Quantifying the Northward Spread of Ticks (Ixodida) as Climate Warms in Northern Russia
Abstract
1. Introduction
2. Data and Methods
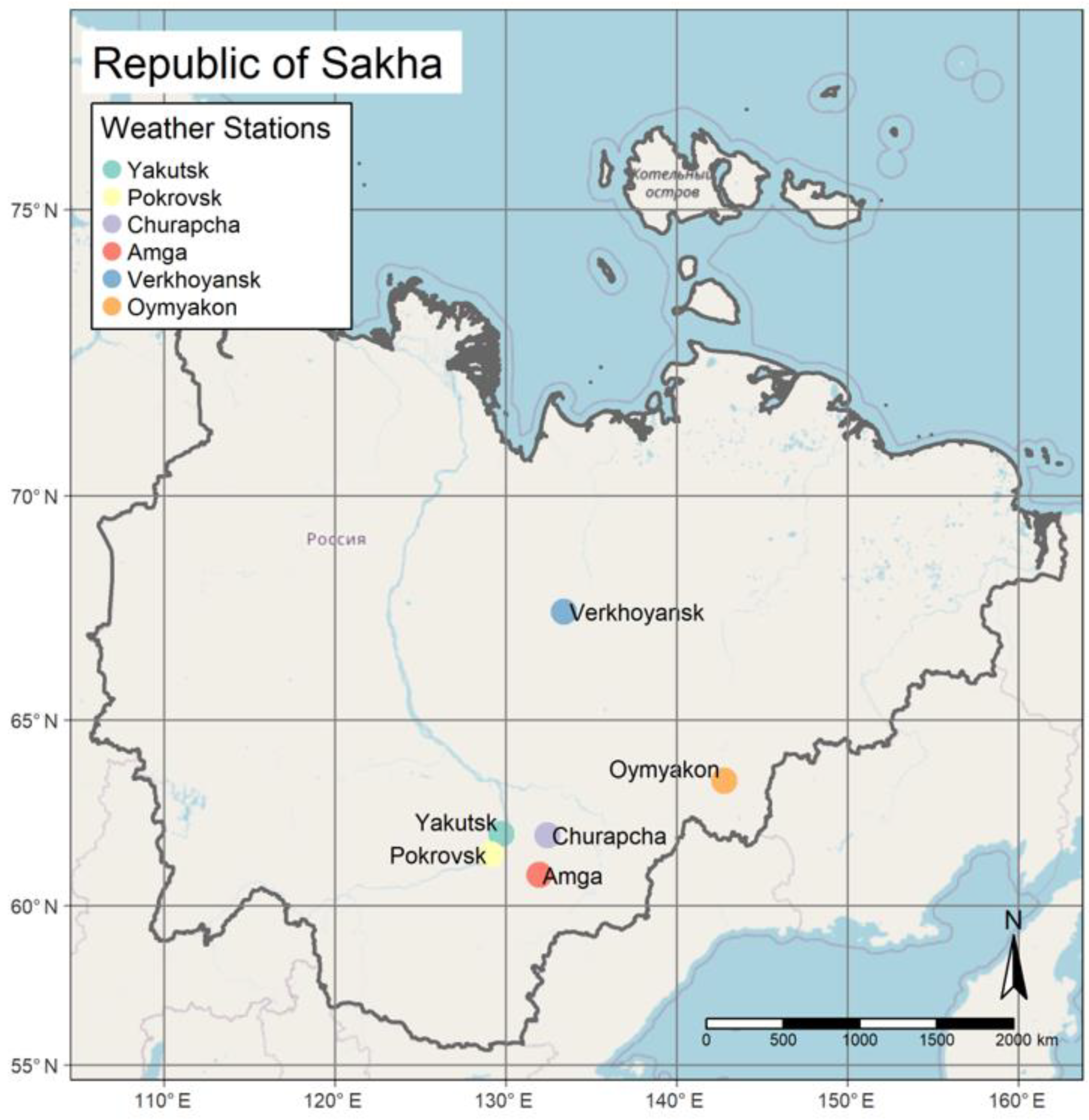
2.1. Study Area
2.2. Data
2.3. Statistical Analysis
- Average Annual Temperature (°C).
- Accumulated daily temperatures on days with an average temperature above 5 °C; this serves as a proxy for the warmth and length of the summer season, when ticks are most active.
- Average temperature in the hottest month of the year; this is also a proxy for the intensity of the warm season.
- Average temperature in the coldest month; the harshness of the winter might influence tick larvae survival and might dictate the presence of host species.
- The observation year (2006–2018); to account/control for possible non-climatic factors that change with time and influence the number of reported tick bite cases. These could include changing population behavior, or medical reporting quality.
- Selyaninov’s Hydrothermal Coefficient; previous studies observed that Taiga tick abundance in Russia was well explained by this coefficient. It is defined as the sum of daily precipitation (mm) on days with average temperature above 5 °C, divided by the sum of daily average temperatures on the days with temperatures above 5 °C, then multiplied by 10.
3. Results
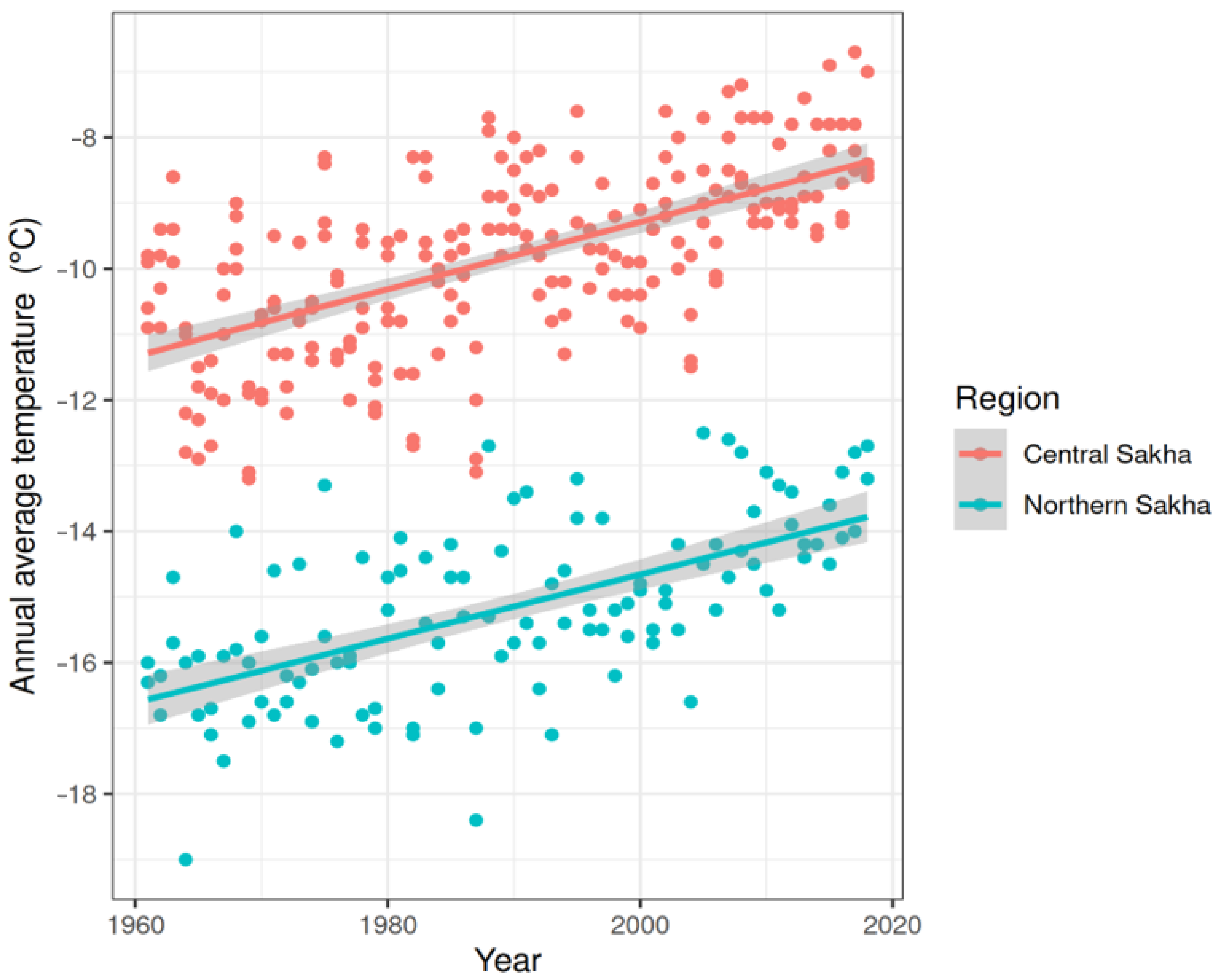
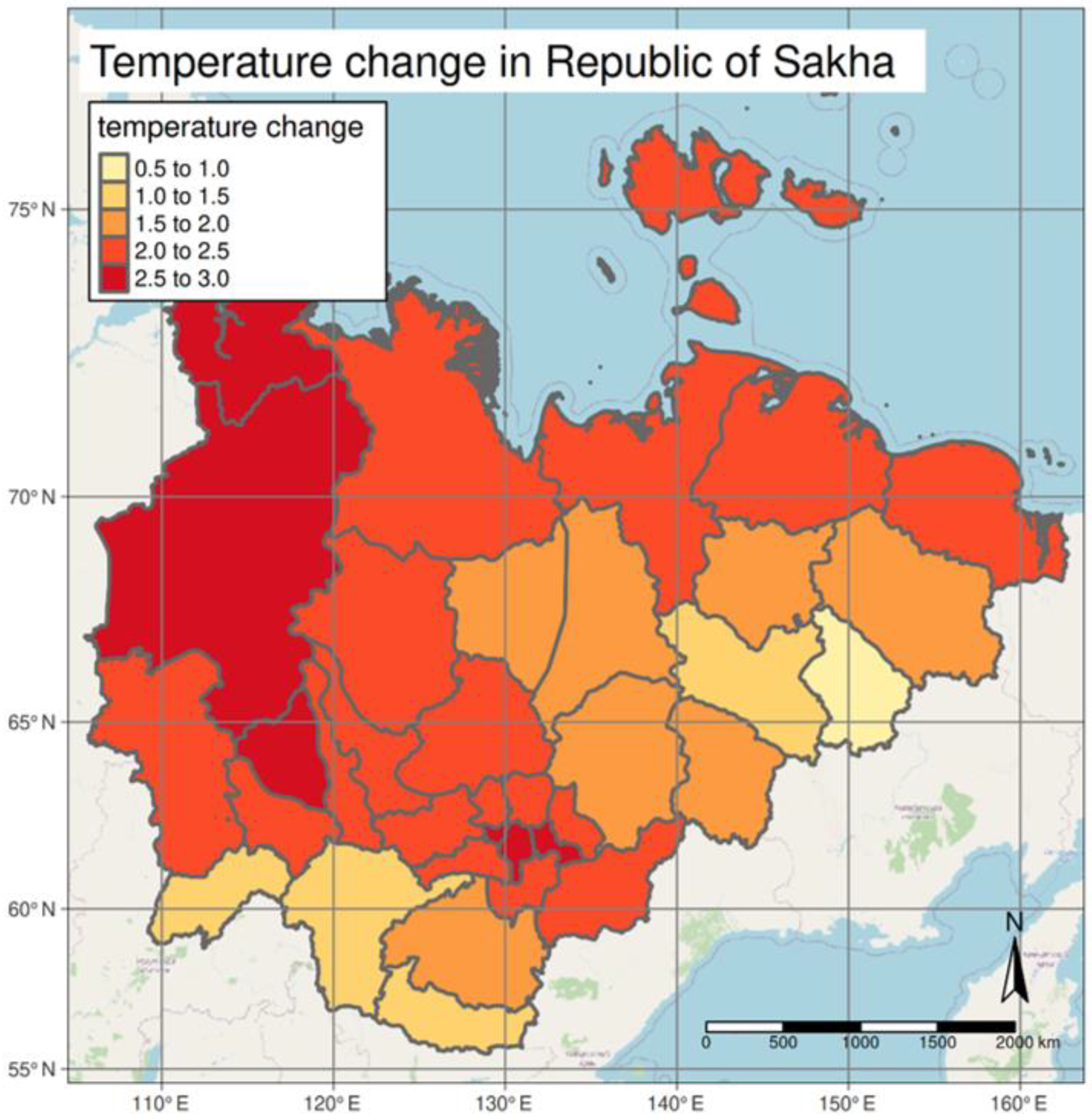
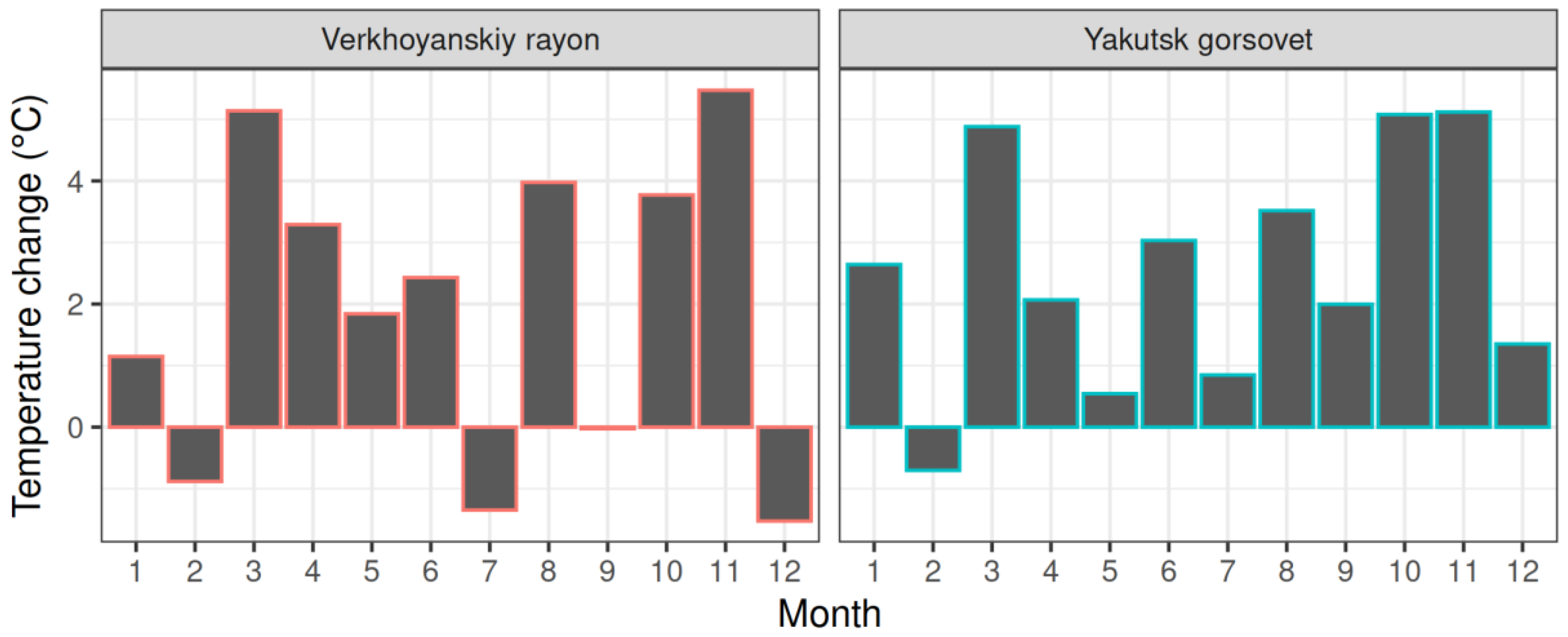
3.1. Climate Trends
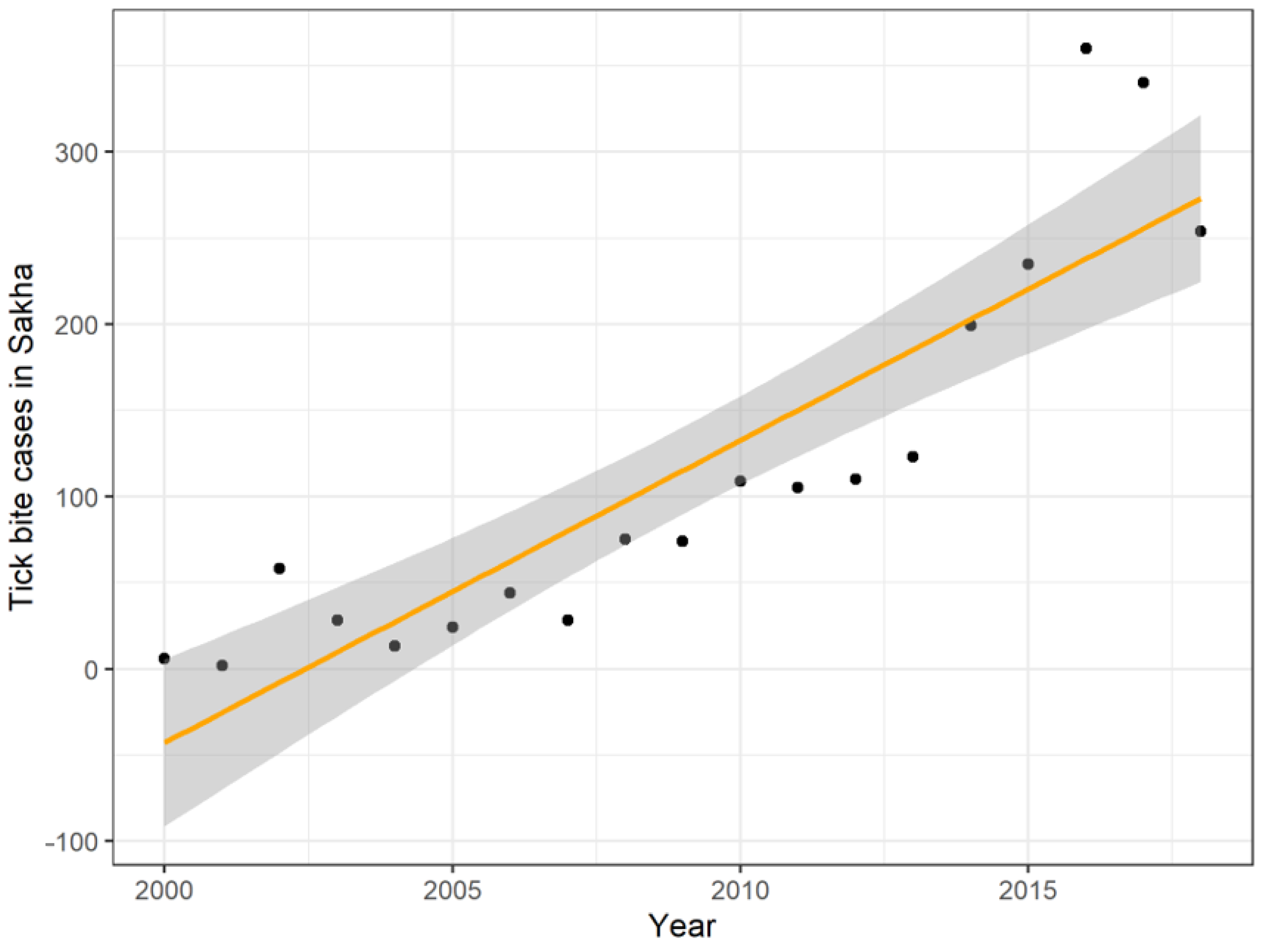
3.2. Tick Bite Results
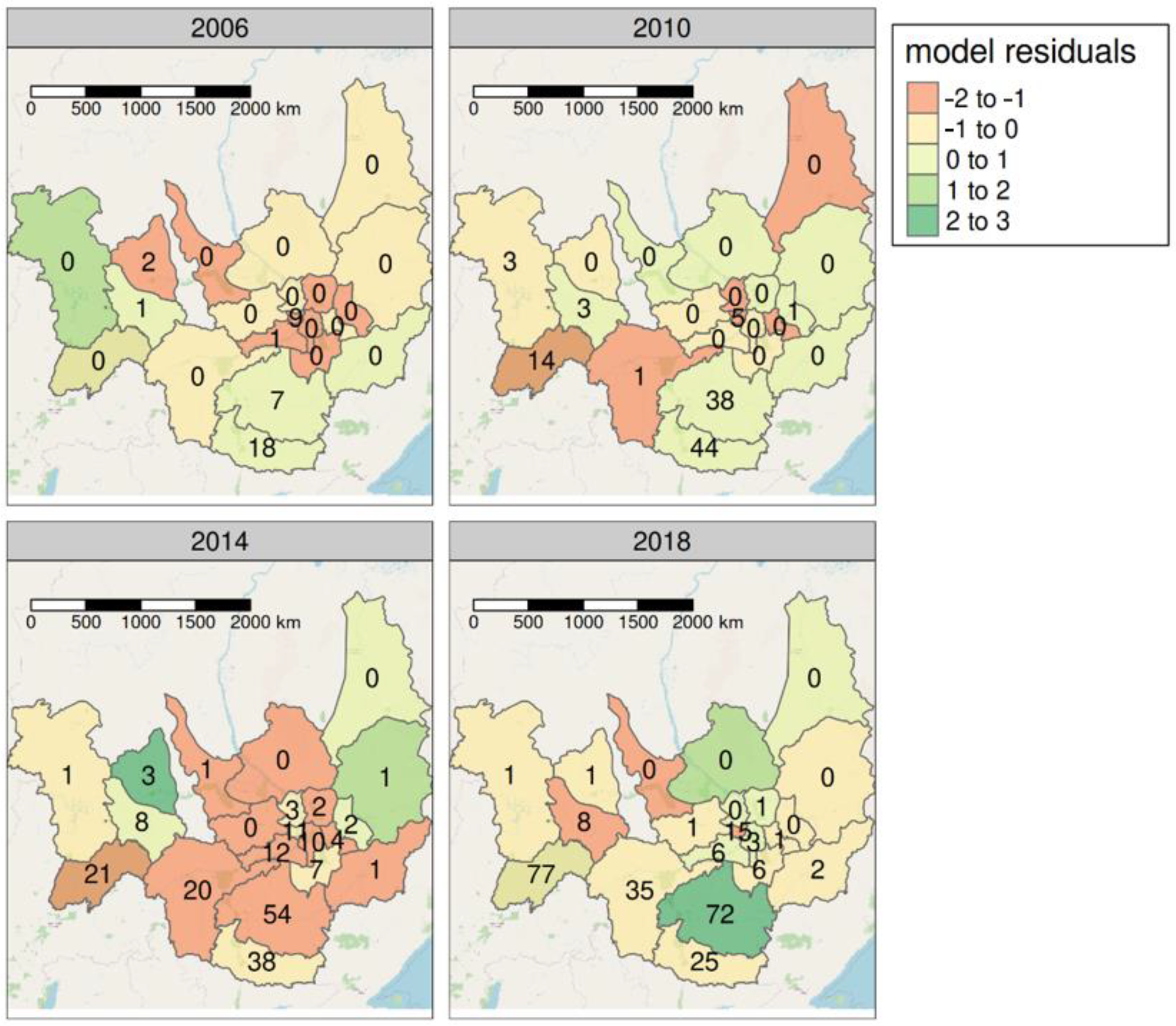
3.3. Spatial Distribution of Tick Bite Cases
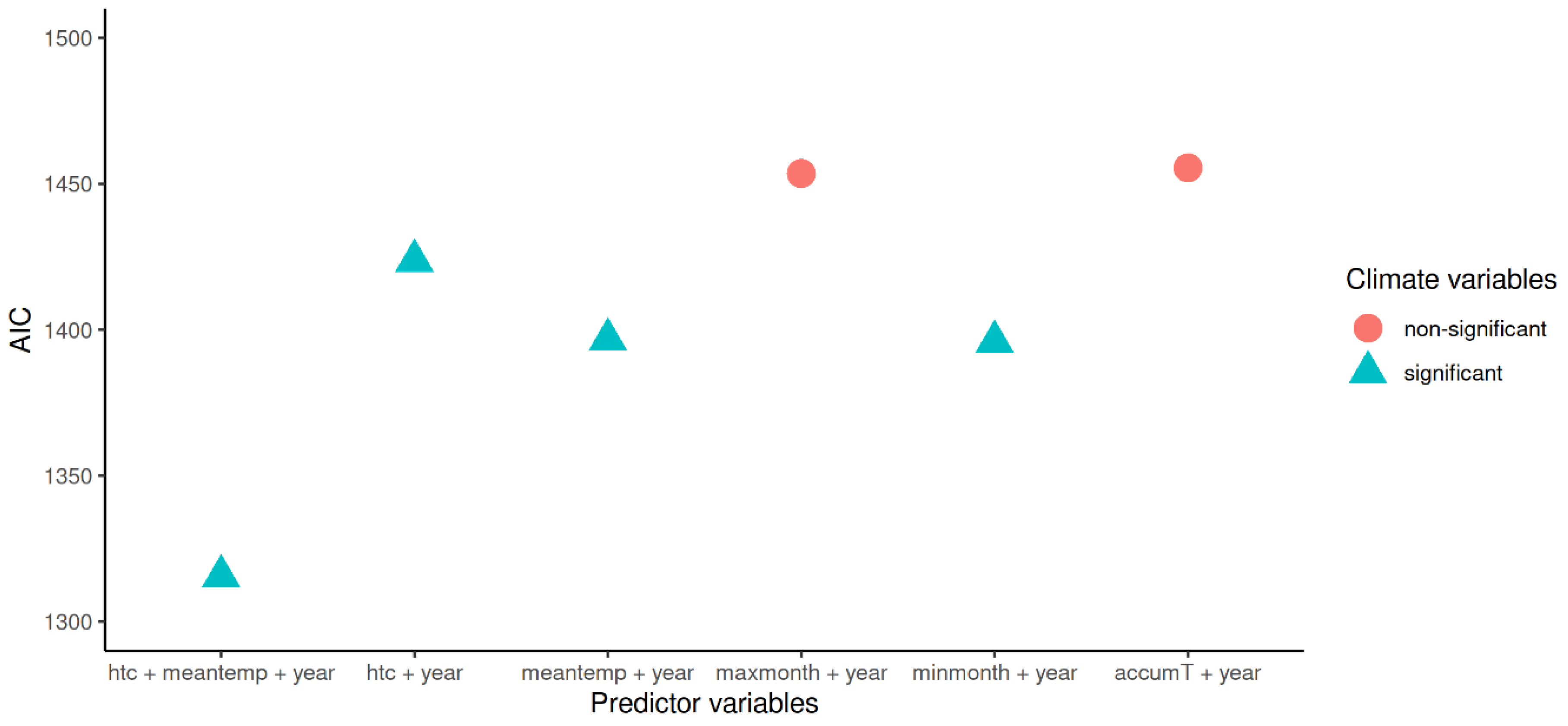
3.4. Model Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Note
- McBean, G.; Alekseev, G.; Chen, D.; Forland, E.; Fyfe, J.; Groisman, P.Y.; King, R.; Melling, H.; Vose, R.; Whitfield, P.H. Arctic climate: Past and present. In Arctic Climate Impact Assessment Overview Report; Cambridge University Press: Cambridge, UK, 2005; pp. 21–60. [Google Scholar]
- Box, J.E.; Colgan, W.T.; Christensen, T.R.; Schmidt, N.M.; Lund, M.; Parmentier, F.-J.W.; Brown, R.; Bhatt, U.S.; Euskirchen, E.S.; Romanovsky, V.E.; et al. Key indicators of Arctic climate change: 1971–2017. Environ. Res. Lett. 2019, 14, 045010. [Google Scholar] [CrossRef]
- Arctic Climate Impact Assessment (ACIA). ACIA Overview Report; Cambridge University Press: Cambridge, UK, 2005; p. 1020. [Google Scholar]
- Revich, B.; Tokarevich, N.; Parkinson, A.J. Climate change and zoonotic infections in the Russian Arctic. Int. J. Circumpolar Health 2012, 71, 18792. [Google Scholar] [CrossRef]
- Parkinson, A.J.; Berner, J. Climate change and impacts on human health in the Arctic: An international workshop on emerging threats and the response of Arctic communities to climate change. Int. J. Circumpolar Health 2009, 68, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Huber, I.; Potapova, K.; Ammosova, E.; Beyer, W.; Blagodatskiy, S.; Desyatkin, R.; Hoelzle, L.E.; Ignateva, M.; Kokolova, L.; Lemke, S.; et al. Symposium report: Emerging threats for human health—Impact of socioeconomic and climate change on zoonotic diseases in the Republic of Sakha (Yakutia), Russia. Int. J. Circumpolar Health 2020, 79, 1715698. [Google Scholar] [CrossRef]
- Gavriliev, P.P.; Ugarov, I.S. The response of ice-rich permafrost in Central Yakutia to climate warming. Криосфера Земли 2009, 13, 24–30. [Google Scholar]
- The Editors of Encyclopaedia Britannica Sakha. Encyclopaedia Britannica; Encyclopaedia Britannica, Inc.: Chicago, IL, USA, 2013; Available online: https://www.britannica.com/place/Sakha-republic-Russia (accessed on 8 November 2020).
- Gorokhov, A.N.; Fedorov, A.N. Current trends in climate change in Yakutia. Geogr. Nat. Resour. 2018, 39, 153–161. [Google Scholar] [CrossRef]
- Кокорин, А.О.; Карелин, Д.В.; Стеценко, А.В. Воздействие Изменения Климата На Российскую Арктику: Анализ и Пути Решения Проблемы; WWF Russia: Moscow, Russian, 2008. [Google Scholar]
- Loiko, S.V.; Lim, A.G.; Manasypov, R.M.; Shirokova, L.S.; Istigechev, G.I.; Kuzmina, D.M.; Kulizhsky, S.P.; Vorobyev, S.N.; Pokrovsky, O.S.; Raudina, T.V. Permafrost thaw and climate warming may decrease the CO2, carbon, and metal concentration in peat soil waters of the Western Siberia Lowland. Sci. Total Environ. 2018, 634, 1004–1023. [Google Scholar]
- Ding, Y.; Zhang, S.; Zhao, L.; Li, Z.; Kang, S. Global warming weakening the inherent stability of glaciers and permafrost. Sci. Bull. 2019, 64, 245–253. [Google Scholar] [CrossRef]
- Hari, P.; Kulmala, L. (Eds.) MicroForest. In Boreal Forest and Climate Change; Springer: Dordrecht, The Netherlands, 2008; pp. 433–478. ISBN 978-1-4020-8718-9. [Google Scholar]
- Semenza, J.C.; Suk, J.E. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2018, 365, fnx224. [Google Scholar] [CrossRef] [PubMed]
- Alfredsson, M.; Olafsson, E.; Eydal, M.; Unnsteinsdottir, E.R.; Hansford, K.; Wint, W.; Alexander, N.; Medlock, J.M. Surveillance of ixodes ricinus ticks (Acari: Ixodidae) in Iceland. Parasit. Vectors 2017, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Descamps, S. Winter temperature affects the prevalence of ticks in an Arctic seabird. PLoS ONE 2013, 8, e65374. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L.; Ogden, N.H.; Beard, C.B. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. J. Med. Entomol. 2016, 53, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Jaenson, T.G.; Hjertqvist, M.; Bergström, T.; Lundkvist, Å. Why is tick-borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden. Parasit. Vectors 2012, 5, 184. [Google Scholar] [CrossRef]
- Sumilo, D.; Asokliene, L.; Bormane, A.; Vasilenko, V.; Golovljova, I.; Randolph, S.E. Climate change cannot explain the upsurge of tick-borne encephalitis in the Baltics. PLoS ONE 2007, 2, e500. [Google Scholar] [CrossRef]
- Grigoryeva, L.; Stanyukovich, M. Life cycle of the taiga tick Ixodes persulcatus (Acari: Ixodidae) in the north-west of Russia. Exp. Appl. Acarol. 2016, 69. [Google Scholar] [CrossRef]
- Osipova, T.N.; Grigoryeva, L.A.; Samoylova, E.P.; Shapar, A.O.; Bychkova, E.M. The influence of meteorological factors on the activity of adult taiga ticks (Ixodes persulcatus Sch., Ixodinae) in St. Petersburg and its environs. Entomol. Rev. 2017, 97, 554–563. [Google Scholar] [CrossRef]
- Selyaninov, G.L. About the agricultural evaluation of the climate. TrudyGGO 1928, 20, 177–185. [Google Scholar]
- Khasnatinov, M.; Liapunov, A.; Manzarova, E.; Liapunova, N.; Solovarov, I.; Salchak, V.; Danchinova, G. The species diversity of ticks that attack human hosts in Eastern Siberia (Russian Federation) and prevalence of tick-borne pathogens. Int. J. Infect. Dis. 2019, 79, 137. [Google Scholar] [CrossRef]
- Eisen, L. Climate change and tick-borne diseases: A research field in need of long-term empirical field studies. Int. J. Med. Microbiol. 2008, 298, 12–18. [Google Scholar] [CrossRef]
- Stjpanova, N.A. On the lowest temperatures on Earth. Mon. Weather Rev. 1958, 86, 6–10. [Google Scholar] [CrossRef]
- Sabater, M. ERA5-Land Monthly Averaged Data from 1981 to Present. Available online: https://cds.climate.copernicus.eu/cdsapp#!/dataset/10.24381/cds.68d2bb30?tab=overview (accessed on 19 January 2021).
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Microsoft Corporation Microsoft Excel. 2018.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Pebesma, E. Simple features for R: Standardized support for spatial vector data. R J. 2018, 10, 439–446. [Google Scholar] [CrossRef]
- Tennekes, M. Tmap: Thematic maps in R. J. Stat. Softw. 2018, 84, 1–39. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Allen, M.P. (Ed.) The problem of multicollinearity. In Understanding Regression Analysis; Springer: Boston, MA, USA, 1997; pp. 176–180. ISBN 978-0-585-25657-3. [Google Scholar]
- Hueffer, K.; Parkinson, A.J.; Gerlach, R.; Berner, J. Zoonotic infections in Alaska: Disease prevalence, potential impact of climate change and recommended actions for earlier disease detection, research, prevention and control. Int. J. Circumpolar Health 2013, 72, 19562. [Google Scholar] [CrossRef] [PubMed]
- Noce, S.; Caporaso, L.; Santini, M. Climate change and geographic ranges: The implications for Russian forests. Front. Ecol. Evol. 2019, 7, 57. [Google Scholar] [CrossRef]
- Piesman, J. Transmission of Lyme disease spirochetes (Borrelia burgdorferi). Exp. Appl. Acarol. 1989, 7, 71–80. [Google Scholar] [CrossRef]
- Тикунова, Н.В.; Власов, В.В.; Рар, В.А.; Ткачев, С.Е. Клещи, которые нас кусают. Наука Из Первых Рук 2019, 85, 32–51. [Google Scholar]
- Tokarevich, N.K.; Tronin, A.A.; Blinova, O.V.; Buzinov, R.V.; Boltenkov, V.P.; Yurasova, E.D.; Nurse, J. The impact of climate change on the expansion of Ixodes persulcatus habitat and the incidence of tick-borne encephalitis in the north of European Russia. Glob. Health Action 2011, 4, 8448. [Google Scholar] [CrossRef]
- Beck, L.R.; Lobitz, B.M.; Wood, B.L. Remote sensing and human health: New sensors and new opportunities. Emerg. Infect. Dis. 2000, 6, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marti, I.; Zurita-Milla, R.; Harms, M.G.; Swart, A. Using volunteered observations to map human exposure to ticks. Sci. Rep. 2018, 8, 15435. [Google Scholar] [CrossRef]
| Name of District | Years | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| Yakutsk city | 9 3.8 | 3 1.2 | 2 0.8 | 8 3.0 | 5 1.8 | 11 4..1 | 13 4.7 | 23 8.0 | 11 3.7 | 21 7.0 | 33 10.9 | 33 10.7 | 15 4.7 |
| Aldansky | 7 29.2 | 5 20.9 | 7 29.4 | 14 32.5 | 38 89.1 | 29 68.2 | 19 44.5 | 32 75.8 | 54 129.7 | 60 146.2 | 102 252.3 | 75 188.2 | 72 187.3 |
| Neryungrinsky | 18 20.2 | 8 9.1 | 40 40.0 | 25 29.0 | 44 53.2 | 29 35.1 | 18 22.2 | 13 16.2 | 38 48.4 | 23 29.8 | 64 84.2 | 26 34.7 | 25 33.8 |
| Suntarsky | 1 3.9 | 3 11.7 | 3 11.9 | 6 23.6 | 3 11.9 | 2 7.96 | 10 40.5 | 3 12.3 | 8 33.2 | 3 12.6 | 28 117.8 | 12 50.6 | 8 33.9 |
| Khangalassky | 1 5.7 | - | 2 5.8 | 3 8.8 | - | 2 5.9 | 5 15.0 | 5 15.3 | 12 37.0 | 15 46.3 | 6 18.6 | 14 43.2 | 6 18.4 |
| Nyurbinsky | 2 7.9 | - | - | - | - | 2 7.9 | 4 16.0 | 1 4.1 | 3 12.2 | - | 5 20.6 | 5 20.7 | 1 4.2 |
| Lensky | - | 6 15.6 | 12 30.4 | 11 28.3 | 14 35.2 | 18 45.4 | 19 48.1 | 25 64.1 | 21 54.6 | 24 63.3 | 35 93.6 | 79 211.6 | 77 207.7 |
| Mirninsky | - | 2 2.4 | - | 1 2.3 | 3 3.9 | 2 2.6 | 1 1.4 | 4 5.6 | 1 1.4 | 4 5.6 | 9 12.5 | 1 1.4 | 1 1.3 |
| Megino-Kangalassky | - | 1 3.2 | - | - | - | - | - | 1 3.3 | 10 32.8 | 11 36.0 | 1 3.3 | 9 29.2 | 3 9.7 |
| Olyokminsky | - | - | 3 11.1 | 1 4.0 | 1 3.7 | 3 11.2 | 16 60.8 | 8 30.8 | 20 195.0 | 14 55.0 | 45 177.6 | 26 103.4 | 35 140.6 |
| Tattinsky | - | - | 1 5.9 | - | 1 5.8 | 1 5.8 | - | 3 18.3 | 2 42.3 | 9 54.9 | 5 30.6 | 5 30.6 | - |
| Amginsky | - | - | 2 11.6 | 3 18.7 | - | - | 1 5.9 | - | 7 41.9 | 26 155.9 | 9 5.4 | 19 113.6 | 6 35.9 |
| Kobyaysky | - | - | 1 7.3 | 2 15.2 | - | 1 7.3 | 1 7.5 | - | - | 2 15.5 | - | 1 7.9 | - |
| Gorny | - | - | 1 8.7 | - | - | - | 1 8.6 | - | - | - | 2 16.8 | 1 8.4 | 1 8.4 |
| Tomponsky | - | - | 1 6.9 | - | - | - | - | - | 1 7.4 | 2 14.8 | - | 3 23.0 | - |
| Churapchinsky | - | - | - | - | - | 2 9.8 | 1 4.9 | 3 14.6 | 4 19.4 | 16 77.5 | 10 48.3 | 15 70.9 | 1 4.7 |
| Ust-Maysky | - | - | - | - | - | 3 35.1 | - | - | 1 12.9 | 2 26.2 | 3 40.8 | 8 109.7 | 2 26.8 |
| Vilyuysky | - | - | - | - | - | - | 1 4.0 | 1 4.0 | 1 4.1 | 1 4.1 | 1 4.0 | 1 3.9 | - |
| Namsky | - | - | - | - | - | - | - | 1 4.3 | 3 12.8 | 1 4.2 | 1 4.1 | 3 12.3 | - |
| Ust-Aldansky | - | - | - | - | - | - | - | - | 2 9.5 | 1 4.8 | - | 4 19.2 | 1 4.8 |
| Verkhoyansky | - | - | - | - | - | - | - | - | - | - | 1 8.8 | - | - |
| Total in the Republic of Sakha | 44 4.6 | 28 2.9 | 75 7.8 | 74 7.7 | 109 11.4 | 105 11.0 | 110 11.5 | 123 12.9 | 199 20.8 | 235 24.6 | 360 37.5 | 340 35.3 | 254 26.3 |
| Model Parameter | Estimate | 2.5% | 97.5% |
|---|---|---|---|
| Intercept | 4.75 × 10−176 | 1.55 × 10−219 | 3.84 × 10−133 |
| Average temperature (annual) | 1.75 | 1.59 | 1.93 |
| Selyaninov’s HTC | 3.42 | 2.62 | 4.54 |
| Year | 1.22 | 1.17 | 1.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladimirov, L.N.; Machakhtyrov, G.N.; Machakhtyrova, V.A.; Louw, A.S.; Sahu, N.; Yunus, A.P.; Avtar, R. Quantifying the Northward Spread of Ticks (Ixodida) as Climate Warms in Northern Russia. Atmosphere 2021, 12, 233. https://doi.org/10.3390/atmos12020233
Vladimirov LN, Machakhtyrov GN, Machakhtyrova VA, Louw AS, Sahu N, Yunus AP, Avtar R. Quantifying the Northward Spread of Ticks (Ixodida) as Climate Warms in Northern Russia. Atmosphere. 2021; 12(2):233. https://doi.org/10.3390/atmos12020233
Chicago/Turabian StyleVladimirov, Leonid N., Grigory N. Machakhtyrov, Varvara A. Machakhtyrova, Albertus S. Louw, Netrananda Sahu, Ali P. Yunus, and Ram Avtar. 2021. "Quantifying the Northward Spread of Ticks (Ixodida) as Climate Warms in Northern Russia" Atmosphere 12, no. 2: 233. https://doi.org/10.3390/atmos12020233
APA StyleVladimirov, L. N., Machakhtyrov, G. N., Machakhtyrova, V. A., Louw, A. S., Sahu, N., Yunus, A. P., & Avtar, R. (2021). Quantifying the Northward Spread of Ticks (Ixodida) as Climate Warms in Northern Russia. Atmosphere, 12(2), 233. https://doi.org/10.3390/atmos12020233











