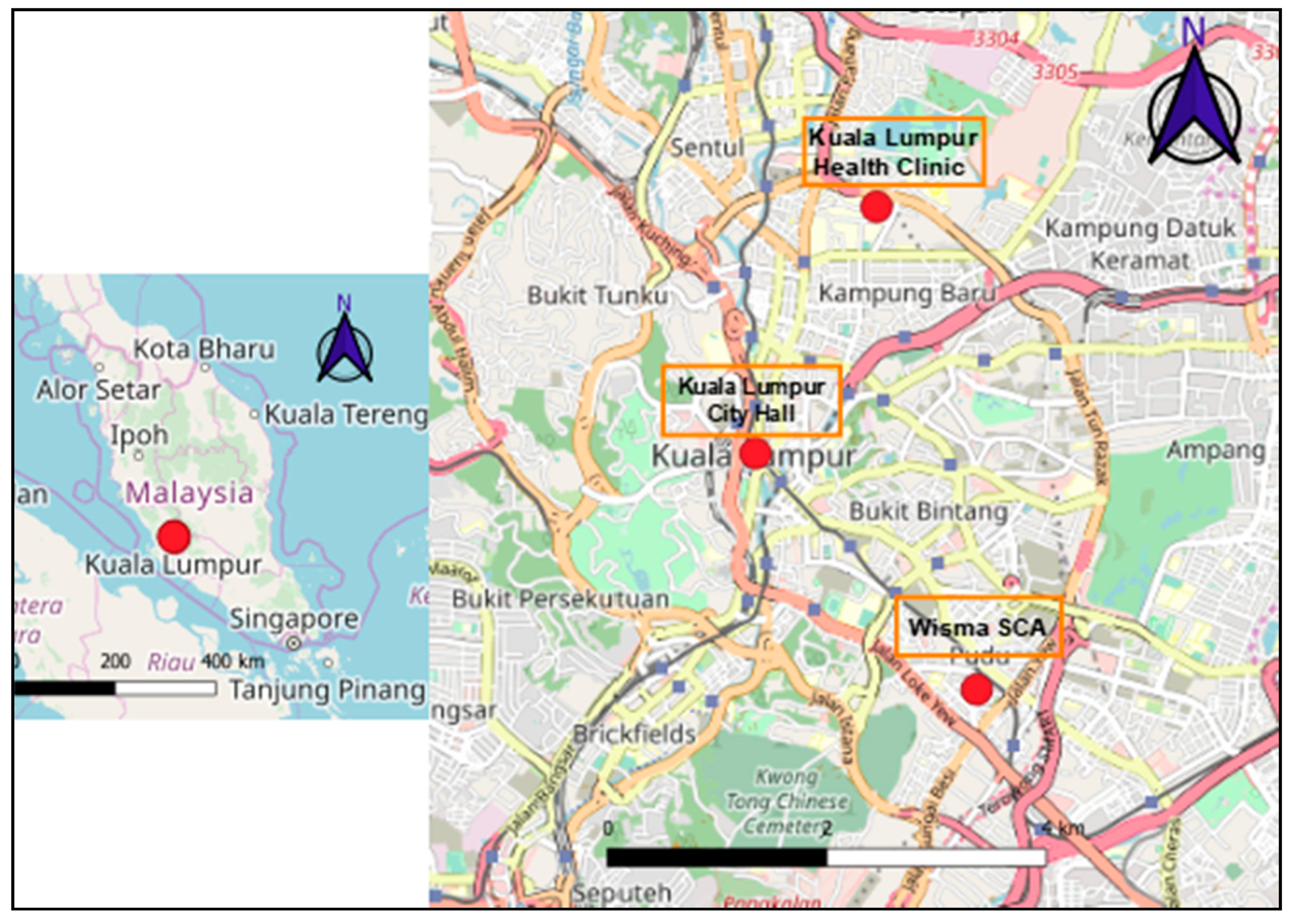
2.1. Study Areas
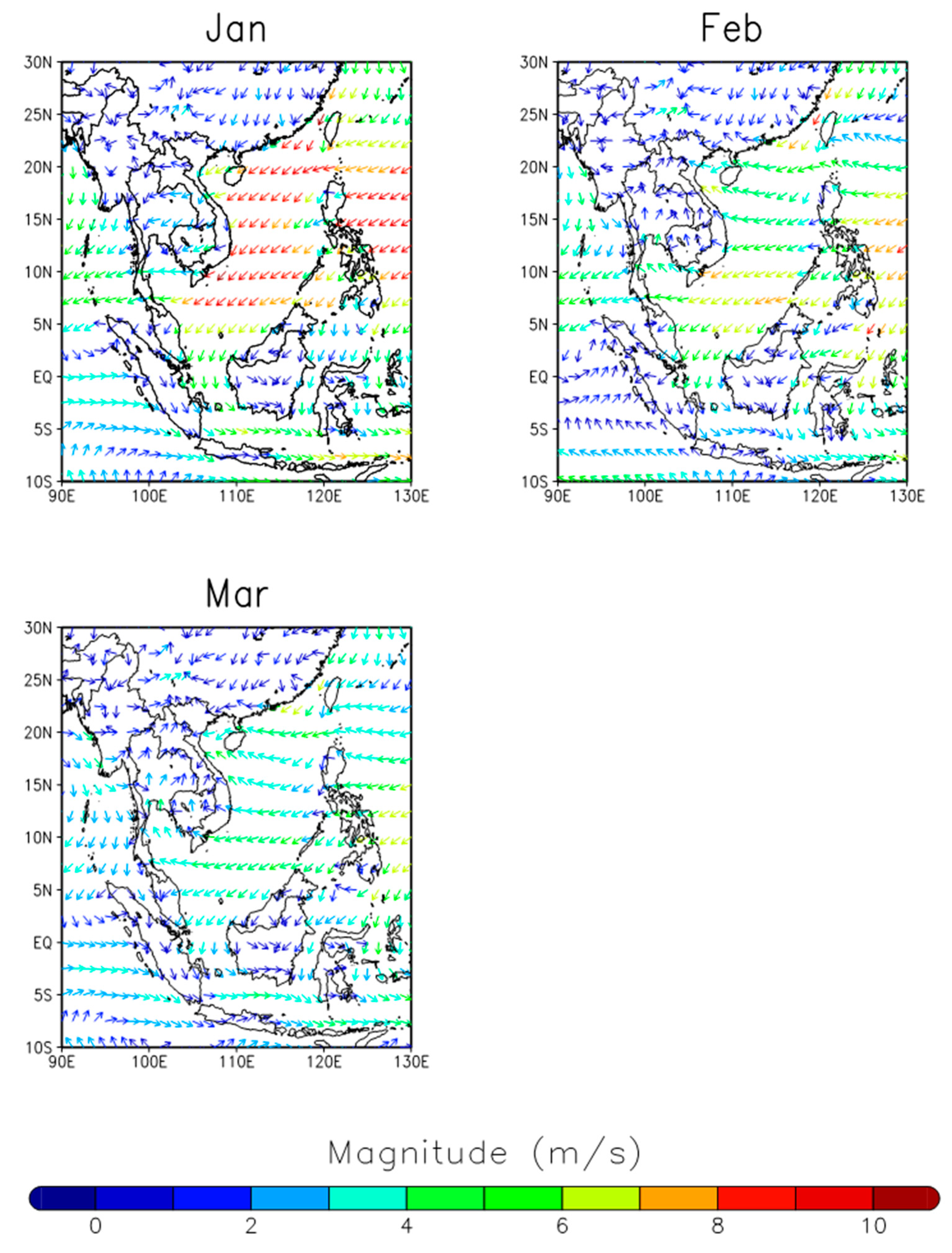
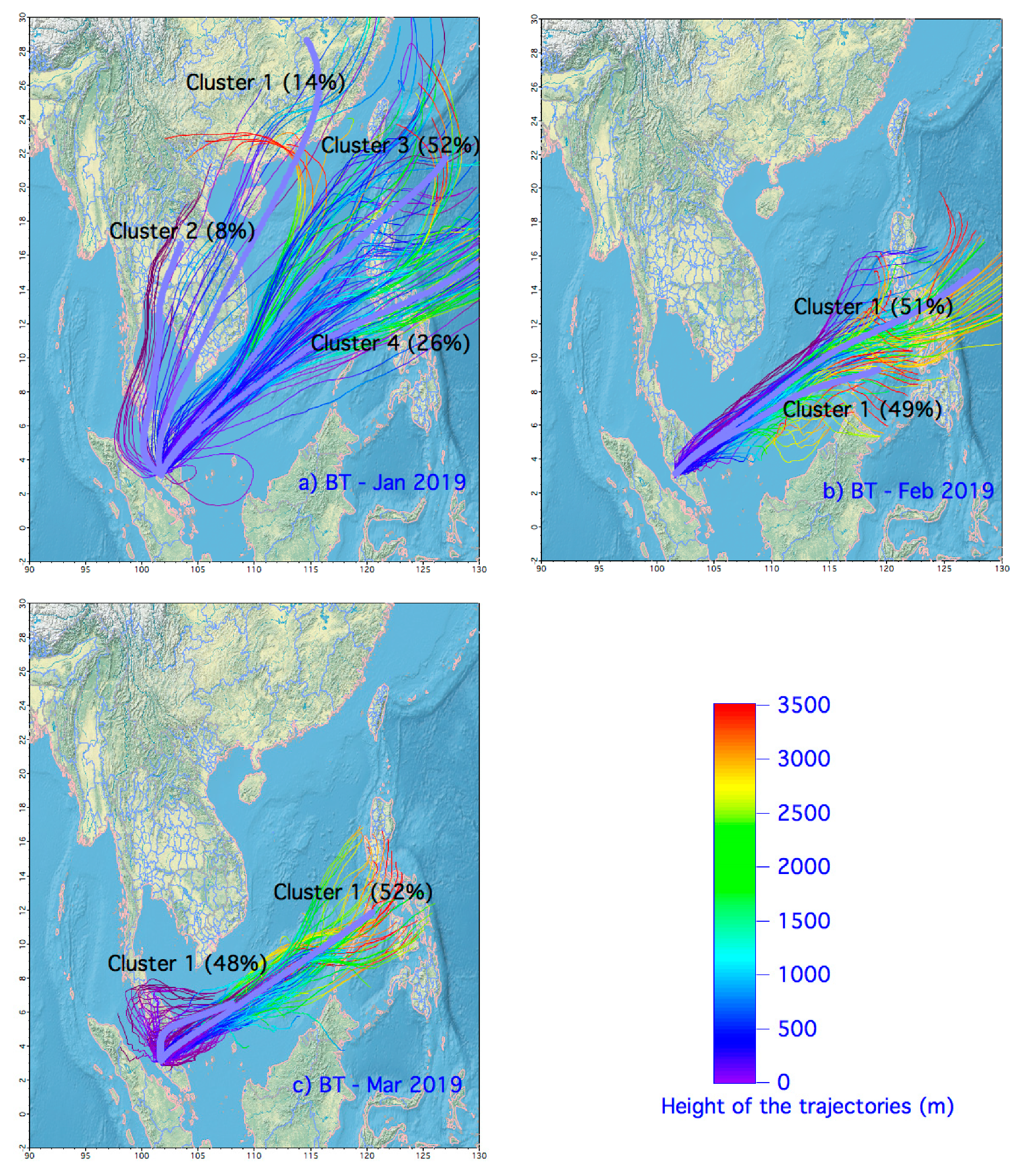
2.2. Local Meteorology and Transport of Air Mass
2.3. Air Sampling Procedures
2.4. Analysis of OC and EC and Estimation of Secondary Organic Carbon (SOC)
2.5. Analysis of Water-Soluble Organic Carbon (WSOC)
2.6. Extraction Procedures for PAHs in PM2.5 Samples
Extraction of the PAHs Composition Using Magnetic Nanoparticles
2.7. Analysis of PAHs Using Gas Chromatography-Flame Ionization Detector (GC-FID)
2.7.1. Preparation of Calibration Standard
2.7.2. Determination of PAHs Using GC-FID
2.8. Quality Assurance and Quality Control (QA/QC)
2.9. Data Analysis and Modelling
2.9.1. Statistical Analysis
2.9.2. Diagnostic Ratio (DR) and Receptor Modelling
Diagnostic Ratio (DR)
Receptor Modelling
2.9.3. US EPA Health Risk Modelling
3. Results and Discussion
3.1. OC, EC, and SOC
3.2. WSOC
3.3. Variation of PAHs
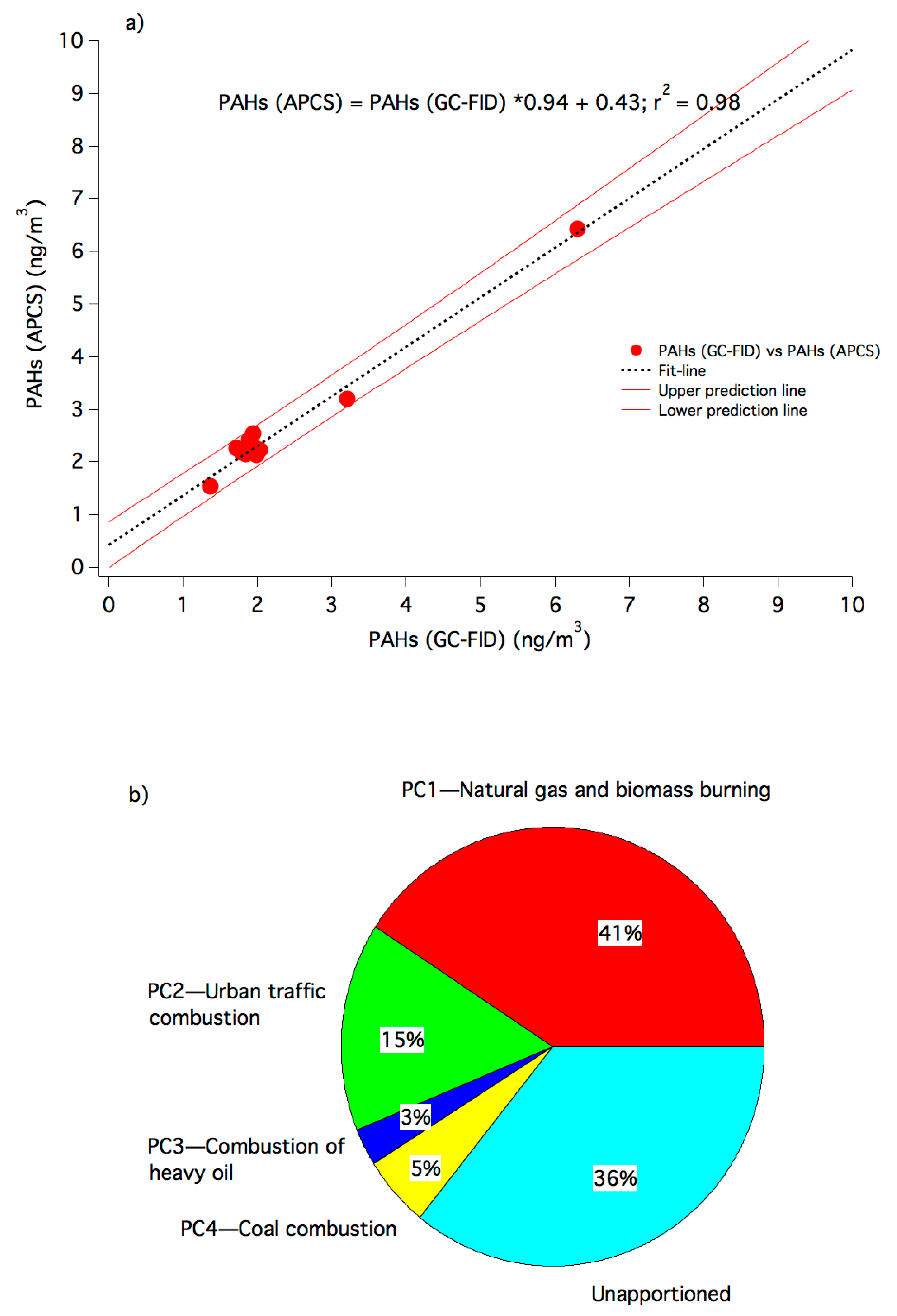
3.4. Identification of Possible Sources
3.4.1. Diagnostic Ratio (DR)
3.4.2. PCA-MLR
3.5. Human Exposure to PAH
3.5.1. Toxicity Risk
3.5.2. Carcinogenic Exposure
Lifetime Average Daily Dose
Excess Lifetime Cancer Risk (ELCR)
3.5.3. Non-Carcinogenic Exposure
Average Daily Dose (ADD) for Non-Carcinogenic Exposure
Hazard Quotient (HQ) and Hazard Index (HI)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brauer, M.; Freedman, G.; Frostad, J.; van Donkelaar, A.; Martin, R.V.; Dentener, F.; Dingenen, R.v.; Estep, K.; Amini, H.; Apte, J.S.; et al. Ambient Air Pollution Exposure Estimation for the Global Burden of Disease 2013. Environ. Sci. Technol. 2016, 50, 79–88. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- WHO. World Health Organization. Global Urban Ambient Air Pollution Database (Update 2016); World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Khan, M.F.; Latif, M.T.; Lim, C.H.; Amil, N.; Jaafar, S.A.; Dominick, D.; Nadzir, M.S.M.; Sahani, M.; Tahir, N.M. Seasonal effect and source apportionment of polycyclic aromatic hydrocarbons in PM2.5. Atmos. Environ. 2015, 106, 178–190. [Google Scholar] [CrossRef]
- Liu, X.; Schnelle-Kreis, J.; Schloter-Hai, B.; Ma, L.; Tai, P.; Cao, X.; Yu, C.; Adam, T.; Zimmermann, R. Analysis of PAHs Associated with PM10 and PM2.5 from Different Districts in Nanjing. Aerosol Air Qual. Res. 2019, 19, 2294–2307. [Google Scholar] [CrossRef]
- Omar, N.Y.M.; Mon, T.C.; Rahman, N.A.; Abas, M.R.B. Distributions and health risks of polycyclic aromatic hydrocarbons (PAHs) in atmospheric aerosols of Kuala Lumpur, Malaysia. Sci. Total Environ. 2006, 369, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Cao, J.; Li, L.; Ho, S.S.H.; Wang, Q.; Zhu, C.; Wang, L. Characteristics and source identification of polycyclic aromatic hydrocarbons and n-alkanes in PM2.5 in Xiamen. Aerosol Air Qual. Res. 2018, 18, 1673–1683. [Google Scholar] [CrossRef]
- WHO. World Health Organization. Particulate Matter, Chapter 7.3; WHO Regional Publications: Copenhagen, Denmark, 2000. [Google Scholar]
- Liu, J.; Man, R.; Ma, S.; Li, J.; Wu, Q.; Peng, J. Atmospheric levels and health risk of polycyclic aromatic hydrocarbons (PAHs) bound to PM2.5 in Guangzhou, China. Mar. Pollut. Bull. 2015, 100, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Seinfield, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Wang, J.; Ogawa, S. Effects of meteorological conditions on PM2.5 concentrations in Nagasaki, Japan. Int. J. Environ. Res. Public Health 2015, 12, 9089–9101. [Google Scholar] [CrossRef]
- Dominick, D.; Latif, M.T.; Juneng, L.; Khan, M.F.; Amil, N.; Mead, M.I.; Nadzir, M.S.M.; Moi, P.S.; Samah, A.A.; Ashfold, M.J. Characterisation of particle mass and number concentration on the east coast of the Malaysian Peninsula during the northeast monsoon. Atmos. Environ. 2015, 117, 187–199. [Google Scholar] [CrossRef]
- Rahim, H.A.; Khan, M.F.; Ibrahim, Z.F.; Shoaib, A.; Suradi, H.; Mohyeddin, N.; Samah, A.A.; Yusoff, S. Coastal meteorology on the dispersion of air particles at the Bachok GAW Station. Sci. Total Environ. 2021, 782, 146783. [Google Scholar] [CrossRef] [PubMed]
- Mohyeddin, N.; Samah, A.A.; Chenoli, S.N.; Ashfold, M.J.; Mead, M.I.; Oram, D.; Latif, M.T.; Sivaprasad, P.; Mohd Nor, M.F.F. The effects of synoptic and local meteorological condition on CO2, CH4, PM10 and PM2.5 at Bachok Marine Research Station (BMRS) in Peninsular Malaysia. Meteorol. Atmos. Phys. 2020, 132, 845–868. [Google Scholar] [CrossRef]
- Farren, N.J.; Dunmore, R.E.; Mead, M.I.; Nadzir, M.S.M.; Samah, A.A.; Phang, S.-M.; Bandy, B.J.; Sturges, W.T.; Hamilton, J.F. Chemical characterisation of water-soluble ions in atmospheric particulate matter on the east coast of Peninsular Malaysia. Atmos. Chem. Phys. 2019, 19, 1537–1553. [Google Scholar] [CrossRef]
- Khan, M.F.; Latif, M.T.; Amil, N.; Juneng, L.; Mohamad, N.; Nadzir, M.S.M.; Hoque, H.M.S. Characterization and source apportionment of particle number concentration at a semi-urban tropical environment. Environ. Sci. Pollut. Res. Int. 2015, 22, 13111–13126. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yu, J.Z. Determination of primary combustion source organic carbon-to-elemental carbon (OC/EC) ratio using ambient OC and EC measurements: Secondary OC-EC correlation minimization method. Atmos. Chem. Phys. 2016, 16, 5453–5465. [Google Scholar] [CrossRef]
- Rajput, P.; Sarin, M.; Kundu, S.S. Atmospheric particulate matter (PM2.5), EC, OC, WSOC and PAHs from NE–Himalaya: Abundances and chemical characteristics. Atmos. Pollut. Res. 2013, 4, 214–221. [Google Scholar] [CrossRef]
- Zhang, H.; Ying, Q. Secondary organic aerosol from polycyclic aromatic hydrocarbons in Southeast Texas. Atmos. Environ. 2012, 55, 279–287. [Google Scholar] [CrossRef]
- Ding, X.; Wang, X.-M.; Gao, B.; Fu, X.-X.; He, Q.-F.; Zhao, X.-Y.; Yu, J.-Z.; Zheng, M. Tracer-based estimation of secondary organic carbon in the Pearl River Delta, south China. J. Geophys. Res. 2012, 117. [Google Scholar] [CrossRef]
- Sulong, N.A.; Latif, M.T.; Sahani, M.; Khan, M.F.; Fadzil, M.F.; Tahir, N.M.; Mohamad, N.; Sakai, N.; Fujii, Y.; Othman, M. Distribution, sources and potential health risks of polycyclic aromatic hydrocarbons (PAHs) in PM2.5 collected during different monsoon seasons and haze episode in Kuala Lumpur. Chemosphere 2019, 219, 1–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, S.; Shen, H.; Ma, J. Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. Proc. Natl. Acad. Sci. USA 2009, 106, 21063–21067. [Google Scholar] [CrossRef] [PubMed]
- USEPA United States Environmental Protection Agency. Risk Assessment Guidance for Superfund: Volume 1, Human Health Evaluation Manual (Part D, Standardized Planning, Reporting, and Review of Superfund Risk Assessments); Office of Emergency and Remedial Response U.S. Environmental Protection Agency: Washington, DC, USA, 2001.
- Chiu, T.R.; Khan, M.F.; Latif, M.T.; Mohd Nadzir, M.S.; Abdul Hamid, H.H.; Yusoff, H.; Mohd Ali, M. Distribution of Polycyclic Aromatic Hydrocarbons (PAHs) in Surface Sediments of Langkawi Island, Malaysia. Sains Malays. 2018, 47, 871–882. [Google Scholar] [CrossRef]
- Harrison, R.M.; Smith, D.J.T.; Luhana, L. Source Apportionment of Atmospheric Polycyclic Aromatic Hydrocarbons Collected from an Urban Location in Birmingham, U.K. Environ. Sci. Technol. 1996, 30, 825–832. [Google Scholar] [CrossRef]
- Khpalwak, W.; Jadoon, W.A.; Abdel-dayem, S.M.; Sakugawa, H. Polycyclic aromatic hydrocarbons in urban road dust, Afghanistan: Implications for human health. Chemosphere 2019, 218, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Yang, W.; Zhu, M.; Gao, B.; Li, S.; Li, G.; Fang, H.; Zhou, H.; Zhang, H.; Wu, Z.; et al. Ambient PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) in rural Beijing: Unabated with enhanced temporary emission control during the 2014 APEC summit and largely aggravated after the start of wintertime heating. Environ. Pollut. 2018, 238, 532–542. [Google Scholar] [CrossRef]
- Peltonen, K.; Kuljukka, T. Air sampling and analysis of polycyclic aromatic hydrocarbons. J. Chromatogr. A 1995, 710, 93–108. [Google Scholar] [CrossRef]
- Gao, B.; Yu, J.-Z.; Li, S.-X.; Ding, X.; He, Q.-F.; Wang, X.-M. Roadside and rooftop measurements of polycyclic aromatic hydrocarbons in PM2.5 in urban Guangzhou: Evaluation of vehicular and regional combustion source contributions. Atmos. Environ. 2011, 45, 7184–7191. [Google Scholar] [CrossRef]
- Pongpiachan, S.; Tipmanee, D.; Khumsup, C.; Kittikoon, I.; Hirunyatrakul, P. Assessing risks to adults and preschool children posed by PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) during a biomass burning episode in Northern Thailand. Sci. Total Environ. 2015, 508, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Geng, N.B.; Xu, Y.F.; Zhang, W.D.; Tang, X.Y.; Zhang, R.Q. PAHs in PM2.5 in Zhengzhou: Concentration, carcinogenic risk analysis, and source apportionment. Environ. Monit. Assess. 2014, 186, 7461–7473. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Huang, Q.-h.; Li, W.-y.; Tang, Y.-j.; Zhao, J.-f. Source apportionment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Huangpu River, Shanghai, China. Sci. Total Environ. 2009, 407, 2931–2938. [Google Scholar] [CrossRef]
- Goel, A.; Ola, D.; Veetil, A.V. Burden of disease for workers attributable to exposure through inhalation of PPAHs in RSPM from cooking fumes. Environ. Sci. Pollut. Res. 2019, 26, 8885–8894. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, H.; Zhang, L.; Cheng, M.; Guo, L.; He, Q.; Wang, X.; Wang, Y. High cancer risk from inhalation exposure to PAHs in Fenhe Plain in winter: A particulate size distribution-based study. Atmos. Environ. 2019, 216, 116924. [Google Scholar] [CrossRef]
- Sarkar, S.; Khillare, P. Profile of PAHs in the inhalable particulate fraction: Source apportionment and associated health risks in a tropical megacity. Environ. Monit. Assess. 2013, 185, 1199–1213. [Google Scholar] [CrossRef]
- Hong, W.-J.; Jia, H.; Ma, W.-L.; Sinha, R.K.; Moon, H.-B.; Nakata, H.; Minh, N.H.; Chi, K.H.; Li, W.-L.; Kannan, K. Distribution, fate, inhalation exposure and lung cancer risk of atmospheric polycyclic aromatic hydrocarbons in some Asian countries. Environ. Sci. Technol. 2016, 50, 7163–7174. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Latif, M.T.; Saw, W.H.; Amil, N.; Nadzir, M.S.M.; Sahani, M.; Tahir, N.M.; Chung, J.X. Fine particulate matter in the tropical environment: Monsoonal effects, source apportionment, and health risk assessment. Atmos. Chem. Phys. 2016, 16, 597–617. [Google Scholar] [CrossRef]
- Hopke, P.K. Review of receptor modeling methods for source apportionment. J. Air Waste Manag. Assoc. 2016, 66, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Jamhari, A.A.; Sahani, M.; Latif, M.T.; Chan, K.M.; Tan, H.S.; Khan, M.F.; Tahir, N.M. Concentration and source identification of polycyclic aromatic hydrocarbons (PAHs) in PM10 of urban, industrial and semi-urban areas in Malaysia. Atmos. Environ. 2014, 86, 16–27. [Google Scholar] [CrossRef]
- Thurston, G.D.; Spengler, J.D. A quantitative assessment of source contributions to inhalable particulate matter pollution in metropolitan Boston. Atmos. Environ. 1985, 19, 9–25. [Google Scholar] [CrossRef]
- Alias, N.F.; Khan, M.F.; Sairi, N.A.; Zain, S.M.; Suradi, H.; Rahim, H.A.; Banerjee, T.; Bari, M.A.; Othman, M.; Latif, M.T. Characteristics, Emission Sources, and Risk Factors of Heavy Metals in PM2.5 from Southern Malaysia. ACS Earth Space Chem. 2020, 4, 1309–1323. [Google Scholar] [CrossRef]
- Worldometers. Malaysia Population. Available online: https://www.worldometers.info/world-population/malaysia-population/ (accessed on 31 December 2019).
- ChooChuay, C.; Pongpiachan, S.; Tipmanee, D.; Suttinun, O.; Deelaman, W.; Wang, Q.; Xing, L.; Li, G.; Han, Y.; Palakun, J. Impacts of PM2.5 sources on variations in particulate chemical compounds in ambient air of Bangkok, Thailand. Atmos. Pollut. Res. 2020, 11, 1657–1667. [Google Scholar] [CrossRef]
- Khan, M.F.; Maulud, K.N.A.; Latif, M.T.; Chung, J.X.; Amil, N.; Alias, A.; Nadzir, M.S.M.; Sahani, M.; Mohammad, M.; Jahaya, M.F.; et al. Physicochemical factors and their potential sources inferred from long-term rainfall measurements at an urban and a remote rural site in tropical areas. Sci. Total Environ. 2018, 613–614, 1401–1416. [Google Scholar] [CrossRef]
- De La Torre-Roche, R.J.; Lee, W.Y.; Campos-Díaz, S.I. Soil-borne polycyclic aromatic hydrocarbons in El Paso, Texas: Analysis of a potential problem in the United States/Mexico border region. J. Hazard. Mater. 2009, 163, 946–958. [Google Scholar] [CrossRef]
- Fujii, Y.; Iriana, W.; Oda, M.; Puriwigati, A.; Tohno, S.; Lestari, P.; Mizohata, A.; Huboyo, H.S. Characteristics of carbonaceous aerosols emitted from peatland fire in Riau, Sumatra, Indonesia. Atmos. Environ. 2014, 87, 164–169. [Google Scholar] [CrossRef]
- Khan, M.F.; Sulong, N.A.; Latif, M.T.; Nadzir, M.S.M.; Amil, N.; Hussain, D.F.M.; Lee, V.; Hosaini, P.N.; Shaharom, S.; Yusoff, N.A.Y.M. Comprehensive assessment of PM2.5 physicochemical properties during the Southeast Asia dry season (southwest monsoon). J. Geophys. Res. Atmos. 2016, 121, 14589–14611. [Google Scholar] [CrossRef]
- Turpin, B.J.; Huntzicker, J.J. Secondary formation of organic aerosol in the Los Angeles basin: A descriptive analysis of organic and elemental carbon concentrations. Atmos. Environ. 1991, 25, 207–215. [Google Scholar] [CrossRef]
- Turpin, B.J.; Lim, H.-J. Species Contributions to PM2.5 Mass Concentrations: Revisiting Common Assumptions for Estimating Organic Mass. Aerosol Sci. Technol. 2001, 35, 602–610. [Google Scholar] [CrossRef]
- Khan, M.F.; Shirasuna, Y.; Hirano, K.; Masunaga, S. Characterization of PM2.5, PM2.5–10 and PM>10 in ambient air, Yokohama, Japan. Atmos. Res. 2010, 96, 159–172. [Google Scholar] [CrossRef]
- Thuy, N.T.T.; Dung, N.T.; Sekiguchi, K.; Yamaguchi, R.; Thuy, L.B.; Hien, N.T.T. Levels and water soluble organic carbon of atmospheric nanoparticles in a location of Ha Noi, Vietnam. Vietnam J. Sci. Technol. 2017, 55, 745–755. [Google Scholar] [CrossRef][Green Version]
- Tay, K.S.; Rahman, N.A.; Abas, M.R.B. Magnetic nanoparticle assisted dispersive liquid–liquid microextraction for the determination of 4-n-nonylphenol in water. Anal. Methods 2013, 5, 2933–2938. [Google Scholar] [CrossRef]
- Lasarte-Aragonés, G.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Effervescence assisted dispersive liquid–liquid microextraction with extractant removal by magnetic nanoparticles. Anal. Chim. Acta 2014, 807, 61–66. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Jiang, Q.; Zhao, W.; Yu, A.; Chang, H.; Lu, X.; Xie, F.; Ye, B.; Zhang, S. Tetraazacalix[2]arence[2]triazine Coated Fe3O4/SiO2 Magnetic Nanoparticles for Simultaneous Dispersive Solid Phase Extraction and Determination of Trace Multitarget Analytes. Anal. Chem. 2016, 88, 10523–10532. [Google Scholar] [CrossRef]
- Shahriman, M.S.; Ramachandran, M.R.; Zain, N.N.M.; Mohamad, S.; Manan, N.S.A.; Yaman, S.M. Polyaniline-dicationic ionic liquid coated with magnetic nanoparticles composite for magnetic solid phase extraction of polycyclic aromatic hydrocarbons in environmental samples. Talanta 2018, 178, 211–221. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Meng, W.; Wang, P.; Wu, F.; Tang, Z.; Han, X.; Giesy, J.P. Cetyltrimethylammonium bromide-coated Fe3O4 magnetic nanoparticles for analysis of 15 trace polycyclic aromatic hydrocarbons in aquatic environments by ultraperformance, liquid chromatography with fluorescence detection. Anal. Chem. 2015, 87, 7667–7675. [Google Scholar] [CrossRef]
- Brändli, R.C.; Bucheli, T.D.; Ammann, S.; Desaules, A.; Keller, A.; Blum, F.; Stahel, W.A. Critical evaluation of PAH source apportionment tools using data from the Swiss soil monitoring network. J. Environ. Monit. 2008, 10, 1278–1286. [Google Scholar] [CrossRef]
- Manoli, E.; Kouras, A.; Samara, C. Profile analysis of ambient and source emitted particle-bound polycyclic aromatic hydrocarbons from three sites in northern Greece. Chemosphere 2004, 56, 867–878. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Akyüz, M.; Çabuk, H. Gas-particle partitioning and seasonal variation of polycyclic aromatic hydrocarbons in the atmosphere of Zonguldak, Turkey. Sci. Total Environ. 2010, 408, 5550–5558. [Google Scholar] [CrossRef] [PubMed]
- USEPA United States Environmental Protection Agency. Risk Assessment Guidance for Superfund Volume 1, Human Health Evaluation Manual (Part A) Interim Final; Office of Emergency and Remedial Response U.S. Environmental Protection Agency: Washington, DC, USA, 1989.
- USEPA United States Environmental Protection Agency. Risk Assessment Guidance for Superfund (RAGS), Volume 1, Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment); Office of Emergency and Remedial Response U.S. Environmental Protection Agency: Washington, DC, USA, 2009.
- Fujii, Y.; Mahmud, M.; Tohno, S.; Okuda, T.; Mizohata, A. A Case Study of PM2.5 Characterization in Bangi, Selangor, Malaysia during the Southwest Monsoon Season. Aerosol Air Qual. Res. 2016, 16, 2685–2691. [Google Scholar] [CrossRef]
- Fujii, Y.; Tohno, S.; Amil, N.; Latif, M.T.; Oda, M.; Matsumoto, J.; Mizohata, A. Annual variations of carbonaceous PM2.5 in Malaysia: Influence by Indonesian peatland fires. Atmos. Chem. Phys. 2015, 15, 13319–13329. [Google Scholar] [CrossRef]
- Suradi, H.; Khan, M.F.; Alias, N.F.; Mustapa Kama Shah, S.; Yusoff, S.; Fujii, Y.; Othman, M.; Latif, M.T. Influence of Tropical Weather and Northeasterly Air Mass on Carbonaceous Aerosol in the Southern Malay Peninsula. ACS Earth Space Chem. 2021, 5, 553–565. [Google Scholar] [CrossRef]
- Pani, S.K.; Chantara, S.; Khamkaew, C.; Lee, C.T.; Lin, N.H. Biomass burning in the northern peninsular Southeast Asia: Aerosol chemical profile and potential exposure. Atmos. Res. 2019, 224, 180–195. [Google Scholar] [CrossRef]
- Han, Y.; Cao, J.; Chow, J.C.; Watson, J.G.; An, Z.; Jin, Z.; Fung, K.; Liu, S. Evaluation of the thermal/optical reflectance method for discrimination between char- and soot-EC. Chemosphere 2007, 69, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Le, P.V.V.; Phan-Van, T.; Mai, K.V.; Tran, D.Q. Space–time variability of drought over Vietnam. Int. J. Climatol. 2019, 39, 5437–5451. [Google Scholar] [CrossRef]
- Yabueng, N.; Wiriya, W.; Chantara, S. Influence of zero-burning policy and climate phenomena on ambient PM2.5 patterns and PAHs inhalation cancer risk during episodes of smoke haze in Northern Thailand. Atmos. Environ. 2020, 232, 117485. [Google Scholar] [CrossRef]
- Guo, X.; Li, C.; Gao, Y.; Tang, L.; Briki, M.; Ding, H.; Ji, H. Sources of organic matter (PAHs and n-alkanes) in PM2.5 of Beijing in haze weather analyzed by combining the C-N isotopic and PCA-MLR analyses. Environ. Sci. Process. Impacts 2016, 18, 314–322. [Google Scholar] [CrossRef]
- Simcik, M.F.; Zhang, H.; Eisenreich, S.J.; Franz, T.P. Urban Contamination of the Chicago/Coastal Lake Michigan Atmosphere by PCBs and PAHs during AEOLOS. Environ. Sci. Technol. 1997, 31, 2141–2147. [Google Scholar] [CrossRef]
- Braun, R.A.; Aghdam, M.A.; Bañaga, P.A.; Betito, G.; Cambaliza, M.O.; Cruz, M.T.; Lorenzo, G.R.; MacDonald, A.B.; Simpas, J.B.; Stahl, C.; et al. Long-range aerosol transport and impacts on size-resolved aerosol composition in Metro Manila, Philippines. Atmos. Chem. Phys. 2020, 20, 2387–2405. [Google Scholar] [CrossRef]
- Kayee, J.; Sompongchaiyakul, P.; Sanwlani, N.; Bureekul, S.; Wang, X.; Das, R. Metal Concentrations and Source Apportionment of PM2.5 in Chiang Rai and Bangkok, Thailand during a Biomass Burning Season. ACS Earth Space Chem. 2020, 4, 1213–1226. [Google Scholar] [CrossRef]
- Lee, C.-T.; Ram, S.S.; Nguyen, D.L.; Chou, C.C.K.; Chang, S.-Y.; Lin, N.-H.; Wu, X.-C.; Chang, S.-C.; Hsiao, T.-C.; Sheu, G.-R.; et al. Aerosol Chemical Profile of Near-Source Biomass Burning Smoke in Sonla, Vietnam during 7-SEAS Campaigns in 2012 and 2013. Aerosol Air Qual. Res. 2016, 16, 2603–2617. [Google Scholar] [CrossRef]
- Nguyen, D.L.; Kawamura, K.; Ono, K.; Ram, S.S.; Engling, G.; Lee, C.-T.; Chi, K.H.; Sun, S.-A.; Lin, N.-H.; Chang, S.-C.; et al. Comprehensive PM2.5 Organic Molecular Composition and Stable Carbon Isotope Ratios at Sonla, Vietnam: Fingerprint of Biomass Burning Components. Aerosol Air Qual. Res. 2016, 16, 2618–2634. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, Y.; Qiu, X.; Lin, Y.; Cao, J.; Hu, D. A quantitative assessment of source contributions to fine particulate matter (PM2.5)-bound polycyclic aromatic hydrocarbons (PAHs) and their nitrated and hydroxylated derivatives in Hong Kong. Environ. Pollut. 2016, 219, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, H.; Zhang, L.; Zhang, Z.; Xing, X.; Qi, S. Fine particle-bound polycyclic aromatic hydrocarbons (PAHs) at an urban site of Wuhan, central China: Characteristics, potential sources and cancer risks apportionment. Environ. Pollut. 2019, 246, 319–327. [Google Scholar] [CrossRef]
| Variables | Sampling Sites | Mean | Min | Max | Standard Deviation |
|---|---|---|---|---|---|
| OC (μg/m3) | Overall | 6.88 | 3.12 | 24.1 | 4.94 |
| DBKL | 6.99 | 4.17 | 13.5 | 3.30 | |
| SCA | 8.73 | 3.69 | 24.1 | 7.64 | |
| KKKL | 4.93 | 3.12 | 9.33 | 2.23 | |
| EC (μg/m3) | Overall | 3.68 | 1.32 | 6.82 | 1.58 |
| DBKL | 4.33 | 2.76 | 6.58 | 1.46 | |
| SCA | 3.98 | 2.53 | 6.82 | 1.76 | |
| KKKL | 2.73 | 1.32 | 4.33 | 1.25 | |
| OC/EC | Overall | 1.86 | 1.24 | 3.53 | 0.626 |
| DBKL | 1.60 | 1.24 | 2.04 | 0.343 | |
| SCA | 2.01 | 1.28 | 3.53 | 0.820 | |
| KKKL | 1.95 | 1.24 | 3.04 | 0.649 | |
| SOC (µg/m3) | Overall | 2.33 | - | 15.6 | 3.61 |
| DBKL | 1.64 | - | 5.32 | 1.97 | |
| SCA | 3.81 | 0.13 | 15.6 | 5.87 | |
| KKKL | 1.56 | 0.020 | 3.98 | 1.43 | |
| Soot-EC (µg/m3) | Overall | 18 | 0.28 | 0.21 | 0.43 |
| KKKL | 6 | 0.23 | 0.21 | 0.25 | |
| SCA | 6 | 0.30 | 0.23 | 0.43 | |
| DBKL | 6 | 0.30 | 0.21 | 0.35 | |
| Char-EC (µg/m3) | Overall | 18 | 3.40 | 1.10 | 6.40 |
| KKKL | 6 | 2.50 | 1.10 | 4.08 | |
| SCA | 6 | 3.68 | 2.24 | 6.40 | |
| DBKL | 6 | 4.03 | 2.49 | 6.37 | |
| WSOC (µg/m3) | Overall | 2.73 | 0.627 | 9.12 | 2.17 |
| Compound | N | Mean | Min | Max | Standard Deviation | |
|---|---|---|---|---|---|---|
| Overall | Ace | 3 | 0.33 | 0.05 | 0.64 | 0.30 |
| Flr | 8 | 0.21 | 0.04 | 0.89 | 0.30 | |
| Ant | 10 | 0.23 | 0.07 | 1.06 | 0.32 | |
| Flt | 10 | 0.16 | 0.05 | 0.97 | 0.29 | |
| Pyr | 9 | 0.19 | 0.04 | 0.91 | 0.29 | |
| B[a]A | 5 | 0.39 | 0.14 | 0.96 | 0.33 | |
| Chr | 7 | 0.21 | 0.12 | 0.29 | 0.06 | |
| B[b]F | 12 | 0.30 | 0.15 | 0.38 | 0.07 | |
| B[k]F | 5 | 0.38 | 0.29 | 0.42 | 0.05 | |
| B[a]P | 4 | 0.41 | 0.21 | 0.58 | 0.15 | |
| I[c]P | 2 | 0.95 | 0.81 | 1.08 | 0.19 | |
| B[h]A | 1 | 1.20 | 1.20 | 1.20 | - | |
| B[g]P | 1 | 0.72 | 0.72 | 0.72 | - | |
| Total PAHs | 13 | 1.74 | 0.15 | 9.94 | 2.68 | |
| DBKL | Flr | 1 | 0.06 | 0.06 | 0.06 | - |
| Ant | 2 | 0.12 | 0.07 | 0.16 | 0.06 | |
| Flt | 2 | 0.09 | 0.06 | 0.12 | 0.04 | |
| Pyr | 2 | 0.06 | 0.04 | 0.08 | 0.02 | |
| B[a]A | 1 | 2.38 × 10−1 | 2.38 × 10−1 | 2.38 × 10−1 | - | |
| Chr | 1 | 0.24 | 0.24 | 0.24 | - | |
| B[b]F | 3 | 0.34 | 0.30 | 0.38 | 0.04 | |
| B[k]F | 1 | 0.42 | 0.42 | 0.42 | - | |
| Total PAHs | 3 | 0.84 | 0.30 | 1.46 | 0.58 | |
| SCA | Flr | 2 | 0.06 | 0.05 | 0.07 | 0.01 |
| Ant | 4 | 0.09 | 0.07 | 0.15 | 0.04 | |
| Flt | 4 | 0.07 | 0.05 | 0.13 | 0.04 | |
| Pyr | 3 | 0.06 | 0.04 | 0.09 | 0.03 | |
| B[a]A | 1 | 0.26 | 0.26 | 0.26 | - | |
| Chr | 1 | 0.22 | 0.22 | 0.22 | - | |
| B[b]F | 3 | 0.34 | 0.32 | 0.37 | 0.03 | |
| B[k]F | 1 | 0.40 | 0.40 | 0.40 | - | |
| B[a]P | 1 | 4.03 × 10−1 | 4.03 × 10−1 | 4.03 × 10−1 | - | |
| Total PAHs | 4 | 0.82 | 0.18 | 1.37 | 0.51 | |
| KKKL | Ace | 3 | 0.33 | 0.05 | 0.64 | 0.30 |
| Flr | 5 | 0.30 | 0.04 | 0.89 | 0.37 | |
| Ant | 4 | 0.43 | 0.07 | 1.06 | 0.46 | |
| Flt | 4 | 0.29 | 0.05 | 0.97 | 0.46 | |
| Pyr | 4 | 0.35 | 0.04 | 0.91 | 0.40 | |
| B[a]A | 3 | 0.49 | 0.14 | 0.96 | 0.42 | |
| Chr | 5 | 0.20 | 0.12 | 0.29 | 0.07 | |
| B[b]F | 6 | 0.26 | 0.15 | 0.33 | 0.07 | |
| B[k]F | 3 | 0.35 | 0.29 | 0.39 | 0.06 | |
| B[a]P | 3 | 0.41 | 0.21 | 0.58 | 0.19 | |
| I[c]P | 2 | 0.95 | 0.81 | 1.08 | 0.19 | |
| B[h]A | 1 | 1.20 | 1.20 | 1.20 | - | |
| B[g]P | 1 | 0.72 | 0.72 | 0.72 | - | |
| Total PAHs | 6 | 2.81 | 0.15 | 9.94 | 3.79 |
| Variables | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| Fluorene | 0.955 | 0.049 | 0.058 | 0.271 |
| Anthracene | 0.970 | 0.043 | 0.010 | 0.202 |
| Fluoranthene | 0.976 | 0.052 | 0.081 | −0.080 |
| Pyrene | 0.977 | 0.031 | 0.025 | 0.186 |
| Benzo(a)anthracene | 0.957 | 0.258 | 0.036 | −0.013 |
| Chrysene | 0.196 | 0.271 | 0.314 | 0.884 |
| Benzo(b)fluoranthene | 0.049 | 0.173 | 0.954 | 0.239 |
| Benzo(k)fluoranthene | 0.037 | 0.952 | 0.179 | 0.213 |
| Benzo(a)pyrene | 0.757 | 0.629 | 0.066 | 0.093 |
| Eigen values | 5.787 | 1.824 | 0.803 | 0.462 |
| Variance (%) | 64.301 | 20.267 | 8.926 | 5.133 |
| Cumulative (%) | 64.301 | 84.568 | 93.494 | 98.627 |
| Sources of the pollutants | Natural gas and biomass burning | Urban traffic combustion | Combustion of heavy oil | Coal combustion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suradi, H.; Khan, M.F.; Sairi, N.A.; Rahim, H.A.; Yusoff, S.; Fujii, Y.; Qin, K.; Bari, M.A.; Othman, M.; Latif, M.T. Ambient Levels, Emission Sources and Health Effect of PM2.5-Bound Carbonaceous Particles and Polycyclic Aromatic Hydrocarbons in the City of Kuala Lumpur, Malaysia. Atmosphere 2021, 12, 549. https://doi.org/10.3390/atmos12050549
Suradi H, Khan MF, Sairi NA, Rahim HA, Yusoff S, Fujii Y, Qin K, Bari MA, Othman M, Latif MT. Ambient Levels, Emission Sources and Health Effect of PM2.5-Bound Carbonaceous Particles and Polycyclic Aromatic Hydrocarbons in the City of Kuala Lumpur, Malaysia. Atmosphere. 2021; 12(5):549. https://doi.org/10.3390/atmos12050549
Chicago/Turabian StyleSuradi, Hamidah, Md Firoz Khan, Nor Asrina Sairi, Haasyimah Ab Rahim, Sumiani Yusoff, Yusuke Fujii, Kai Qin, Md. Aynul Bari, Murnira Othman, and Mohd Talib Latif. 2021. "Ambient Levels, Emission Sources and Health Effect of PM2.5-Bound Carbonaceous Particles and Polycyclic Aromatic Hydrocarbons in the City of Kuala Lumpur, Malaysia" Atmosphere 12, no. 5: 549. https://doi.org/10.3390/atmos12050549
APA StyleSuradi, H., Khan, M. F., Sairi, N. A., Rahim, H. A., Yusoff, S., Fujii, Y., Qin, K., Bari, M. A., Othman, M., & Latif, M. T. (2021). Ambient Levels, Emission Sources and Health Effect of PM2.5-Bound Carbonaceous Particles and Polycyclic Aromatic Hydrocarbons in the City of Kuala Lumpur, Malaysia. Atmosphere, 12(5), 549. https://doi.org/10.3390/atmos12050549










