Nitrous Oxide Emission from Grazing Is Low across a Gradient of Plant Functional Diversity and Soil Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Characteristics
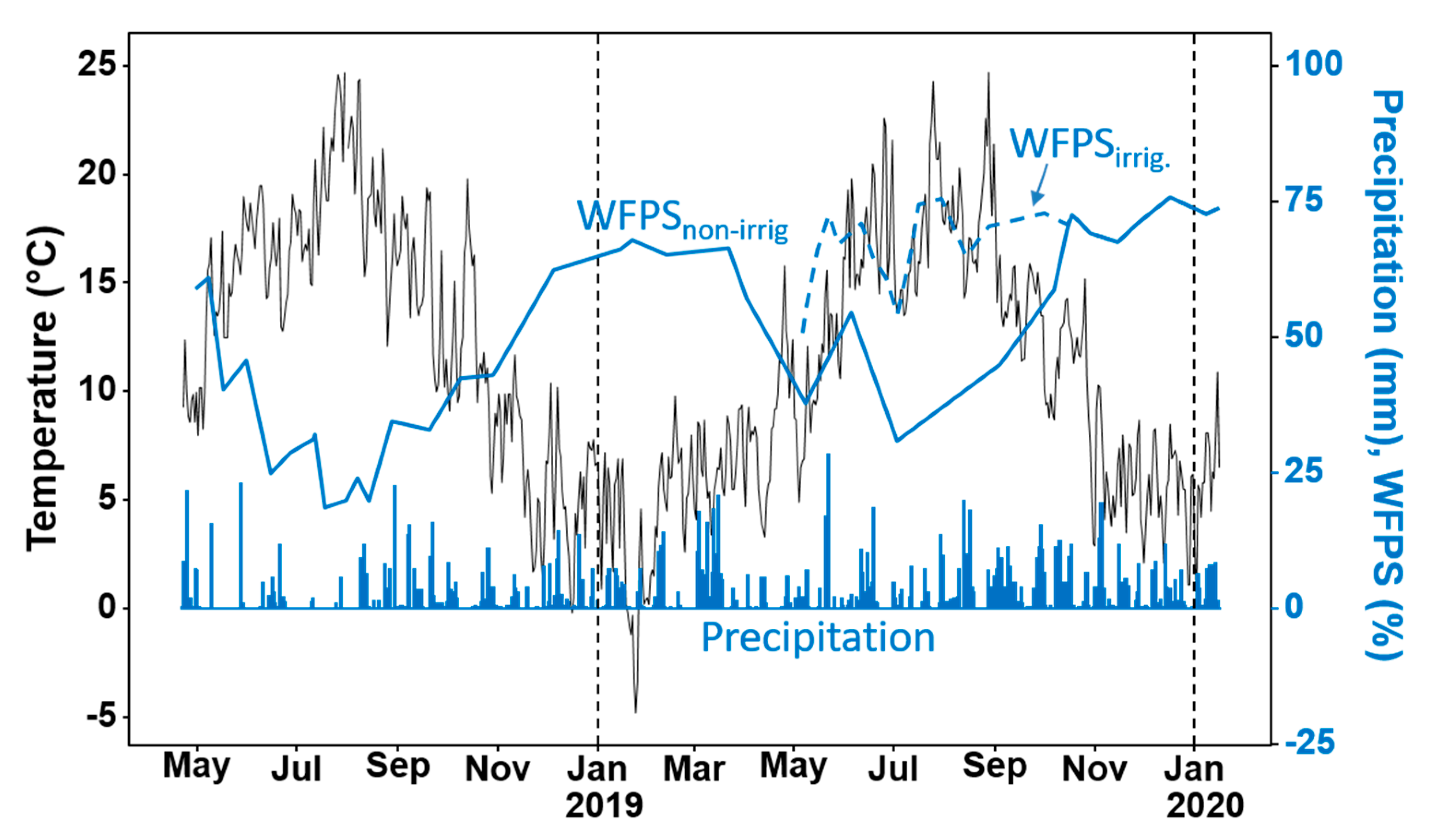
2.2. Environmental Conditions
2.3. Experiments
2.3.1. Urine and Dung Collection, Handling, and Application
2.3.2. N2O Sampling
2.3.3. Soil and Biomass Sampling
2.4. Chemical Analyses
2.5. Calculations
2.6. Statistics
3. Results
3.1. Plant Yields, Botanical Composition, and Soil Residual N
3.2. N2O Emissions
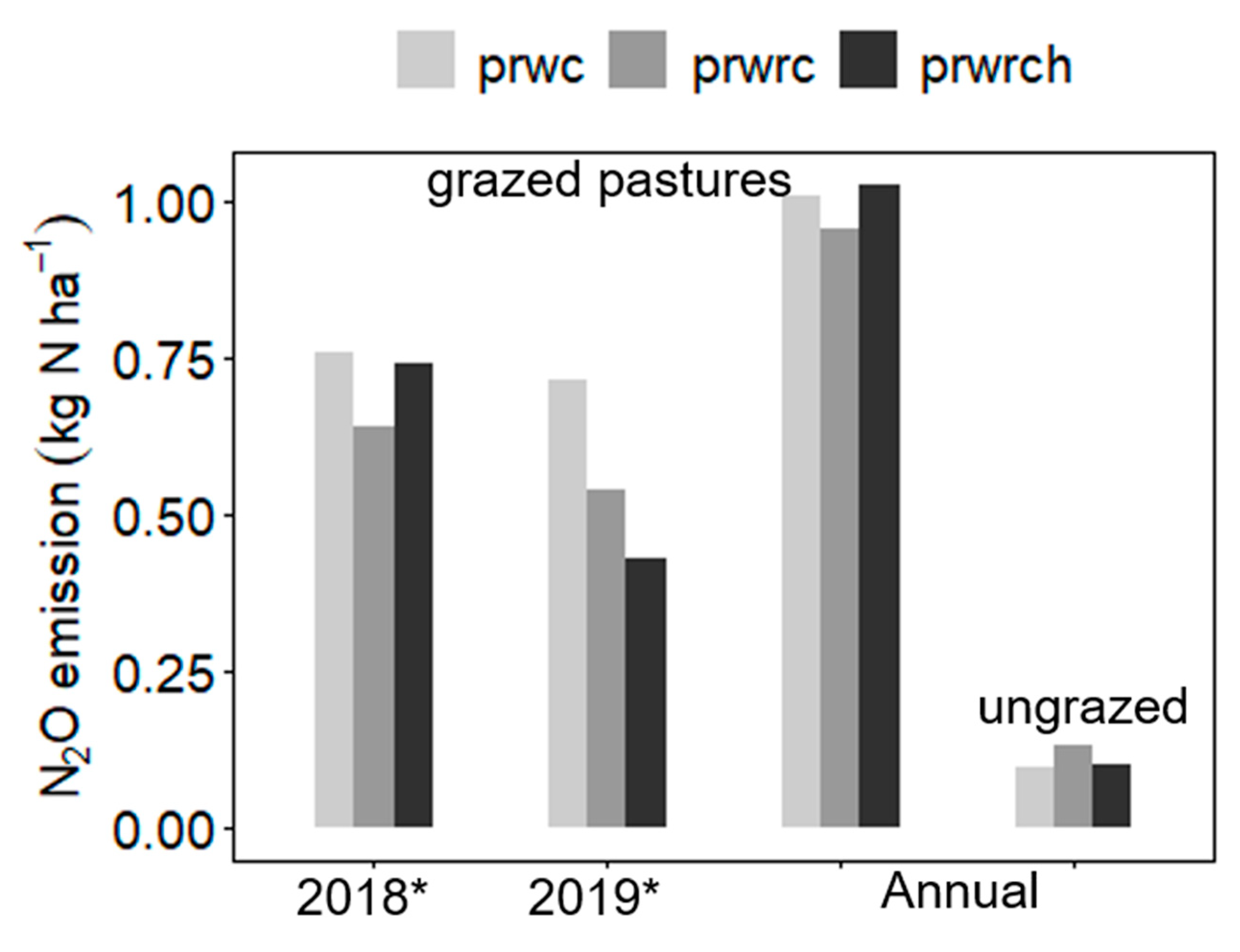
3.2.1. N2O Emissions from Grazed Pastures with a Gradient in Diversity
3.2.2. N2O Emission from Dung and Urine Patches (Simulated Grazing)
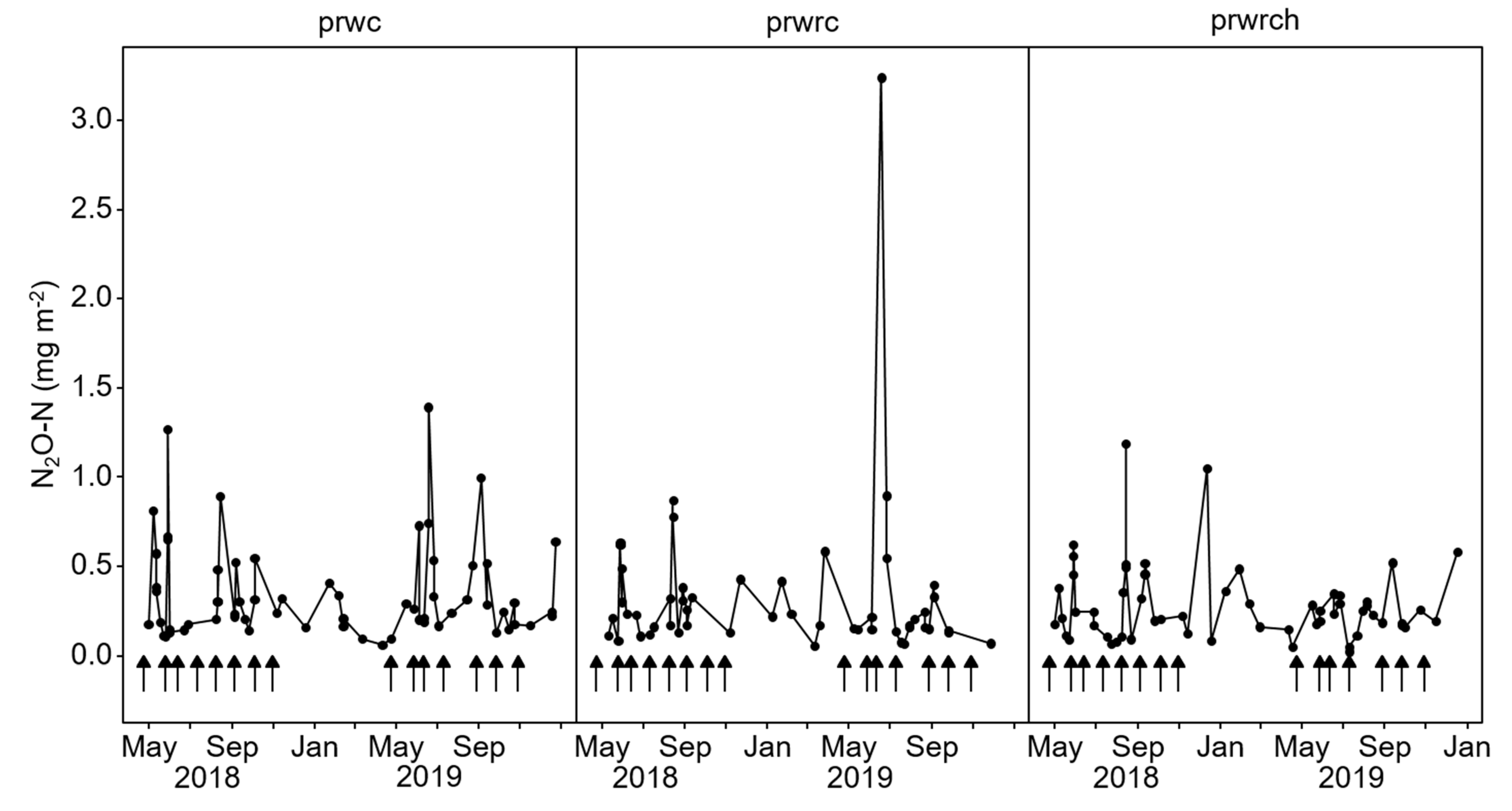
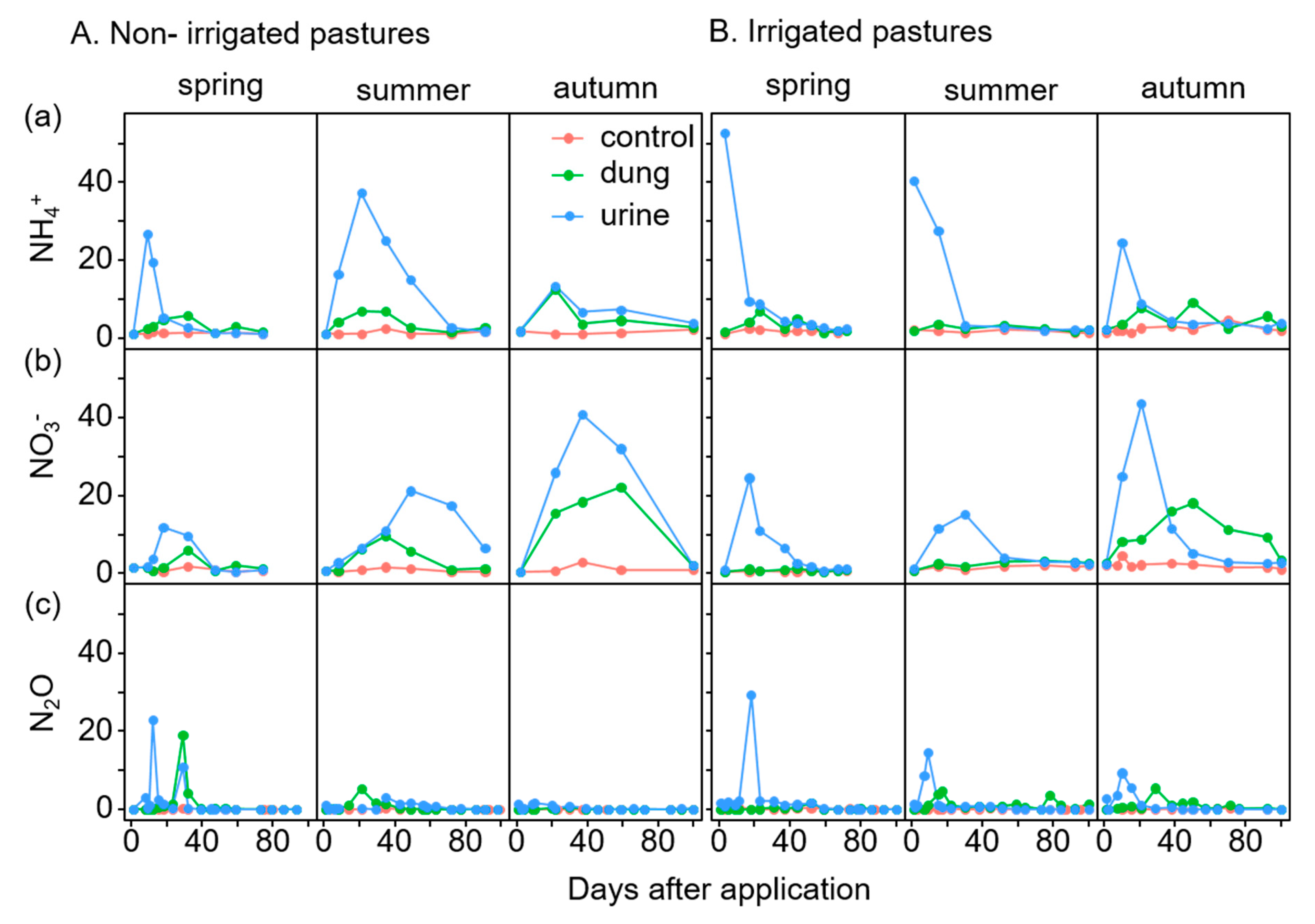
Temporal N Fluxes
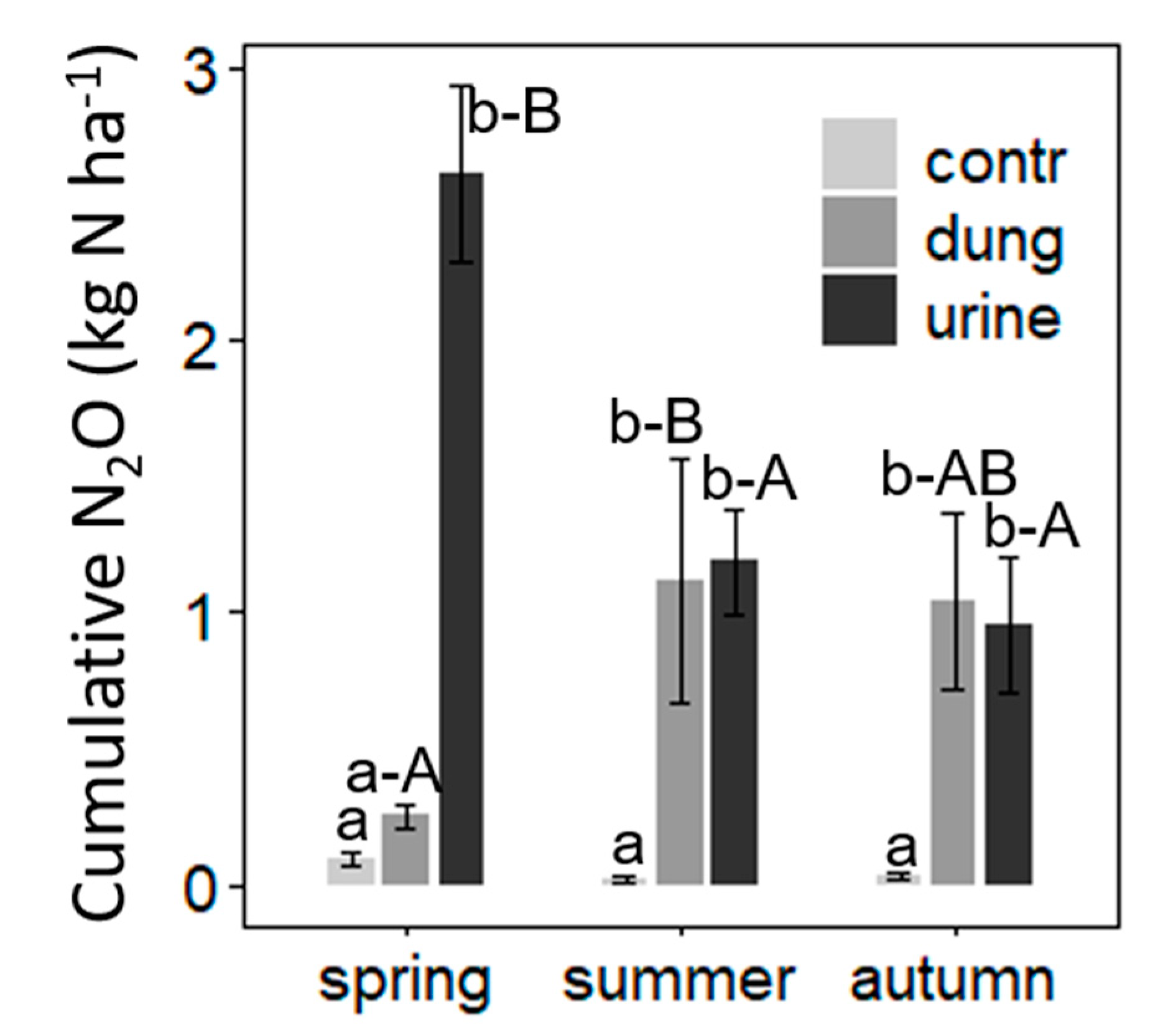
Cumulative N2O Emissions
Drivers of N2O Emission
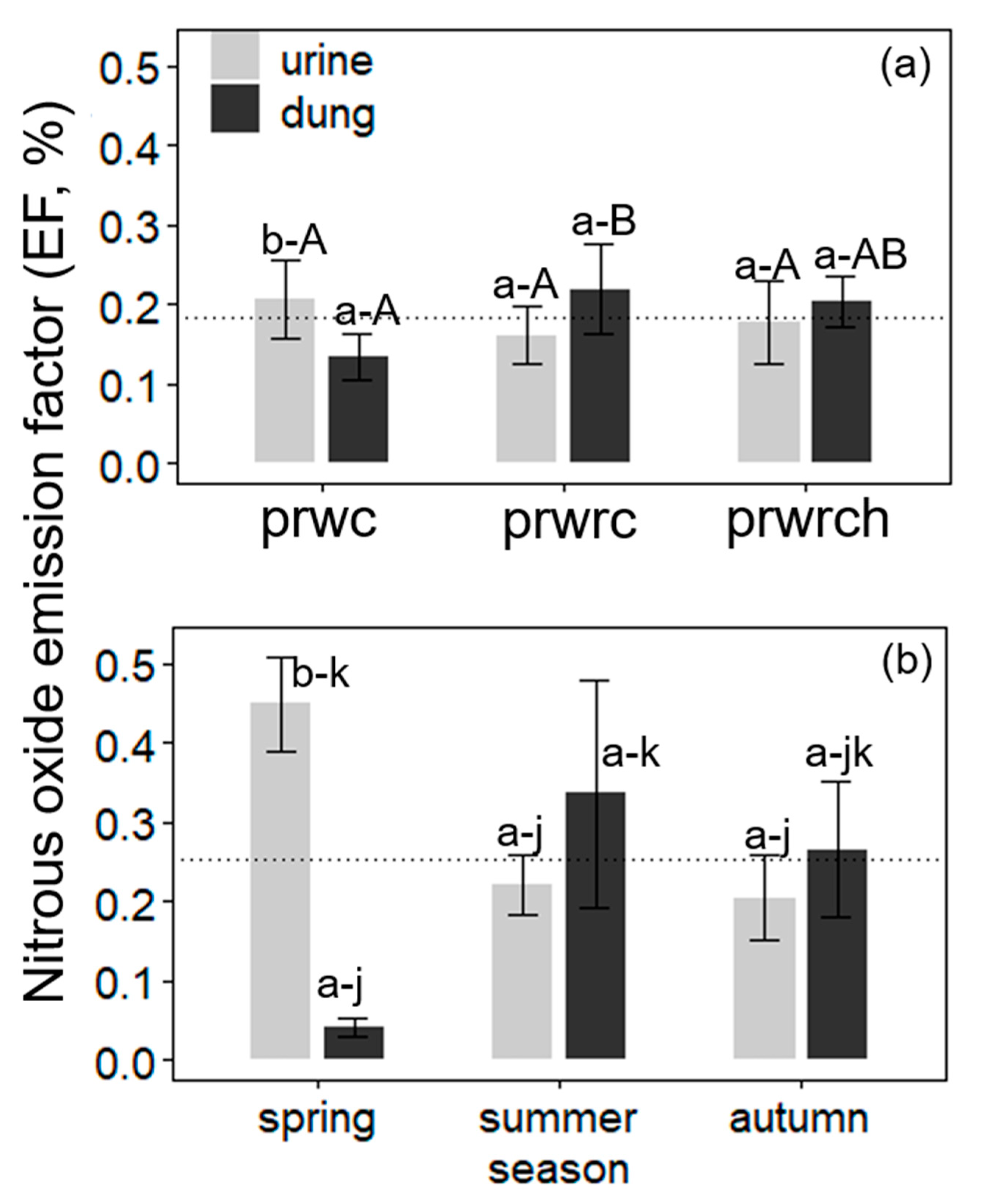
N2O Emission Factors
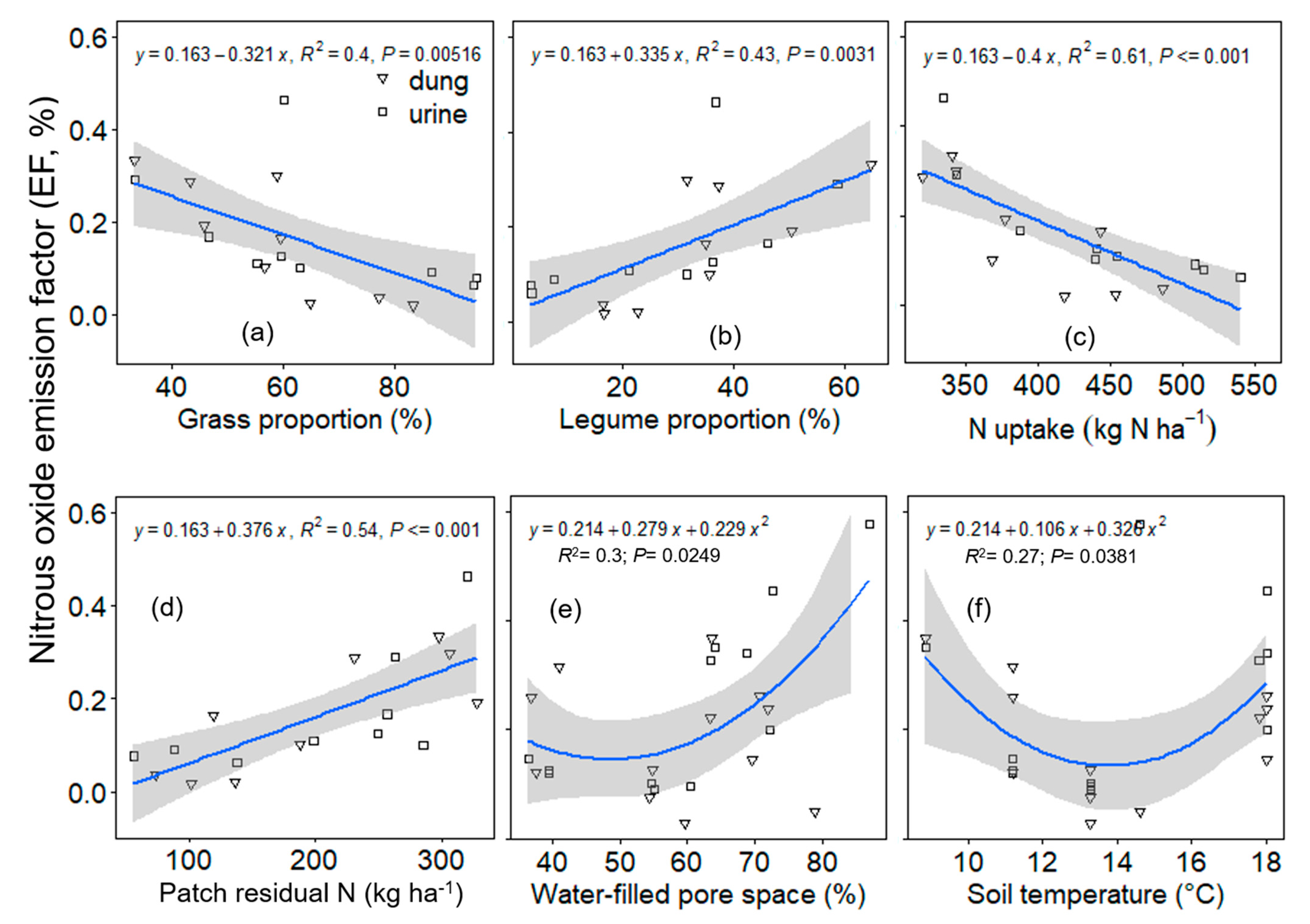
Drivers of N2O-N EF
4. Discussion
4.1. Plant Yields, Botanical Composition, and Soil Residual N
4.2. N2O Emissions and Emission Factors
4.2.1. The Effect of Grassland Diversity
4.2.2. Effect of N Source
4.2.3. Effect of Timing of Excreta Deposition
4.2.4. Effect of Irrigation
4.3. Factors That Controlled N2O Emission and EF at the Site
4.3.1. Botanical Characteristics
4.3.2. Soil Mineral N Availability and Plant N Uptake
4.3.3. Soil Moisture and Temperature
5. Conclusions and Implications of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Properties | |
|---|---|
| Soil texture | Sandy loam |
| Sand | 61.1 |
| Silt | 26.2 |
| Clay | 12.7 |
| Bulk density (g m−3) | 1.56 |
| Organic C (%) | 2.2 |
| pH | 6.4 |
| Total C (%) | 1.29 |
| Total N (%) | 0.12 |
| CN ratio | 10.9 |
| CEC (cmol kg−1) | 17.2 |
| Field capacity (%) | 37.5 |
| Parameters | F-/P-Value | ||||||
|---|---|---|---|---|---|---|---|
| (G)rassland (2,52) | (T)reatment (2,52) | (S)eason (2,52) | G × T (4,52) | G × S (4,52) | T × S (4,52) | G × T × S (8,52) | |
| Experiment 2—Non-irrigated simulated grazing | |||||||
| Grass (%) | 94.4 *** | 52.3 *** | 2.45 ns | 1.47 ns | 2.84 * | 1.88 ns | 0.44 ns |
| Legume (%) | 94.7 *** | 62.7 *** | 4.57 * | 1.25 ns | 5.36 ** | 0.66 ns | 0.38 ns |
| Herb (%) | 143.4 *** | 2.72 ns | 0.62 ns | 3.66 * | 0.62 ns | 0.97 ns | 0.97 ns |
| Unsown (%) | 16.3 *** | 3.76 * | 2.85 ns | 3.65 * | 0.42 ns | 0.24 ns | 0.36 ns |
| DM yield (Mg ha−1) | 44.0 *** | 1.35 ns | 39.3 *** | 2.97 * | 0.76 ns | 15.2 *** | 0.87 ns |
| N (g N kg DM−1) | 9.4 *** | 67.4 *** | 291.0 *** | 4.39 ** | 1.54 ns | 26.9 *** | 0.53 ns |
| β BFN (kg ha−1) | 87.7 *** | 79.0 *** | 29.8 *** | 4.63 ** | 6.96 *** | 13.0 *** | 0.69 ns |
| N input (kg N ha−1) | 87.7 *** | 512.7 *** | 42.7 *** | 4.63 ** | 6.96 *** | 16.9 *** | 0.69 ns |
| N uptake (kg N ha−1) | 22.5 *** | 6.44 ** | 5.77 ** | 2.43 ns | 0.94 ns | 21.8 *** | 1.85 ns |
| PRN (kg N ha−1) | 10.1 *** | 45.7 *** | 46.5 *** | 3.40 * | 3.11 * | 19.7 *** | 2.46 * |
| NO3 (mg kg−1 soil d−1) | 0.44 ns | 64.4 *** | 22.9 *** | 1.06 ns | 0.26 ns | 4.54 ** | 1.03 ns |
| NH4 (mg kg−1 soil d−1) | 0.90 ns | 29.6 *** | 2.70 ns | 1.30 ns | 2.19 ns | 2.27 ns | 0.72 ns |
| N2O-Nexcreta ( kg N ha−1) | 0.28 ns | 51.6 *** | 24.0 *** | 1.80 ns | 1.05 ns | 6.06 *** | 0.90 ns |
| N2O (kg N ha−1 100 day−1) | 0.40 ns | 10.5 ** | 17.6 *** | 1.35 ns | 0.57 ns | 5.05 ** | 0.56 ns |
| N2O-N EF (%) | 0.63 ns (2,34) | 0.005 ns (1,34) | 34.4 *** (2,34) | 3.37 * (2,34) | 1.40 ns (4,34) | 2.44 ns (2,34) | 1.63 ns (4,34) |
| g N2O-N kg−1 N uptake | 0.31 ns | 99.3 *** | 34.0 *** | 1.73 ns | 0.71 ns | 11.1 *** | 1.04 ns |
| N2O-Ngrazed ( kg N ha−1) | 0.19 ns | ||||||
| Experiment 3—Irrigated simulated grazing | |||||||
| Parameters | (T)reatment (2,24) | (S)eason (2,24) | T × S (4,24) | ||||
| NO3 (mg kg−1 soil day−1) | 79.8 *** | 75.3 *** | 16.9 *** | ||||
| NH4 (mg kg−1 soil day−1) | 135.9 *** | 3.22 ns | 3.19 * | ||||
| N2O-Nexcreta (kg N ha−1) | 53.9 *** | 0.44 ns | 8.68 *** | ||||
| N2O-N EF (%) | 8.37 * (1,15) | 2.76 ns (2,15) | 12.5 *** (2,15) | ||||
| Parameter | Estimate | Adj. R2 | F | Pr > F | Estimate | Adj. R2 | F | Pr > F |
|---|---|---|---|---|---|---|---|---|
| Non-Irrigated Simulated Grazing | Irrigated Simulated Grazing | |||||||
| Soil temp. | 0.05 | 0.15 | 5.52 | * | 0.02 | −0.02 | 0.38 | ns |
| Precipitation | −0.01 | 0.14 | 5.27 | * | 2.75 × 10−4 | −0.03 | 0.13 | ns |
| WFPS | −0.002 | 0.002 | 1.04 | ns | −0.03 | 0.02 | 1.82 | ns |
| WFPS^2 | −0.002 | 0.13 | 2.92 | * | ||||
| N content | 1.19 | 0.21 | 9.65 | ** | 6.54 | 0.17 | 5.82 | * |
| C content | −0.10 | −0.06 | 0.07 | ns | −0.23 | 0.26 | 9.18 | ** |
| C content^2 | −0.10 | 0.30 | 7.68 | ** | ||||
| C:N ratio | −0.002 | −0.05 | 0.17 | ns | ||||
| C:N ratio^2 | −0.03 | 0.54 | 19.8 | *** | −0.08 | 0.22 | 7.64 | * |
| N load | 0.002 | 0.42 | 23.7 | *** | 0.01 | 0.22 | 7.38 | * |
| NH4 intensity | −7.94 × 10−5 | 0.07 | 2.29 | ns | 0.003 | 0.83 | 53.4 | *** |
| NO3 intensity | −8.45 × 10−5 | 0.37 | 10.8 | ** | 0.002 | 0.61 | 18.4 | ** |
| Grass | −0.02 | 0.45 | 14.9 | ** | ||||
| Clover | 0.02 | 0.52 | 19.1 | *** | ||||
| Clover:grass | 0.50 | 0.63 | 30.1 | *** | ||||
| Herbs | −0.003 | −0.06 | 0.04 | ns | ||||
| Herbs:clover | −0.28 | 0.12 | 3.25 | ns | ||||
| Herbs:grass | 0.50 | 0.01 | 1.24 | ns | ||||
| Dry matter yield | −1.21 × 10−4 | −0.05 | 0.22 | ns | 4.37 × 10−4 | 0.10 | 4.87 | * |
| Harvested N | −0.01 | −0.002 | 0.98 | ns | ||||
| N input | 0.0003 | 0.03 | 2.85 | ns | ||||
| N uptake | −8.93 × 10−4 | 0.40 | 12.6 | ** | ||||
| PRN | 7.0 × 10−4 | 0.34 | 28.7 | *** | ||||
| Days after Excreta Application | 15 | 30 | 60 | 100 |
|---|---|---|---|---|
| Soil temperature (°C) | 0.08 ns | −0.03 ns | 0.07 ns | 0.26 *** |
| Soil temperature^2 (°C) | 0.38 *** | 0.32 *** | 0.49 *** | 0.43 *** |
| Water-filled pore space (%) | 0.18 ** | 0.19 ** | 0.03 ns | −0.004 ns |
| Water-filled pore space^2 (%) | 0.16 * | 0.18 * | 0.002 ns | −0.03 ns |
References
- Myers, S.S.; Smith, M.R.; Guth, S.; Golden, C.D.; Vaitla, B.; Mueller, N.D.; Dangour, A.D.; Huybers, P. Climate Change and Global Food Systems: Potential Impacts on Food Security and Undernutrition. Annu. Rev. Public Health 2017, 38, 259–277. [Google Scholar] [CrossRef]
- The Intergovernmental Panel on Climate Change. Climate Change 2014 Synthesis Report; The Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014; ISBN 978-92-9169-143-2. [Google Scholar]
- Portmann, R.W.; Daniel, J.S.; Ravishankara, A.R. Stratospheric Ozone Depletion Due to Nitrous Oxide: Influences of Other Gases. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1257–1264. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Lu, C.; Xu, X.; Ren, W.; Zhang, B.; Banger, K.; Tao, B.; Pan, S.; Liu, M.; et al. Global Methane and Nitrous Oxide Emissions from Terrestrial Ecosystems Due to Multiple Environmental Changes. Ecosyst. Health Sustain. 2015, 1, 1–20. [Google Scholar] [CrossRef]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A Comprehensive Quantification of Global Nitrous Oxide Sources and Sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Selbie, D.R.; Buckthought, L.E.; Shepherd, M.A. The Challenge of the Urine Patch for Managing Nitrogen in Grazed Pasture Systems; Elsevier: Amsterdam, The Netherlands, 2015; Volume 129, ISBN 978-0-12-802138-5. [Google Scholar]
- Luo, J.; Balvert, S.F.; Wise, B.; Welten, B.; Ledgard, S.F.; de Klein, C.A.M.; Lindsey, S.; Judge, A. Using Alternative Forage Species to Reduce Emissions of the Greenhouse Gas Nitrous Oxide from Cattle Urine Deposited onto Soil. Sci. Total Environ. 2018, 610–611, 1271–1280. [Google Scholar] [CrossRef]
- Dangal, S.R.S.; Tian, H.; Xu, R.; Chang, J.; Canadell, J.G.; Ciais, P.; Pan, S.; Yang, J.; Zhang, B. Global Nitrous Oxide Emissions From Pasturelands and Rangelands: Magnitude, Spatiotemporal Patterns, and Attribution. Glob. Biogeochem. Cycles 2019, 33, 200–222. [Google Scholar] [CrossRef]
- Reinsch, T.; Loges, R.; Kluß, C.; Taube, F. Renovation and Conversion of Permanent Grass-Clover Swards to Pasture or Crops: Effects on Annual N2O Emissions in the Year after Ploughing. Soil Tillage Res. 2018, 175, 119–129. [Google Scholar] [CrossRef]
- McAuliffe, G.A.; López-Aizpún, M.; Blackwell, M.S.A.; Castellano-Hinojosa, A.; Darch, T.; Evans, J.; Horrocks, C.; Le Cocq, K.; Takahashi, T.; Harris, P.; et al. Elucidating Three-Way Interactions between Soil, Pasture and Animals That Regulate Nitrous Oxide Emissions from Temperate Grazing Systems. Agric. Ecosyst. Environ. 2020, 300, 106978. [Google Scholar] [CrossRef] [PubMed]
- van der Weerden, T.J.; Manderson, A.; Kelliher, F.M.; de Klein, C.A.M. Spatial and Temporal Nitrous Oxide Emissions from Dairy Cattle Urine Deposited onto Grazed Pastures across New Zealand Based on Soil Water Balance Modelling. Agric. Ecosyst. Environ. 2014, 189, 92–100. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C.; Podolyan, A.; Robinson, A. Effect of Soil Moisture Status and a Nitrification Inhibitor, Dicyandiamide, on Ammonia Oxidizer and Denitrifier Growth and Nitrous Oxide Emissions in a Grassland Soil. Soil Biol. Biochem. 2014, 73, 59–68. [Google Scholar] [CrossRef]
- Oenema, O.; Sapek, A. Controlling nitrous oxide emissions from grassland farming systems; the COGANOG project. In Effects of Liming and Nitrogen Fertilizer Application on Soil Acidity and Gaseous Nitrogen Oxide Emissions in Grassland Systems; Falenty IMUZ Publisher: Falenty, Poland, 2000; pp. 7–13. [Google Scholar]
- Burke, I.C.; Lauenroth, W.K.; Cunfer, G.; Barrett, J.E.; Mosier, A.; Lowe, P. Nitrogen in the Central Grasslands Region of the United States. BioScience 2002, 52, 813–823. [Google Scholar] [CrossRef]
- Núñez, P.; Demanet, R.; Matus, F.; Mora, M. Grazing Management, Ammonia and Nitrous Oxide Emissions: A General View. Rev. Cienc. Suelo Y Nutr. Veg. 2007, 7, 61–99. [Google Scholar] [CrossRef]
- Schmeer, M.; Loges, R.; Dittert, K.; Senbayram, M.; Horn, R.; Taube, F. Legume-Based Forage Production Systems Reduce Nitrous Oxide Emissions. Soil Tillage Res. 2014, 143, 17–25. [Google Scholar] [CrossRef]
- Schaub, S.; Buchmann, N.; Lüscher, A.; Finger, R. Economic Benefits from Plant Species Diversity in Intensively Managed Grasslands. Ecol. Econ. 2020, 168, 106488. [Google Scholar] [CrossRef]
- Nyfeler, D.; Huguenin-Elie, O.; Suter, M.; Frossard, E.; Connolly, J.; Lüscher, A. Strong Mixture Effects among Four Species in Fertilized Agricultural Grassland Led to Persistent and Consistent Transgressive Overyielding. J. Appl. Ecol. 2009, 46, 683–691. [Google Scholar] [CrossRef]
- Fuchs, K.; Merbold, L.; Buchmann, N.; Bellocchi, G. Evaluating the Potential of Legumes to Mitigate N 2 O Emissions from Permanent Grassland Using Process-Based Models. Glob. Biogeochem. Cycles 2020, 34, e2020GB006561. [Google Scholar] [CrossRef]
- Cong, W.F.; Jing, J.; Rasmussen, J.; Søegaard, K.; Eriksen, J. Forbs Enhance Productivity of Unfertilised Grass-Clover Leys and Support Low-Carbon Bioenergy. Sci. Rep. 2017, 7, 1422. [Google Scholar] [CrossRef]
- de Klein, C.A.M.; van der Weerden, T.J.; Luo, J.; Cameron, K.C.; Di, H.J. A Review of Plant Options for Mitigating Nitrous Oxide Emissions from Pasture-Based Systems. N. Z. J. Agric. Res. 2019, 63, 29–43. [Google Scholar] [CrossRef]
- Bowatte, S.; Hoogendoorn, C.J.; Newton, P.C.D.; Liu, Y.; Brock, S.C.; Theobald, P.W. Grassland Plant Species and Cultivar Effects on Nitrous Oxide Emissions after Urine Application. Geoderma 2018, 323, 74–82. [Google Scholar] [CrossRef]
- Podolyan, A.; Di, H.J.; Cameron, K.C. Effect of Plantain on Nitrous Oxide Emissions and Soil Nitrification Rate in Pasture Soil under a Simulated Urine Patch in Canterbury, New Zealand. J. Soils Sediments 2020, 20, 1468–1479. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Cattle Grazing Effects on the Environment: Greenhouse Gas Emissions and Carbon Footprint. In Management Strategies for Sustainable Cattle Production in Southern Pastures; Academic Press: Cambridge, MA, USA, 2019; pp. 11–34. [Google Scholar] [CrossRef]
- Chadwick, D.R.; Cardenas, L.M.; Dhanoa, M.S.; Donovan, N.; Misselbrook, T.; Williams, J.R.; Thorman, R.E.; McGeough, K.L.; Watson, C.J.; Bell, M.; et al. The Contribution of Cattle Urine and Dung to Nitrous Oxide Emissions: Quantification of Country Specific Emission Factors and Implications for National Inventories. Sci. Total Environ. 2018, 635, 607–617. [Google Scholar] [CrossRef]
- Marsden, K.A.; Jones, D.L.; Chadwick, D.R. The Urine Patch Diffusional Area: An Important N2O Source? Soil Biol. Biochem. 2016, 92, 161–170. [Google Scholar] [CrossRef]
- Simon, P.L.; Dieckow, J.; de Klein, C.A.M.; Zanatta, J.A.; van der Weerden, T.J.; Ramalho, B.; Bayer, C. Nitrous Oxide Emission Factors from Cattle Urine and Dung, and Dicyandiamide (DCD) as a Mitigation Strategy in Subtropical Pastures. Agric. Ecosyst. Environ. 2018, 267, 74–82. [Google Scholar] [CrossRef]
- Thomas, B.W.; Gao, X.; Beck, R.; Hao, X. Are Distinct Nitrous Oxide Emission Factors Required for Cattle Urine and Dung Deposited on Pasture in Western Canada? Environ. Sci. Pollut. Res. 2017, 24, 26142–26147. [Google Scholar] [CrossRef]
- Voglmeier, K.; Six, J.; Jocher, M.; Ammann, C. Grazing-Related Nitrous Oxide Emissions: From Patch Scale to Field Scale. Biogeosciences 2019, 16, 1685–1703. [Google Scholar] [CrossRef]
- Krol, D.J.; Carolan, R.; Minet, E.; McGeough, K.L.; Watson, C.J.; Forrestal, P.J.; Lanigan, G.J.; Richards, K.G. Improving and Disaggregating N 2 O Emission Factors for Ruminant Excreta on Temperate Pasture Soils. Sci. Total Environ. 2016, 568, 327–338. [Google Scholar] [CrossRef] [PubMed]
- IPCC. IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies (IGES): Tokyo, Japan, 2006; pp. 1–49. [Google Scholar]
- IPCC 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. In Agriculture, Forestryand Other Land Use; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; Volume 4, ISBN 978-4-88788-232-4.
- FAO. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014; ISBN 978-92-5-108369-7. [Google Scholar]
- Loges, R.; Loza, C.; Voss, P.; Kluß, C.; Malisch, C.; Taube, F. The Potential of Multispecies Swards for Eco-Efficient Dairy Production in Northern Germany. In Proceedings of the Grassland Science in Europe, Helsinki, Finland, 19–22 October 2020; pp. 312–314. [Google Scholar]
- Cardoso, A.D.S.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M. Effect of Volume of Urine and Mass of Faeces on N2O and CH4 Emissions of Dairy-Cow Excreta in a Tropical Pasture. Anim. Prod. Sci. 2018, 58, 1079–1086. [Google Scholar] [CrossRef]
- Cichota, R.; Vogeler, I.; Snow, V.; Shepherd, M.; Mcauliffe, R.; Welten, B. Science of the Total Environment Lateral Spread Affects Nitrogen Leaching from Urine Patches. Sci. Total Environ. 2018, 635, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Høgh-Jensen, H.; Loges, R.; Jørgensen, F.V.; Vinther, F.P.; Jensen, E.S. An Empirical Model for Quantification of Symbiotic Nitrogen Fixation in Grass-Clover Mixtures. Agric. Syst. 2004, 82, 181–194. [Google Scholar] [CrossRef]
- Ministry of Energy, Agriculture, the Environment, Nature and Digitalization of Schleswig Holstein Annual Balance of Nutrients and Alkali/Alkaline or Trace Metals of Wet Deposition in Schleswig-Holstein. Available online: https://www.schleswig-holstein.de/DE/Fachinhalte/H/hydrologie_niederschlag/Downloads/JahresbilanzNaehrstoffe.pdf (accessed on 4 February 2021).
- Parkin, T.B.; Venterea, R.T. USDA-ARS GRACEnet Project Protocols Chapter 3. Chamber-Based Trace Gas Flux Measurements. Flux 2010, 2010, 1–39. [Google Scholar]
- R Development Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing Vienna Austria: Vienna, Austria, 2019; ISBN 3-900051-07-0. [CrossRef]
- Bretz, F.; Hothorn, T.; Westfall, P. Multiple Comparisons Using R; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4200-1090-9. [Google Scholar]
- Burnham, K.P.; Anderson, D.R.; Burnham, K.P.; Anderson, D.R. Burnham-Anderson, Model Selection. 2004. Available online: http://spider.ipac.caltech.edu/staff/fmasci/home/astro_refs/AIC_in_modelselection.pdf (accessed on 4 February 2021).
- Lorenz, H.; Reinsch, T.; Kluß, C.; Taube, F.; Loges, R. Does the Admixture of Forage Herbs Affect the Yield Performance, Yield Stability and Forage Quality of a Grass Clover Ley? Sustainability 2020, 12, 5842. [Google Scholar] [CrossRef]
- Komainda, M.; Küchenmeister, F.; Küchenmeister, K.; Kayser, M.; Wrage-Mönnig, N.; Isselstein, J. Drought Tolerance Is Determined by Species Identity and Functional Group Diversity Rather than by Species Diversity within Multi-Species Swards. Eur. J. Agron. 2020, 119, 126116. [Google Scholar] [CrossRef]
- Reinsch, T.; Malisch, C.; Loges, R.; Taube, F. Nitrous Oxide Emissions from Grass–Clover Swards as Influenced by Sward Age and Biological Nitrogen Fixation. Grass Forage Sci. 2020, 75, 372–384. [Google Scholar] [CrossRef]
- Vinther, F.P. Biological Nitrogen Fixation in Grass-Clover Affected by Animal Excreta. Plant Soil 2020, 203, 207–215. [Google Scholar] [CrossRef]
- Ledgard, S.F.; Steele, K.W. Biological Nitrogen Fixation in Mixed Legume/Grass Pastures. Plant Soil 1992, 141, 137–153. [Google Scholar] [CrossRef]
- Lüscher, A.; Mueller-Harvey, I.; Soussana, J.F.; Rees, R.M.; Peyraud, J.L. Potential of Legume-Based Grassland-Livestock Systems in Europe: A Review. Grass Forage Sci. 2014, 69, 206–228. [Google Scholar] [CrossRef]
- Ledgard, S.F. Nitrogen Cycling in Low Input Legume-Based Agriculture, with Emphasis on Legume/Grass Pastures. Plant Soil 2001, 228, 43–59. [Google Scholar] [CrossRef]
- Rafique, R.; Hennessy, D.; Kiely, G. Nitrous Oxide Emission from Grazed Grassland Under Different Management Systems. Ecosystems 2011, 14, 563–582. [Google Scholar] [CrossRef]
- Saggar, S.; Andrew, R.M.; Tate, K.R.; Hedley, C.B.; Rodda, N.J.; Townsend, J.A. Modelling Nitrous Oxide Emissions from Dairy-Grazed Pastures. Nutr. Cycl. Agroecosyst. 2004, 68, 243–255. [Google Scholar] [CrossRef]
- Rees, R.M.; Augustin, J.; Alberti, G.; Ball, B.C.; Boeckx, P.; Cantarel, A.; Castaldi, S.; Chirinda, N.; Chojnicki, B.; Giebels, M.; et al. Nitrous Oxide Emissions from European Agriculture—An Analysis of Variability and Drivers of Emissions from Field Experiments. Biogeosciences 2013, 10, 2671–2682. [Google Scholar] [CrossRef]
- de Klein, C.A.M.; Shepherd, M.A.; van der Weerden, T.J. Nitrous Oxide Emissions from Grazed Grasslands: Interactions between the N Cycle and Climate Change—A New Zealand Case Study. Curr. Opin. Environ. Sustain. 2014, 9–10, 131–139. [Google Scholar] [CrossRef]
- Holtan-Hartwig, L.; Dörsch, P.; Bakken, L.R. Low Temperature Control of Soil Denitrifying Communities: Kinetics of N2O Production and Reduction. Soil Biol. Biochem. 2002, 34, 1797–1806. [Google Scholar] [CrossRef]
- Cavigelli, M.A.; Robertson, G.P. Role of Denitrifier Diversity in Rates of Nitrous Oxide Consumption in a Terrestrial Ecosystem. Soil Biol. Biochem. 2001, 33, 297–310. [Google Scholar] [CrossRef]
- Levy-Booth, D.J.; Prescott, C.E.; Grayston, S.J. Microbial Functional Genes Involved in Nitrogen Fixation, Nitrification and Denitrification in Forest Ecosystems. Soil Biol. Biochem. 2014, 75, 11–25. [Google Scholar] [CrossRef]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Kim, D.G.; Zaman, M.; Tillman, R.W. Denitrification and N2O: N2 Production in Temperate Grasslands: Processes, Measurements, Modelling and Mitigating Negative Impacts. Sci. Total Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef]
- Hoeft, I.; Steude, K.; Wrage, N.; Veldkamp, E. Response of Nitrogen Oxide Emissions to Grazer Species and Plant Species Composition in Temperate Agricultural Grassland. Agric. Ecosyst. Environ. 2012, 151, 34–43. [Google Scholar] [CrossRef]
- Niklaus, P.A.; Le Roux, X.; Poly, F.; Buchmann, N.; Scherer-Lorenzen, M.; Weigelt, A.; Barnard, R.L. Plant Species Diversity Affects Soil–Atmosphere Fluxes of Methane and Nitrous Oxide. Oecologia 2016, 181, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Van Groenigen, J.W.; Kuikman, P.J.; De Groot, W.J.M.; Velthof, G.L. Nitrous Oxide Emission from Urine-Treated Soil as Influenced by Urine Composition and Soil Physical Conditions. Soil Biol. Biochem. 2005, 37, 463–473. [Google Scholar] [CrossRef]
- Van der Weerden, T.J.; Luo, J.; de Klein, C.A.M.; Hoogendoorn, C.J.; Littlejohn, R.P.; Rys, G.J. Disaggregating Nitrous Oxide Emission Factors for Ruminant Urine and Dung Deposited onto Pastoral Soils. Agric. Ecosyst. Environ. 2011, 141, 426–436. [Google Scholar] [CrossRef]
- Biernat, L.; Taube, F.; Vogeler, I.; Reinsch, T.; Kluß, C.; Loges, R. Is Organic Agriculture in Line with the EU-Nitrate Directive? On-Farm Nitrate Leaching from Organic and Conventional Arable Crop Rotations. Agric. Ecosyst. Environ. 2020, 298, 106964. [Google Scholar] [CrossRef]
- Smit, H.P.J.; Reinsch, T.; Swanepoel, P.A.; Kluß, C. Grazing under Irrigation Affects N 2 O-Emissions Substantially in South Africa. Atmosphere 2020, 11, 925. [Google Scholar] [CrossRef]
- Bracken, C.J.; Lanigan, G.J.; Richards, K.G.; Müller, C.; Tracy, S.R.; Grant, J.; Krol, D.J.; Sheridan, H.; Lynch, M.B.; Grace, C.; et al. Sward Composition and Soil Moisture Conditions Affect Nitrous Oxide Emissions and Soil Nitrogen Dynamics Following Urea-Nitrogen Application. Sci. Total Environ. 2020, 722, 137780. [Google Scholar] [CrossRef]
- Barneze, A.S.; Whitaker, J.; McNamara, N.P.; Ostle, N.J. Legumes Increase Grassland Productivity with No Effect on Nitrous Oxide Emissions. Plant Soil 2020, 446, 163–177. [Google Scholar] [CrossRef]
- Abalos, D.; De Deyn, G.B.; Kuyper, T.W.; van Groenigen, J.W. Plant Species Identity Surpasses Species Richness as a Key Driver of N2O Emissions from Grassland. Glob. Chang. Biol. 2014, 20, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Vogeler, I.; Hansen, E.M.; Thomsen, I.K.; Østergaard, H.S. Legumes in Catch Crop Mixtures: Effects on Nitrogen Retention and Availability, and Leaching Losses. J. Environ. Manag. 2019, 239, 324–332. [Google Scholar] [CrossRef]
- Bessler, H.; Oelmann, Y.; Roscher, C.; Buchmann, N.; Scherer-Lorenzen, M.; Schulze, E.-D.; Temperton, V.M.; Wilcke, W.; Engels, C. Nitrogen Uptake by Grassland Communities: Contribution of N2 Fixation, Facilitation, Complementarity, and Species Dominance. Plant Soil 2012, 358, 301–322. [Google Scholar] [CrossRef]
- Chen, S.; Lin, S.; Reinsch, T.; Loges, R.; Hasler, M.; Taube, F. Comparison of Ingrowth Core and Sequential Soil Core Methods for Estimating Belowground Net Primary Production in Grass–Clover Swards. Grass Forage Sci. 2016, 71, 515–528. [Google Scholar] [CrossRef]
- Lü, X.T.; Dijkstra, F.A.; Kong, D.L.; Wang, Z.W.; Han, X.G. Plant Nitrogen Uptake Drives Responses of Productivity to Nitrogen and Water Addition in a Grassland. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Plant Physiological Ecology, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-0-387-78340-6. [Google Scholar]
- de Klein, C.A.; Van Logtestijn, R.S. Denitrification in Grassland Soils in The Netherlands in Relation to Irrigation, N-Application Rate, Soil Water Content and Soil Temperature. Soil Biol. Biochem. 1996, 28, 231–237. [Google Scholar] [CrossRef]
- Davidson, E.A. Fluxes of nitrous oxide and nitric oxide from terrestrial ecosystems. In Microbial Production and Consumption of Greenhouse Gases: Methane, Nitrogen Oxides and Halomethanes; American Society for Microbiology: Washington, DC, USA, 1991; pp. 219–235. [Google Scholar]
- de Klein, C.A.M.; Eckard, R.J. Targeted Technologies for Nitrous Oxide Abatement from Animal Agriculture. Aust. J. Exp. Agric. 2008, 48, 14–20. [Google Scholar] [CrossRef]
- Maag, M.; Vinther, F.P. Nitrous Oxide Emission by Nitrification and Denitrification in Different Soil Types and at Different Soil Moisture Contents and Temperatures. Appl. Soil Ecol. 1996, 4, 5–14. [Google Scholar] [CrossRef]
- Granli, T.; Bøckman, O.C. Nitrous Oxide (N2O) Emissions from Soils in Warm Climates. Fertil. Res. 1995, 42, 159–163. [Google Scholar] [CrossRef]
- Flessa, H.; Dorsch, P.; Beese, F. Seasonal Variation of N2O and CH4 Fluxes in Differently Managed Arable Soils in Southern Germany. J. Geophys. Res. 1995, 100, 23115–23124. [Google Scholar] [CrossRef]
- Burton, D.L.; Beauchamp, E.G. Profile Nitrous Oxide and Carbon Dioxide Concentrations in a Soil Subject to Freezing. Soil Sci. Soc. Am. J. 1994, 58, 115–122. [Google Scholar] [CrossRef]
- Nyameasem, J.K.; Malisch, C.S.; Loges, R.; Taube, F.; Kluß, C.; Vogeler, I.; Reinsch, T. Nitrous Oxide Emission from Grazing Is Low across a Gradient of PlantS Functional Diversity and Soil Conditions-Dataset. Figshare 2021. [Google Scholar] [CrossRef]

| Experimental Design | Measurements |
|---|---|
| Experiment 1: Grassland diversity affects N2O emission from pastures under grazing stress | |
| Static chambers established on the three diverse pastures (prwc, prwrc, and prwrch); in a randomized complete block design, laid out in three replicate blocks (18 experimental units in all); each replicate plot measured 12 × 9 m; Jersey dairy cows rotationally grazed on a 3–5-week cycle depending on the pastures’ growth rate. | Gas sampling was done at least once a week to measure N2O; gas sampling started from April 2018 to January 2020, weather parameters were monitored. |
| Experiment 2: Botanical and environmental factors determine N2O emission from soils | |
| Static chambers arranged in a split-split-split plot design; the diverse grasslands as main plots, excreta treatments as split-split plots, and season as split-split-split plots, laid out in three replicated blocks (27 experimental units per each set-up); urine and dung applied once in spring, summer and autumn; each application was measured for one year; duplicate plots for soil sampling. | Gas sampling started immediately after excreta application; was done at least once a week for one year, except in December where only three samples were taken; weather parameters, soil mineral N concentration, biomass dry matter and N yields, species composition, water-filled pore space (WFPS) were monitored. |
| Experiment 3: N2O EF for cow excreta deposited on irrigated pastures is lower for systems with legume as a sole N source compared with the IPCC default. | |
| Static chambers were arranged in a split-split plot design, using the prwc swards; the season of excreta application as a split-plot and excreta treatment as a split-split plot, all laid out randomly in one block with four replicates (12 experimental units per each set-up); duplicate plots were used for soil sampling. | Gas sampling was done at least once a week for 100 days; weather parameters, mineral N concentration, biomass dry matter and WFPS were monitored; sampling duration was 100 days. |
| Parameters | Experiment 2 | Experiment 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | Spring | Summer | Autumn | |||||||
| U | D | U | D | U | D | U | D | U | D | U | D | |
| N (%) | 0.44 | 0.49 | 0.52 | 0.42 | 0.46 | 0.39 | 0.54 | 0.45 | 0.51 | 0.36 | 0.43 | 0.41 |
| C:N | 2.7 | 12.2 | 2.2 | 14.2 | 2.5 | 15.3 | 2.5 | 14.5 | 3.4 | 14.5 | 4.0 | 12.5 |
| Application rate (kg m−2) * | 2.5 | 2.2 | 2.5 | 2.2 | 2.5 | 2.2 | 2.5 | 2.2 | 2.5 | 2.2 | 2.5 | 2.2 |
| N loading rate (kg ha−1) | 458 | 449 | 542 | 385 | 479 | 358 | 563 | 413 | 529 | 326 | 451 | 380 |
| Date of application | 30.04.2018 | 11.07.2018 | 08.10.2018 | 06.05.2019 | 16.07.2019 | 09.10.2019 | ||||||
| Parameters | Grassland | Treatment | Season | SEM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| prwc | prwrc | prwrch | Control | Dung | Urine | Spring | Summer | Autumn | ||
| Experiment 2—Non-irrigated simulated grazing | ||||||||||
| Grass (%) | 74 c | 55 b | 47 a | 48 a | 60 b | 67 c | 57 | 61 | 58 | 1.77 |
| Legume (%) | 18 a | 42 c | 33 b | 41 c | 30 b | 22 a | 34 b | 29 a | 30 ab | 1.62 |
| Herb (%) | - | - | 18 | 4 | 7 | 7 | 6 | 6 | 7 | 1.14 |
| Unsown (%) | 8 b | 3 a | 2 a | 6 | 3 | 3 | 3 | 5 | 5 | 0.65 |
| DM yield (Mg ha−1) | 9.8 a | 11.7 b | 12.4 b | 11.8 | 10.7 | 11.3 | 12.7 c | 11.2 b | 9.9 a | 0.26 |
| Herbage N (g N kg DM−1) | 35.4 a | 37.3 b | 36.7 b | 33.2 a | 36.7 b | 39.5 c | 31.2 a | 34.7 b | 43.4 c | 0.74 |
| β BFN (kg ha−1 year−1) | 106 a | 279 c | 241 b | 294 c | 187 b | 146 a | 263 b | 190 a | 174 a | 13.8 |
| N input (kg N ha−1 year−1) | 416 a | 589 c | 550 b | 307 a | 597 b | 651 c | 578 c | 512 b | 465 a | 21.0 |
| N uptake (kg N ha−1 year−1) | 344 a | 432 b | 447 b | 389 a | 394 a | 440 b | 397 a | 391 a | 435 b | 10.6 |
| PRN (kg N ha−1 year−1) | 67 a | 152 b | 98 a | -87 a | 198 b | 206 b | 176 c | 116 b | 26 a | 18.9 |
| NO3 (mg N kg−1 dry soil) | 3.8 | 3.3 | 3.5 | 1.4 a | 3.5 b | 5.7 c | 2.2 a | 3.1 a | 5.4 b | 0.37 |
| NH4 (mg N kg−1 dry soil) | 3.5 | 2.9 | 3.2 | 1.5 a | 2.9 b | 5.3 c | 2.8 | 3.9 | 2.9 | 0.26 |
| α N2O (kg N ha−1 year−1) | 0.61 | 0.67 | 0.68 | 0.11 a | 0.87 b | 0.99 b | 1.01 c | 0.59b | 0.36a | 0.07 |
| N2O (kg N ha−1 100 d−1) | 0.46 | 0.57 | 0.53 | 0.03 a | 0.71 b | 0.83 b | 0.92 b | 0.48 a | 0.17 a | 0.07 |
| g N2O-N kg−1 N uptake year−1 | 1.83 | 1.73 | 1.67 | 0.29 a | 2.38 b | 2.56 b | 2.88 c | 1.55 b | 0.80 a | 0.21 |
| N2O-N EF (%) | 0.17 | 0.19 | 0.19 | - | 0.19 | 0.18 | 0.30 c | 0.16 b | 0.09 a | 0.02 |
| N2O-N EF1 (%) | 0.13 | 0.10 | 0.11 | 0.04 a | 0.14 b | 0.16 b | 0.16 b | 0.11 a | 0.08 a | 0.01 |
| Experiment 3—Irrigated simulated grazing | ||||||||||
| NO3 (mg kg−1 dry soil) | 4.64 | - | - | 1.2 a | 4.7 b | 8.1 c | 2.2 a | 3.2 a | 8.5 b | 0.77 |
| NH4 (mg kg−1 dry soil) | 4.80 | - | - | 1.8 a | 3.5 b | 9.1 c | 4.6 | 5.0 | 4.8 | 0.65 |
| α N2O (kg N ha−1 100 d−1) | 0.81 | - | - | 0.05 a | 0.81 b | 1.59 c | 0.99 | 0.78 | 0.68 | 0.15 |
| N2O-N EF (%) | 0.25 | - | - | - | 0.21 a | 0.29 b | 0.24 | 0.28 | 0.23 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyameasem, J.K.; Malisch, C.S.; Loges, R.; Taube, F.; Kluß, C.; Vogeler, I.; Reinsch, T. Nitrous Oxide Emission from Grazing Is Low across a Gradient of Plant Functional Diversity and Soil Conditions. Atmosphere 2021, 12, 223. https://doi.org/10.3390/atmos12020223
Nyameasem JK, Malisch CS, Loges R, Taube F, Kluß C, Vogeler I, Reinsch T. Nitrous Oxide Emission from Grazing Is Low across a Gradient of Plant Functional Diversity and Soil Conditions. Atmosphere. 2021; 12(2):223. https://doi.org/10.3390/atmos12020223
Chicago/Turabian StyleNyameasem, John Kormla, Carsten S. Malisch, Ralf Loges, Friedhelm Taube, Christof Kluß, Iris Vogeler, and Thorsten Reinsch. 2021. "Nitrous Oxide Emission from Grazing Is Low across a Gradient of Plant Functional Diversity and Soil Conditions" Atmosphere 12, no. 2: 223. https://doi.org/10.3390/atmos12020223
APA StyleNyameasem, J. K., Malisch, C. S., Loges, R., Taube, F., Kluß, C., Vogeler, I., & Reinsch, T. (2021). Nitrous Oxide Emission from Grazing Is Low across a Gradient of Plant Functional Diversity and Soil Conditions. Atmosphere, 12(2), 223. https://doi.org/10.3390/atmos12020223








