Transmission of SARS-CoV-2 Indoor and Outdoor Environments
Abstract
:1. Introduction
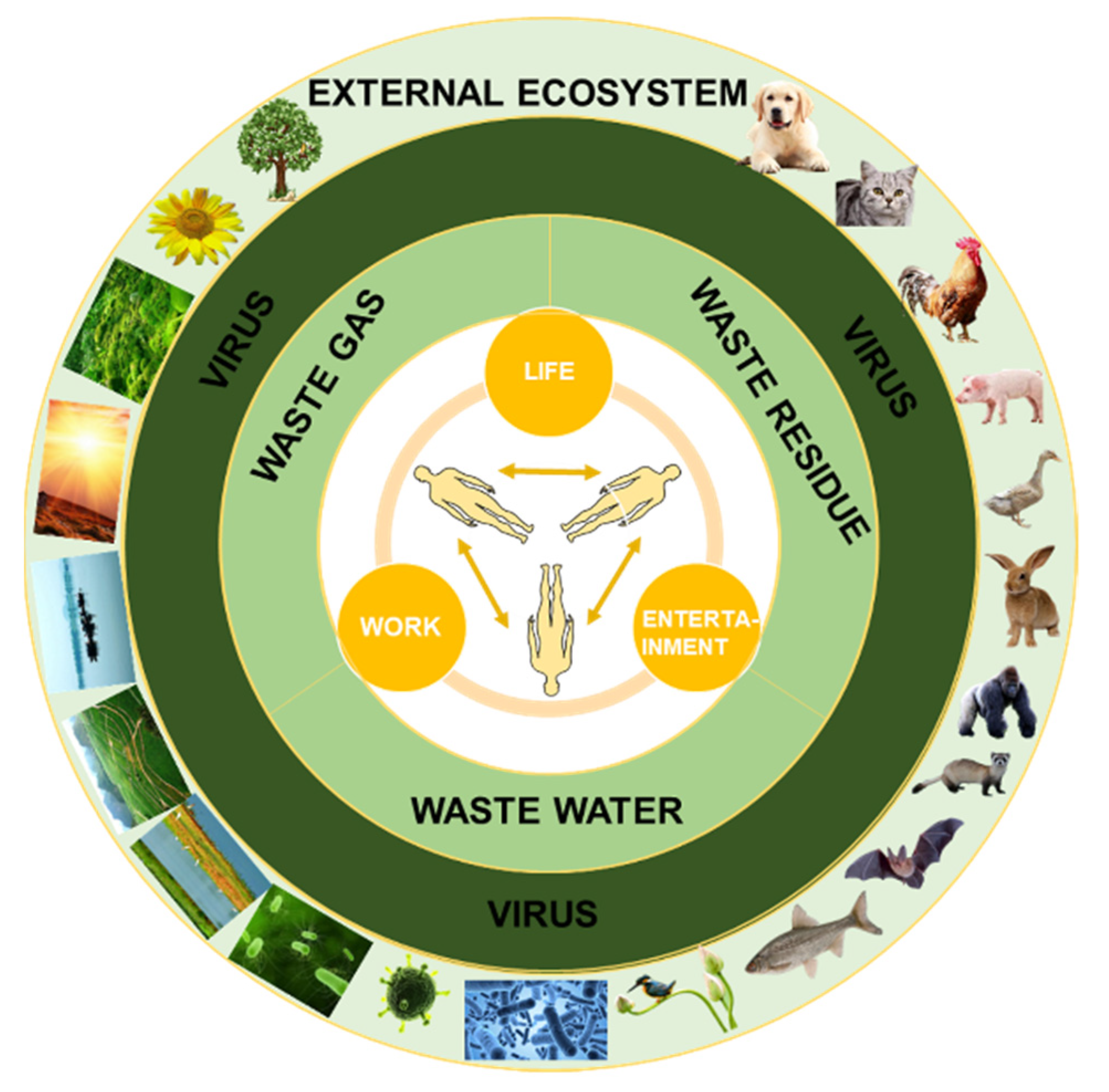
2. The Transmission Routes of the Virus
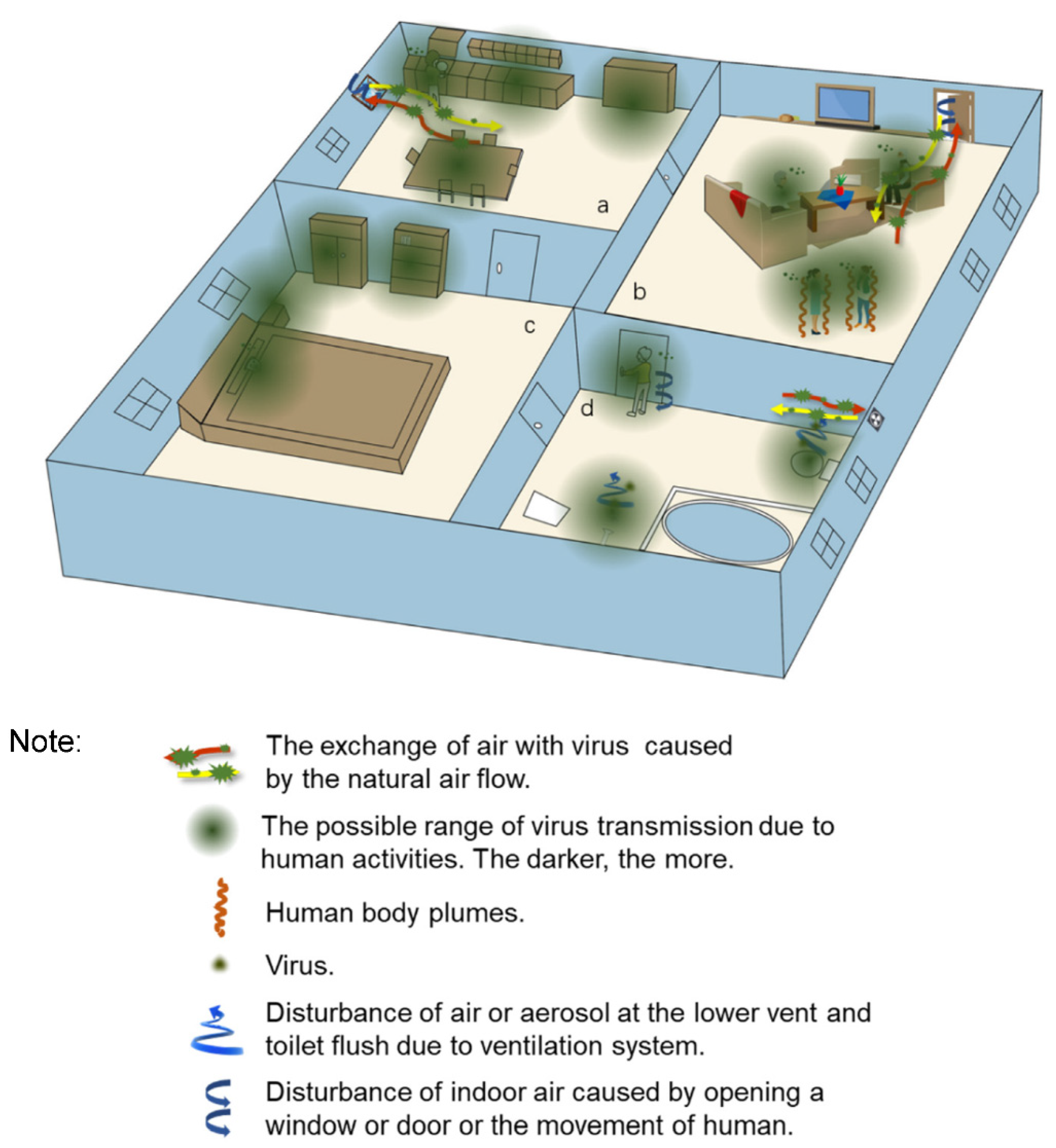
2.1. Transmission Routes of the Virus in the Indoor Environment
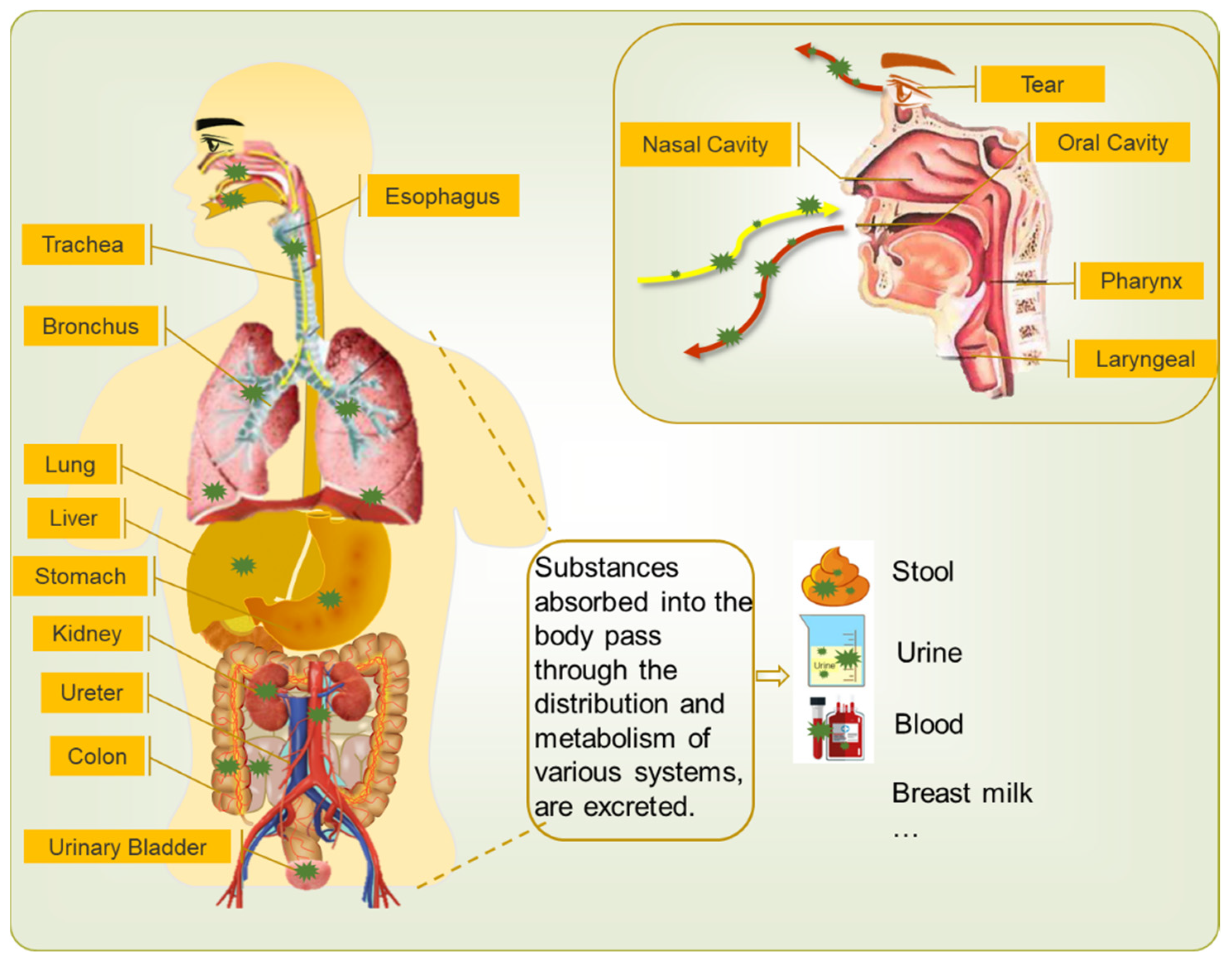
2.1.1. Virus Shedding from Hosts
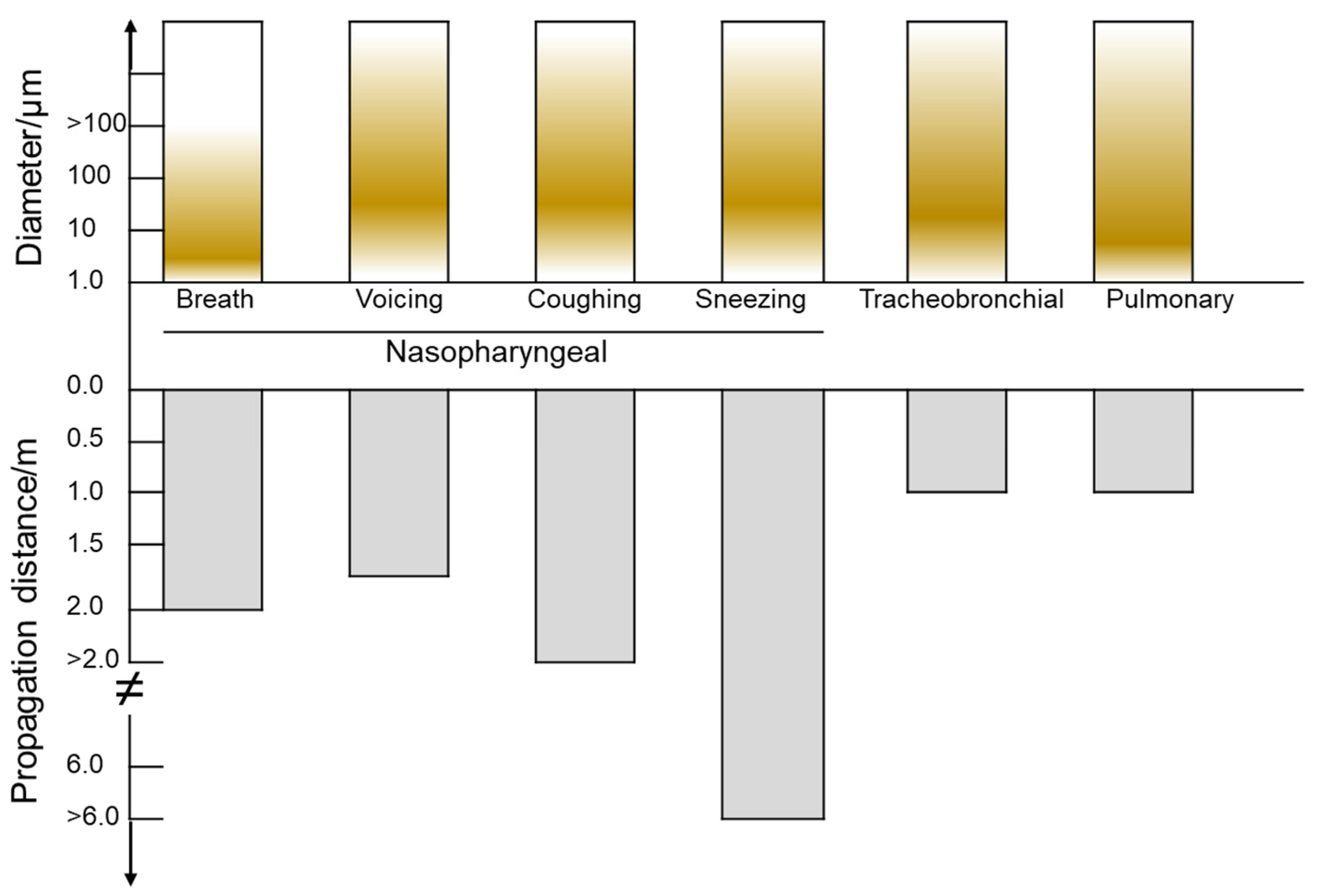
2.1.2. The Spread of Droplets Carrying Virus in the Indoor Environment
2.1.3. The Present Form and Survival Time of Virus on Solid Surfaces
Persistence of Virus on Nonporous Surfaces
Persistence of Virus on Porous Surfaces
2.2. Transmission Routes of Virus in the Outdoor Environment
2.3. The Impact of Environmental Factors on the Stability and Transmission of Virus
2.4. The Emergence and Spread of SARS-CoV-2 Variants of Concern
3. The Existence Form and Survival Time of Virus in Different Environments
3.1. The Existence Form and Survival Time of Virus in Water
3.2. Airborne Form and Survival Time of Virus
4. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rehman, S.U.; Shafique, L.; Ihsan, A.; Liu, Q. Evolutionary Trajectory for the Emergence of Novel Coronavirus SARS-CoV-2. Pathogens 2020, 9, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, L.; Horve, P.F.; Coil, D.A.; Fretz, M.; Eisen, J.A.; Van Den Wymelenberg, K. 2019 Novel Coronavirus (COVID-19) Pandemic: Built Environment Considerations To Reduce Transmission. mSystems 2020, 5, e00245-20. [Google Scholar] [CrossRef] [Green Version]
- Acuti Martellucci, C.; Flacco, M.E.; Cappadona, R.; Bravi, F.; Mantovani, L.; Manzoli, L. SARS-CoV-2 pandemic: An overview. Adv. Biol. Regul. 2020, 77, 100736. [Google Scholar] [CrossRef]
- Faridi, S.; Niazi, S.; Sadeghi, K.; Naddafi, K.; Yavarian, J.; Shamsipour, M.; Jandaghi, N.Z.S.; Sadeghniiat, K.; Nabizadeh, R.; Yunesian, M.; et al. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total Environ. 2020, 725, 138401. [Google Scholar] [CrossRef]
- Woolhouse, M.E.J.; Gowtage-Sequeria, S. Host Range and Emerging and Reemerging Pathogens. Emerg. Infect. Dis. 2005, 11, 1842. [Google Scholar] [CrossRef]
- Kan, B.; Wang, M.; Jing, H.; Xu, H.; Jiang, X.; Yan, M.; Liang, W.; Zheng, H.; Wan, K.; Liu, Q.; et al. Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. J. Virol. 2005, 79, 11892–11900. [Google Scholar] [CrossRef] [Green Version]
- Ji, W.; Wang, W.; Zhao, X.; Zai, J.; Li, X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020, 92, 433–440. [Google Scholar] [CrossRef]
- Kayali, G.; Peiris, M. A more detailed picture of the epidemiology of Middle East respiratory syndrome coronavirus. Lancet Infect. Dis. 2015, 15, 495–497. [Google Scholar] [CrossRef] [Green Version]
- Wolfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Muller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadman, M.; Couzin-Frankel, J.; Kaiser, J.; Matacic, C. How Does Coronavirus kill? Clinicians Trace a Ferocious Rampage through the Body, from Brain to Toes. Available online: https://www.science.org/content/article/how-does-coronavirus-kill-clinicians-trace-ferocious-rampage-through-body-brain-toes (accessed on 17 April 2020).
- Belhadjer, Z.; Méot, M.; Bajolle, F.; Khraiche, D.; Legendre, A.; Abakka, S.; Auriau, J.; Grimaud, M.; Oualha, M.; Beghetti, M.; et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation 2020, 142, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.; Pepper, G.; Wener, M.H.; Fink, S.L.; Morishima, C.; Chaudhary, A.; Jerome, K.R.; Mathias, P.C.; Greninger, A.L. Performance Characteristics of the Abbott Architect SARS-CoV-2 IgG Assay and Seroprevalence in Boise, Idaho. J. Clin. Microbiol. 2020, 58, e00941-20. [Google Scholar] [CrossRef]
- Lei, Z.; Cao, H.; Jie, Y.; Huang, Z.; Guo, X.; Chen, J.; Peng, L.; Cao, H.; Dai, X.; Liu, J.; et al. A cross-sectional comparison of epidemiological and clinical features of patients with coronavirus disease (COVID-19) in Wuhan and outside Wuhan, China. Travel. Med. Infect. Dis. 2020, 35, 101664. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Xu, S.B.; Lin, Y.X.; Tian, D.; Zhu, Z.Q.; Dai, F.H.; Wu, F.; Song, Z.G.; Huang, W.; Chen, J.; et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin. Med. J.-Peking 2020, 133, 1039–1043. [Google Scholar] [CrossRef]
- Peng, L.; Liu, J.; Xu, W.; Luo, Q.; Chen, D.; Lei, Z.; Huang, Z.; Li, X.; Deng, K.; Lin, B.; et al. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y. Airborne spread of infectious agents in the indoor environment. Am. J. Infect. Control. 2016, 44 (Suppl. 9), S102–S108. [Google Scholar] [CrossRef]
- Corman, V.M.; Albarrak, A.M.; Omrani, A.S.; Albarrak, M.M.; Farah, M.E.; Almasri, M.; Muth, D.; Sieberg, A.; Meyer, B.; Assiri, A.M.; et al. Viral Shedding and Antibody Response in 37 Patients With Middle East Respiratory Syndrome Coronavirus Infection. Clin. Infect. Dis. 2016, 62, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Colavita, F.; Lapa, D.; Carletti, F.; Lalle, E.; Bordi, L.; Marsella, P.; Nicastri, E.; Bevilacqua, N.; Giancola, M.L.; Corpolongo, A.; et al. SARS-CoV-2 Isolation From Ocular Secretions of a Patient With COVID-19 in Italy With Prolonged Viral RNA Detection. Ann. Intern. Med. 2020, 173, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A.; et al. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef]
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.T.; Wong, M.S.Y.; Marimuthu, K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA 2020, 323, 1610–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, B.E.; Ong, S.W.X.; Kalimuddin, S.; Low, J.G.; Tan, S.Y.; Loh, J.S.; Ng, O.T.; Marimuthu, K.; Ang, L.W.; Mak, T.M.; et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA-J. Am. Med. Assoc. 2020, 323, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Cheng, P.K.; Wong, D.A.; Tong, L.K.; Ip, S.-M.; Lo, A.C.; Lau, C.-S.; Yeung, E.Y.; Lim, W.W. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet 2004, 363, 1699–1700. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xu, Y.; Gao, R. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843. [Google Scholar] [CrossRef] [Green Version]
- Seah, I.; Agrawal, R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals. Ocul. Immunol. Inflamm. 2020, 28, 391–395. [Google Scholar] [CrossRef]
- Yan, A. Chinese Expert Who Came down with Wuhan Coronavirus after Saying It was Controllable Thinks He Was Infected through His Eyes. Available online: https://www.scmp.com/news/china/article/3047394/chinese-expert-who-came-down-wuhan-coronavirus-after-saying-it-was (accessed on 23 January 2020).
- Kumar, K.; Prakash, A.A.; Gangasagara, S.B.; Rathod, B.L.S.; Ravi, K.; Rangaiah, A.; Shankar, S.M.; Basawarajappa, S.G.; Bhushan, S.; Neeraja, T.G.; et al. Presence of viral RNA of SARS-CoV-2 in conjunctival swab specimens of COVID-19 patients. Indian J. Ophthalmol. 2020, 68, 1015–1017. [Google Scholar] [CrossRef] [PubMed]
- Loon, S.C.; Teoh, S.C.B.; Oon, L.L.E.; Se-Thoe, S.-Y.; Ling, A.-E.; Leo, Y.-S. The severe acute respiratory syndrome coronavirus in tears. Br. J. Ophthalmol. 2004, 88, 861–863. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Wang, J.; Mo, Y.; Duan, W.; Xiang, G.; Yi, M.; Bao, L.; Shi, Y. Unlikely SARS-CoV-2 vertical transmission from mother to child: A case report. J. Infect. Public Health 2020, 13, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Alzamora, M.C.; Paredes, T.; Caceres, D.; Webb, C.M.; Valdez, L.M.; La Rosa, M. Severe COVID-19 during Pregnancy and Possible Vertical Transmission. Am. J. Perinatol. 2020, 37, 861–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Liu, C.; Dong, L.; Zhang, C.; Chen, Y.; Liu, J.; Zhang, C.; Duan, C.; Zhang, H.; Mol, B.W.; et al. Coronavirus disease 2019 among pregnant Chinese women: Case series data on the safety of vaginal birth and breastfeeding. BJOG 2020, 127, 1109–1115. [Google Scholar] [CrossRef]
- Davanzo, R.; Moro, G.; Sandri, F.; Agosti, M.; Moretti, C.; Mosca, F. Breastfeeding and coronavirus disease-2019: Ad interim indications of the Italian Society of Neonatology endorsed by the Union of European Neonatal & Perinatal Societies. Matern. Child. Nutr. 2020, 16, e13010. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, D.W.; Stagno, S. Can SARS-CoV-2 Infection Be Acquired In Utero?: More Definitive Evidence Is Needed. JAMA 2020, 323, 1788–1789. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, C.; Fan, J.; Tang, Y.; Deng, Q.; Zhang, W.; Long, X. Antibodies in Infants Born to Mothers with COVID-19 Pneumonia. JAMA 2020, 323, 1848–1849. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Li, W.B.; Zhou, Z.X.; Liu, S.Y.; Rong, Z.H. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front. Med-Prc. 2020, 14, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Tian, J.; He, S.; Zhu, C.; Wang, J.; Liu, C.; Yang, J. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA 2020, 323, 1846–1848. [Google Scholar] [CrossRef] [Green Version]
- Detection Dogs as a Help in the Detection of COVID-19 Can the Dog Alert on COVID-19 Positive Persons by Sniffing Axillary Sweat Samples? Proof-of-Concept Study. Available online: https://www.biorxiv.org/content/10.1101/2020.06.03.132134v1.full (accessed on 25 November 2021).
- Holtmann, N.; Edimiris, P.; Andree, M.; Doehmen, C.; Baston-Buest, D.; Adams, O.; Kruessel, J.S.; Bielfeld, A.P. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril 2020, 114, 233–238. [Google Scholar] [CrossRef]
- Paoli, D.; Pallotti, F.; Colangelo, S.; Basilico, F.; Mazzuti, L.; Turriziani, O.; Antonelli, G.; Lenzi, A.; Lombardo, F. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J. Endocrinol. Invest. 2020, 43, 1819–1822. [Google Scholar] [CrossRef]
- Soetikno, R.; Teoh, A.Y.B.; Kaltenbach, T.; Lau, J.Y.W.; Asokkumar, R.; Cabral-Prodigalidad, P.; Shergill, A. Considerations in performing endoscopy during the COVID-19 pandemic. Gastrointest. Endosc. 2020, 92, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, S.R.; O’Shea, T.J.; Oko, L.M.; Holmes, K.V. Detection of Group 1 Coronaviruses in Bats in North America. Emerg. Infect. Dis. 2007, 13, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; Zhao, Y.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef] [Green Version]
- Cole, E.C.; Cook, C.E. Characterization of infectious aerosols in health care facilities An aid to effective engineering controls and preventive strategies. Am. J. Infect. Control 1998, 26, 453–464. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Chwang, A.T.Y.; Ho, P.L.; Seto, W.H. How far droplets can move in indoor environments – revisiting the Wells evaporation–falling curve. INDOOR AIR 2007, 17, 211–225. [Google Scholar] [CrossRef]
- Roy, C.J.; Milton, D.K. Airborne transmission of communicable infection--the elusive pathway. N. Engl. J. Med. 2004, 350, 1710–1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-Allendes, A.; Contreras-Ruston, F.; Cantor-Cutiva, L.C.; Codino, J.; Guzman, M.; Malebran, C.; Manzano, C.; Pavez, A.; Vaiano, T.; Wilder, F.; et al. Voice Therapy in the Context of the COVID-19 Pandemic: Guidelines for Clinical Practice. J. Voice 2020, 35, 717–727. [Google Scholar] [CrossRef]
- Mizumoto, K.; Chowell, G. Transmission potential of the novel coronavirus (COVID-19) onboard the diamond Princess Cruises Ship, 2020. Infect. Dis. Model. 2020, 5, 264–270. [Google Scholar] [CrossRef]
- Chowell, G.; Abdirizak, F.; Lee, S.; Lee, J.; Jung, E.; Nishiura, H.; Viboud, C. Transmission characteristics of MERS and SARS in the healthcare setting: A comparative study. BMC Med. 2015, 13, 210. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Yang, J.; Xue, J.; Zhu, P.; Liu, L.; Li, S. Detection of SARS-CoV-2 RNA residue on object surfaces in nucleic acid testing laboratory using droplet digital PCR. Sci. Total Environ. 2020, 742, 140370. [Google Scholar] [CrossRef]
- Olsen, S.J.; Chang, H.-L.; Cheung, T.Y.-Y.; Tang, A.F.-Y.; Fisk, T.L.; Ooi, S.P.-L.; Kuo, H.-W.; Jiang, D.D.-S.; Chen, K.-T.; Lando, J.; et al. Transmission of the Severe Acute Respiratory Syndrome on Aircraft. N. Engl. J. Med. 2003, 394, 2416–2422. [Google Scholar] [CrossRef]
- Conte, M.; Feltracco, M.; Chirizzi, D.; Trabucco, S.; Dinoi, A.; Gregoris, E.; Barbaro, E.; La Bella, G.; Ciccarese, G.; Belosi, F.; et al. Airborne concentrations of SARS-CoV-2 in indoor community environments in Italy. Environ. Sci. Pollut. Res. Int. 2021. [Google Scholar] [CrossRef] [PubMed]
- Correia, G.; Rodrigues, L.; Gameiro da Silva, M.; Goncalves, T. Airborne route and bad use of ventilation systems as non-negligible factors in SARS-CoV-2 transmission. Med Hypotheses 2020, 141, 109781. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.T.S.; Li, Y.; Wong, T.W.; Tam, W.; Chan, A.T.; Lee, J.H.W.; Leung, D.Y.C.; Ho, T. Evidence of Airborne Transmission of the Severe Acute Respiratory Syndrome Virus. N. Engl. J. Med. 2004, 350, 1731–1739. [Google Scholar] [CrossRef] [Green Version]
- Bhowmick, G.D.; Dhar, D.; Nath, D.; Ghangrekar, M.M.; Banerjee, R.; Das, S.; Chatterjee, J. Coronavirus disease 2019 (COVID-19) outbreak some serious consequences with urban and rural water cycle. npj Clean. Water 2020, 3. [Google Scholar] [CrossRef]
- Rim, D.; Novoselac, A. Transport of particulate and gaseous pollutants in the vicinity of a human body. Build. Environ. 2009, 44, 1840–1849. [Google Scholar] [CrossRef]
- Xu, R.; Cui, B.; Duan, X.; Zhang, P.; Zhou, X.; Yuan, Q. Saliva: Potential diagnostic value and transmission of 2019-nCoV. Int. J. Oral Sci. 2020, 12, 11. [Google Scholar] [CrossRef]
- Tang, J.W. The effect of environmental parameters on the survival of airborne infectious agents. J. R. Soc. Interface 2009, 6 (Suppl. 6), S737–S746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of HCoV-19 (SARS-CoV-2) compared to SARS-CoV-1. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Sizun, J.; Yu, M.W.; Talbot, P.J. Survival of human coronaviruses 229E and OC43 in suspension and after drying onsurfaces: A possible source ofhospital-acquired infections. J. Hosp. Infect. 2000, 46, 55–60. [Google Scholar] [CrossRef]
- Lai, M.Y.; Cheng, P.K.; Lim, W.W. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 2005, 41, e67–e71. [Google Scholar] [CrossRef] [Green Version]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials. mBio 2015, 6, e01697-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabenau, H.F.; Cinatl, J.; Morgenstern, B.; Bauer, G.; Preiser, W.; Doerr, H.W. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005, 194, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, M.; Sattar, S.; Adegbunrin, O.; Tetro, J. Environmental survival and microbicide inactivation of coronaviruses. In Coronaviruses with Special Emphasis on First Insights Concerning SARS; Springer: Birkhäuser, Basel, 2006; pp. 201–212. [Google Scholar] [CrossRef] [Green Version]
- Michels, H.; Wilks, S.A.; Noyce, J.C.; Keevil, C.W. Copper Alloys for Human Infectious Disease Control. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.559.9650&rep=rep1&type=pdf (accessed on 25 November 2021).
- Warnes, S.L.; Keevil, C. Inactivation of Norovirus on Dry Copper Alloy Surfaces. PLoS ONE 2013, 8, e75017. [Google Scholar]
- Liu, W.; Tang, F.; Fontanet, A.; Zhan, L.; Zhao, Q.-M.; Zhang, P.-H.; Wu, X.-M.; Zuo, S.-Q.; Baril, L.; Vabret, A.; et al. Long-term SARS Coronavirus Excretion from Patient Cohort, China. Emerg. Infect. Dis. 2004, 10, 1841–1843. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.H.; Peiris, J.S.; Lam, S.Y.; Poon, L.L.; Yuen, K.Y.; Seto, W.H. The Effects of Temperature and Relative Humidity on the Viability of the SARS Coronavirus. Adv. Virol. 2011, 2011, 734690. [Google Scholar] [CrossRef]
- Casanova, L.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Survival of surrogate coronaviruses in water. Water Res. 2009, 43, 1893–1898. [Google Scholar] [CrossRef]
- Mallapaty, S. How sewage could reveal true scale of coronavirus outbreak. Nature 2020, 580, 176–177. [Google Scholar] [CrossRef] [Green Version]
- Bibby, K.; Peccia, J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environ. Sci. Technol. 2013, 47, 1945–1951. [Google Scholar] [CrossRef] [Green Version]
- Franklin, A.B.; Bevins, S.N. Spillover of SARS-CoV-2 into novel wild hosts in North America: A conceptual model for perpetuation of the pathogen. Sci. Total Environ. 2020, 733, 139358. [Google Scholar] [CrossRef] [PubMed]
- Korine, C.; Adams, R.; Russo, D.; Fisher-Phelps, M.; Jacobs, D. Bats and Water: Anthropogenic Alterations Threaten Global Bat Populations. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Springer: Cham, Switzerland, 2016; pp. 215–241. [Google Scholar] [CrossRef] [Green Version]
- Ragazzi, M.; Rada, E.C.; Schiavon, M. Municipal solid waste management during the SARS-COV-2 outbreak and lockdown ease: Lessons from Italy. Sci. Total Environ. 2020, 745, 141159. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Gao, C.X.; Cowling, B.J.; Koh, G.C.; Chu, D.; Heilbronn, C.; Lloyd, B.; Pantelic, J.; Nicolle, A.D.; Klettner, C.A.; et al. Absence of detectable influenza RNA transmitted via aerosol during various human respiratory activities--experiments from Singapore and Hong Kong. PLoS ONE 2014, 9, e107338. [Google Scholar]
- Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19 (accessed on 25 November 2021).
- Johnson, G.R.; Morawska, L.; Ristovski, Z.D.; Hargreaves, M.; Mengersen, K.; Chao, C.Y.H.; Wan, M.P.; Li, Y.; Xie, X.; Katoshevski, D.; et al. Modality of human expired aerosol size distributions. J. Aerosol Sci. 2011, 42, 839–851. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. J. Emerg. Med. 2020, 58, 711–712. [Google Scholar] [CrossRef] [PubMed]
- Marr, L.C.; Tang, J.W.; Van Mullekom, J.; Lakdawala, S.S. Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J. R. Soc. Interface 2019, 16, 20180298. [Google Scholar] [CrossRef]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza Virus Transmission Is Dependent on Relative Humidity and Temperature. PLoS Pathog. 2007, 3, e1470–e1476. [Google Scholar] [CrossRef]
- Moriyama, M.; Ichinohe, T. High ambient temperature dampens adaptive immune responses to influenza A virus infection. Proc. Natl. Acad. Sci. USA 2019, 116, 3118–3125. [Google Scholar] [CrossRef] [Green Version]
- Shaman, J.; Pitzer, V.E.; Viboud, C.; Grenfell, B.T.; Lipsitch, M. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol. 2010, 8, e1000316. [Google Scholar] [CrossRef]
- Kudo, E.; Song, E.; Yockey, L.J.; Rakib, T.; Wong, P.W.; Homer, R.J.; Iwasaki, A. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc. Natl. Acad. Sci. USA 2019, 116, 10905–10910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otter, J.A.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.D.; Weber, D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2016, 92, 235–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaman, J.; Kohn, M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc. Natl. Acad. Sci. USA 2009, 106, 3243–3248. [Google Scholar] [CrossRef] [Green Version]
- Peci, A.; Winter, A.L.; Li, Y.; Gnaneshan, S.; Liu, J.; Mubareka, S.; Gubbay, J.B. Effects of Absolute Humidity, Relative Humidity, Temperature, and Wind Speed on Influenza Activity in Toronto, Ontario, Canada. Appl. Environ. Microbiol. 2019, 85, e02426-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, A.; Yin, J. Short-Term Effects of Ambient Ozone, PM2.5, and Meteorological Factors on COVID-19 Confirmed Cases and Deaths in Queens, New York. Int. J. Environ. Res. Public Health 2020, 17, 4047. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.T.; Wu, P.; Cauchemez, S.; He, D.; Fang, V.J.; Cowling, B.J.; Tian, L. Ambient ozone and influenza transmissibility in Hong Kong. Eur. Respir. J. 2018, 51, 1800369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chennakesavulu, K.; Reddy, G.R. The effect of latitude and PM2.5 on spreading of SARS-CoV-2 in tropical and temperate zone countries. Environ Pollut 2020, 266, 115176. [Google Scholar] [CrossRef]
- Gauderman, W.J.; Avol, E.; Gilliland, F.; Vora, H.; Thomas, D.; Berhane, K.; McConnell, R.; Kuenzli, N.; Lurmann, F.; Rappaport, E.; et al. The Effect of Air Pollution on Lung Development from 10 to 18 Years of Age. N. Engl. J. Med. 2004, 351, 1057–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Liu, H.; Wu, Y.; Si, Y.; Song, J.; Cao, Y.; Li, M.; Wu, Y.; Wang, X.; Chen, L.; et al. Association between ambient fine particulate pollution and hospital admissions for cause specific cardiovascular disease: Time series study in 184 major Chinese cities. BMJ 2019, 367, l6572. [Google Scholar] [CrossRef] [Green Version]
- Takagi, H.; Kuno, T.; Yokoyama, Y.; Ueyama, H.; Matsushiro, T.; Hari, Y.; Ando, T. Higher Temperature, Pressure, and Ultraviolet Are Associated With Less COVID-19 Prevalence: Meta-Regression of Japanese Prefectural Data. Asia Pac. J. Public Health 2020, 38, 520–522. [Google Scholar] [CrossRef]
- Tosepu, R.; Gunawan, J.; Effendy, D.S.; Ahmad, O.A.I.; Lestari, H.; Bahar, H.; Asfian, P. Correlation between weather and Covid-19 pandemic in Jakarta, Indonesia. Sci. Total Environ. 2020, 725, 138436. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.; Huang, H.; Avellan-Llaguno, R.D.; Lazo, M.M.L.; Gaggero, A.; Soto-Rifo, R.; Patino, L.; Valencia-Avellan, M.; Diringer, B.; et al. Meteorological impact on the COVID-19 pandemic: A study across eight severely affected regions in South America. Sci. Total Environ. 2020, 744, 140881. [Google Scholar] [CrossRef]
- Raman, R.; Patel, K.J.; Ranjan, K. COVID-19: Unmasking Emerging SARS-CoV-2 Variants, Vaccines and Therapeutic Strategies. Biomolecules 2021, 11, 993. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827.e9. [Google Scholar] [CrossRef] [PubMed]
- Rochelle, P.; Walensky, M.; Henry, T.; Walke, M.; Anthony, S.; Fauci, M. SARS-CoV-2 Variants of Concern in the United States—Challenges and Opportunities. JAMA. 2021, 325. [Google Scholar] [CrossRef]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Gupta, R.K.; de Oliveira, T.; Kosakovsky Pond, S.L.; Fera, D.; Shafer, R.W. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021, 22, 757–773. [Google Scholar] [CrossRef]
- Mwenda, M.; Saasa, N.; Sinyange, N.; Busby, G.; Chipimo, P.J.; Hendry, J.; Kapona, O.; Samuel Yingst, D.; Jonas, Z.; Hines, M.; et al. Detection of B.1.351 SARS-CoV-2 Variant Strain—Zambia, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 280–282. [Google Scholar] [CrossRef]
- Horby, P.; Huntley, C.; Davies, N.; Edmunds, J.; Ferguson, N.; Medley, G.; Semple, C. NERVTAG_note_on_B.1.1.7_severity_for_SAGE_77__1_. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/961037/NERVTAG_note_on_B.1.1.7_severity_for_SAGE_77__1_.pdf (accessed on 25 November 2021).
- Romano, C.M.; Felix, A.C.; Paula, A.V.; Jesus, J.G.; Andrade, P.S.; Candido, D.; Oliveira, F.M.; Ribeiro, A.C.; Silva, F.C.D.; Inemami, M.; et al. SARS-CoV-2 reinfection caused by the P.1 lineage in Araraquara city, Sao Paulo State, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e36. [Google Scholar] [CrossRef]
- Volz, E.; Mishra, S.; Chand, M.; Barrett, J.C.; Johnson, R.; Geidelberg, L.; Hinsley, W.R.; Laydon, D.J.; Dabrera, G.; O’Toole, A.; et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature 2021, 593, 266–269. [Google Scholar] [CrossRef]
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; Candido, D.D.S.; Mishra, S.; Crispim, M.A.E.; Sales, F.C.; Hawryluk, I.; McCrone, J.T.; et al. Genomics and epidemiology of a novel SARS-CoV-2 lineage in Manaus, Brazil. medRxiv 2021. [Google Scholar] [CrossRef]
- Hamza, I.A.; Bibby, K. Critical issues in application of molecular methods to environmental virology. J. Virol. Methods 2019, 266, 11–24. [Google Scholar] [CrossRef]
- Haramoto, E.; Kitajima, M.; Hata, A.; Torrey, J.R.; Masago, Y.; Sano, D.; Katayama, H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018, 135, 168–186. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Fratini, M.; della Libera, S.; Iaconelli, M.; Muscillo, M. Emerging and potentially emerging viruses in water environments. Ann. Ist. Super Sanita 2012, 48, 397–406. [Google Scholar] [CrossRef]
- Moreira, N.A.; Bondelind, M. Safe drinking water and waterborne outbreaks. J. Water Health 2017, 15, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Rusiñol, M.; Gironés, R. Summary of Excreted and Waterborne Viruses. 2018. Available online: https://www.waterpathogens.org/book/summary-of-excreted-and-waterborne-viruses (accessed on 25 November 2021).
- Cotruvo, J. 2017 WHO Guidelines for Drinking Water Quality: First Addendum to the Fourth Edition. J. Am. Water Work. Assoc. 2017, 109, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Carducci, A.; Federigi, I.; Liu, D.; Thompson, J.R.; Verani, M. Making Waves: Coronavirus detection, presence and persistence in the water environment: State of the art and knowledge needs for public health. Water Res 2020, 179, 115907. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.M.; Zhao, X.S.; Wen, R.F.; Huang, J.J.; Pi, G.H.; Zhang, S.X.; Han, J.; Bi, S.L.; Ruan, L.; Dong, X.P.; et al. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed. Environ. Sci. 2003, 16, 246–255. [Google Scholar]
- Wang, X.W.; Li, J.S.; Jin, M.; Zhen, B.; Kong, Q.X.; Song, N.; Xiao, W.J.; Yin, J.; Wei, W.; Wang, G.J.; et al. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods 2005, 126, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Wurtzer, S.; Marechal, V.; Mouchel, J.-M.; Moulin, L. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. MedRxiv 2020. [Google Scholar] [CrossRef]
- Calvet, G.; Aguiar, R.; Melo, A.; Sampaio, S.; de Filippis, I.; Fabri, A.; Araujo, E.; Sequeira, P.; Mendonça, M.; Oliveira, L.; et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect. Dis. 2016, 6, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Guery, B.; Poissy, J.; Mansouf, L.; Séjourné, C.; Ettahar, N.; Lemaire, X.; Vuotto, F.; Goffard, A.; Behillil, S.; Enouf, V.; et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: A report of nosocomial transmission. Lancet 2013, 381, 2265–2272. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.-Y.; Yan, J.-Y.; Qu, J.-M. Interpretations of “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7)”. Chin. Med. J. 2020, 133, 1. [Google Scholar] [CrossRef]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.; Yang, L.; et al. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiology 2020, 295, 200370. [Google Scholar] [CrossRef] [Green Version]
- Bouguezzi, A. COVID-19: Special Precautions in Dentistry. Open Access J. Biomed. Sci. 2020, 2. [Google Scholar] [CrossRef]
- Fennelly, K.P. Particle sizes of infectious aerosols: Implications for infection control. Lancet Respir. Med. 2020, 8, 914–924. [Google Scholar] [CrossRef]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L.; Health Care Infection Control Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am. J. Infect. Control 2007, 35, S65–S164. [Google Scholar] [CrossRef]
- Dutra, F. Airborne Contagion and Air Hygiene: An Ecological Study of Droplet Infections. Am. J. Clin. Pathol. 1955, 25, 1301. [Google Scholar] [CrossRef]
- Fabian, P.; McDevitt, J.; DeHaan, W.; Fung, R.; Cowling, B.; Chan, K.-H.; Leung, G.; Milton, D. Influenza Virus in Human Exhaled Breath: An Observational Study. PLoS ONE 2008, 3, e2691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gralton, J.; Tovey, E.; McLaws, M.L.; Rawlinson, W. The role of particle size in aerosolised pathogen transmission: A review. J. Infect. 2011, 62, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, T.; Valway, S.; Ihle, W.; Onorato, I.; Castro, K. Transmission of Multidrug-Resistant Mycobacterium tuberculosis during a Long Airplane Flight. N. Engl. J. Med. 1996, 334, 933–938. [Google Scholar] [CrossRef]
- Macintyre, C.; Carnie, J.; Randall, M. Risk of transmission of tuberculosis among inmates of an Australian prison. Epidemiol. Infect. 2000, 123, 445–450. [Google Scholar] [CrossRef]
- Mandalakas, A.; Kirchner, H.L.; Lombard, C.; Walzl, G.; Grewal, H.; Gie, R.; Hesseling, A. Well-quantified tuberculosis exposure is a reliable surrogate measure of tuberculosis infection. Int. J. Tuberc. Lung Dis. 2012, 16, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Reese, A.L.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; et al. Increasing Temperature and Relative Humidity Accelerates Inactivation of SARS-CoV-2 on Surfaces. mSphere 2020, 5, e00441-20. [Google Scholar] [CrossRef]
- Kormuth, K.; Lin, K.; Prussin Ii, A.; Vejerano, E.; Tiwari, A.; Cox, S.; Myerburg, M.; Lakdawala, S.; Marr, L. Influenza Virus Infectivity Is Retained in Aerosols and Droplets Independent of Relative Humidity. J. Infect. Dis. 2018, 218, 739–747. [Google Scholar] [CrossRef]
| Indoor Environment | Furniture/Equipment | Texture | Virus Survival Time (d) | Influence Factor | Reference |
|---|---|---|---|---|---|
| House | sofa | leather, fabric, wood | 1–2 (woodiness and fabric) | temperature and humidity | [57] |
| chair | leather, wood, steal, fabric, wood-plastic | 3–4 (stainless steel) 1–2 (woodiness) | temperature and humidity | [57,58] | |
| table | artificial marble, solid wood, glass, | 1–2 (woodiness) 2 (glass) | temperature and humidity | [57] | |
| bed/mattress | sponge, latex | 3 (sponge) 6 (latex) | temperature and humidity | [59] | |
| carpet | wool, propylene, nylon, hemp, and mixed fabric | 1 (cotton) | temperature and humidity | [60] | |
| cup | glass, porcelain, stainless steel, plastic | 3–4 (plastic and stainless steel) 5 (porcelain) | temperature and humidity | [57,58,61] | |
| toothbrush | willow, bristle, artificial wool brush | 4–6 | temperature and humidity | [61,62] | |
| Lab | centrifugal machine | stainless steel | 3–4 (stainless steel) | temperature and humidity | [58] |
| electronic balance | stainless steel | 3–4 (stainless steel) | temperature and humidity | [58] | |
| beaker | glass | 2 (glass) | temperature and humidity | [57] | |
| conical flask | glass | 2 (glass) | temperature and humidity | [57] | |
| thermometer | glass surface | 2 (glass) | temperature and humidity | [57] | |
| acid/base burette | glass surface | 2 (glass) | temperature and humidity | [57] | |
| Hospital | stethoscope | metal | - | - | - |
| chest X-ray machine | compound material | - | - | - | |
| public seat | plastic | 3–4 (plastic) | temperature and humidity | [57] | |
| Office Block | public printer | plastic | 3–4 (plastic) | temperature and humidity | [57] |
| public water dispenser | plastic | 3–4 (plastic) | temperature and humidity | [57] | |
| public toilet | marble |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Zhang, J.; Zhu, L.; Huang, Q. Transmission of SARS-CoV-2 Indoor and Outdoor Environments. Atmosphere 2021, 12, 1640. https://doi.org/10.3390/atmos12121640
Xu X, Zhang J, Zhu L, Huang Q. Transmission of SARS-CoV-2 Indoor and Outdoor Environments. Atmosphere. 2021; 12(12):1640. https://doi.org/10.3390/atmos12121640
Chicago/Turabian StyleXu, Xueli, Jing Zhang, Liting Zhu, and Qiansheng Huang. 2021. "Transmission of SARS-CoV-2 Indoor and Outdoor Environments" Atmosphere 12, no. 12: 1640. https://doi.org/10.3390/atmos12121640
APA StyleXu, X., Zhang, J., Zhu, L., & Huang, Q. (2021). Transmission of SARS-CoV-2 Indoor and Outdoor Environments. Atmosphere, 12(12), 1640. https://doi.org/10.3390/atmos12121640







