Bioaerosol Emission from Biofilters: Impact of Bed Material Type and Waste Gas Origin
Abstract
:1. Introduction
- Colonization by bacteria and fungi measured by culture-dependent and independent methods that may be easy to apply on-site;
- Activity of microorganisms inhabiting the biofilters beds;
- Bioaerosol emissions from the biofilters.
2. Materials and Methods
2.1. Test Sites and Biofilters
2.2. Bed Material Sampling
- Point 1 was located symmetrically (centrally) in relation to the longer walls of the biofilter, but 0.5 m from the shorter wall of the biofilter;
- Point 2 was located in the center of the biofilter;
- Point 3 was located symmetrically (centrally) in relation to the longer walls of the biofilter, but 2.50 m from the shorter wall of the biofilter.
2.3. Gas Sampling
2.4. Quantification of the Number of Microorganisms
- cATP—intracellular ATP, reflecting total live biomass;
- tATP—total ATP;
- fATP—free-available ATP.
2.5. Determination of Microbial Activity
2.6. Statistical Measures and Methods
3. Results
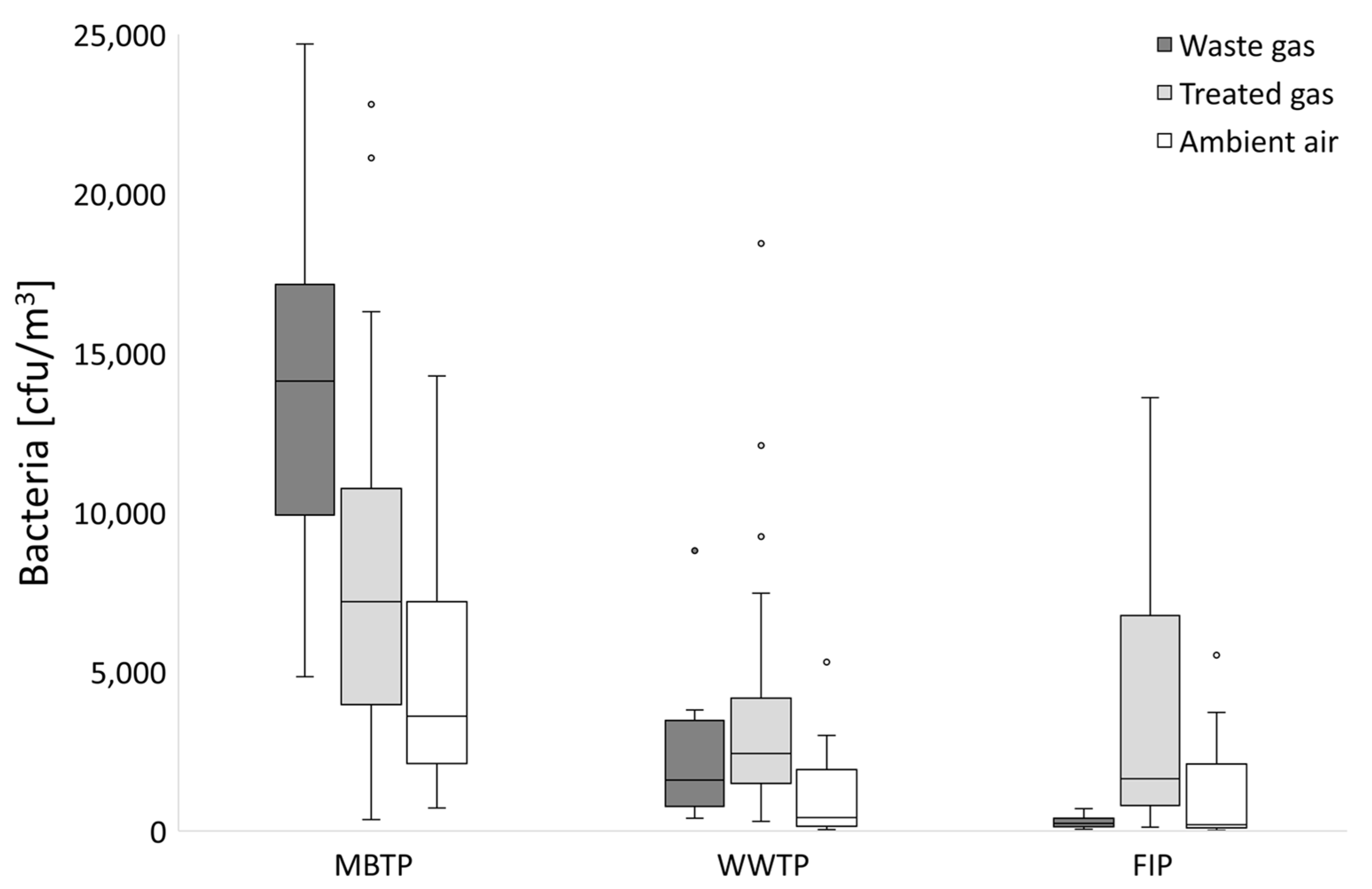
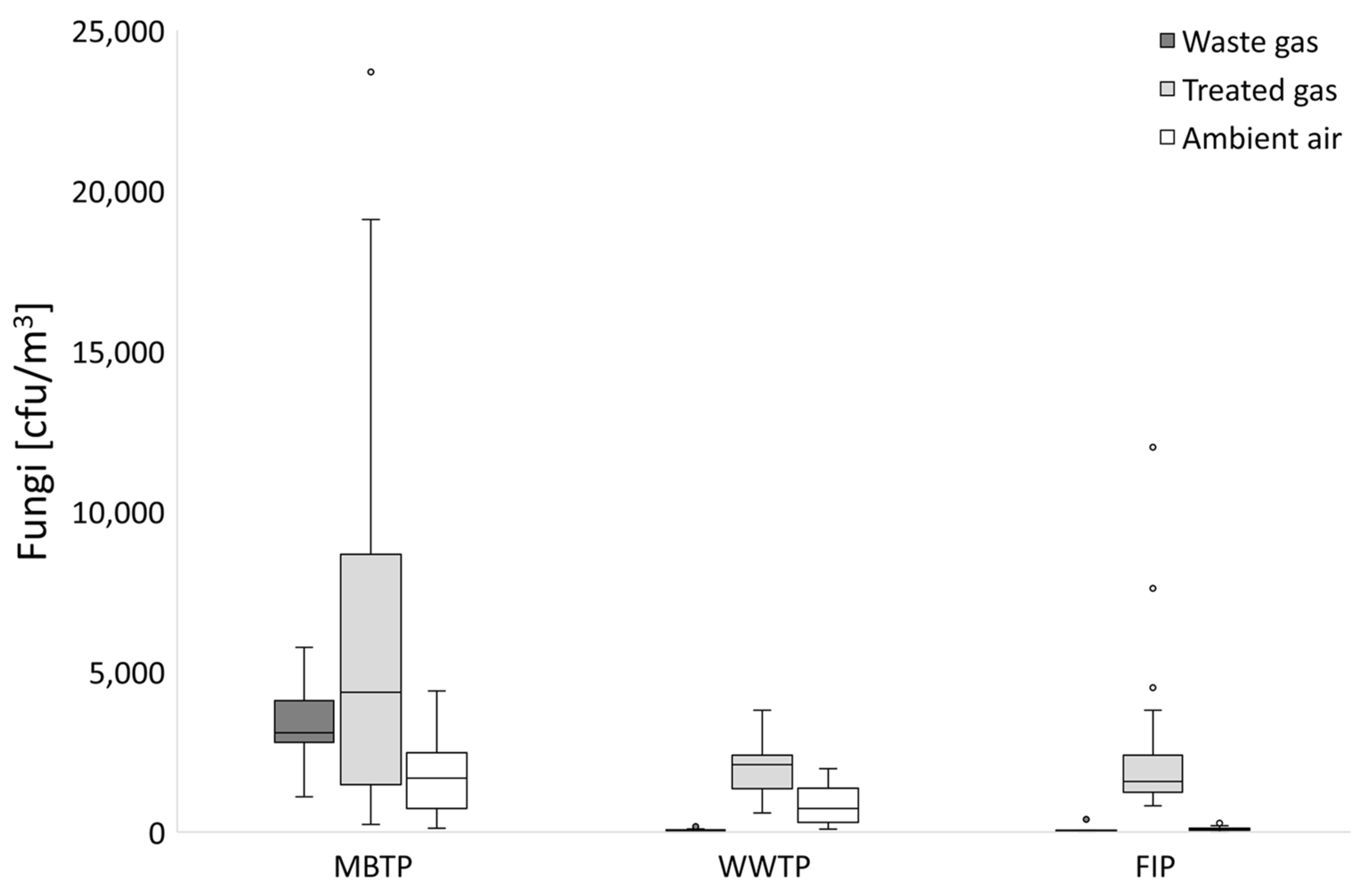
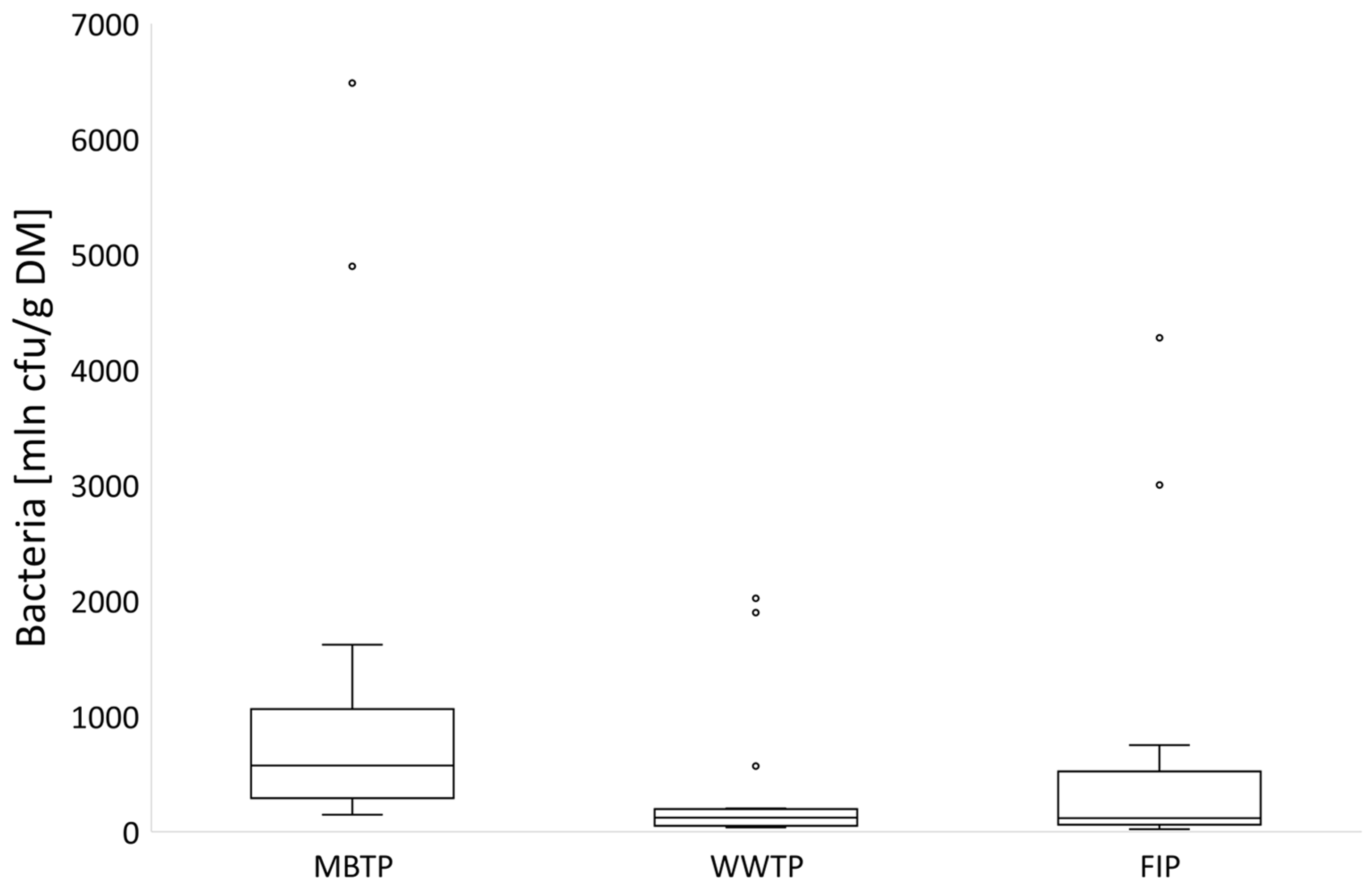
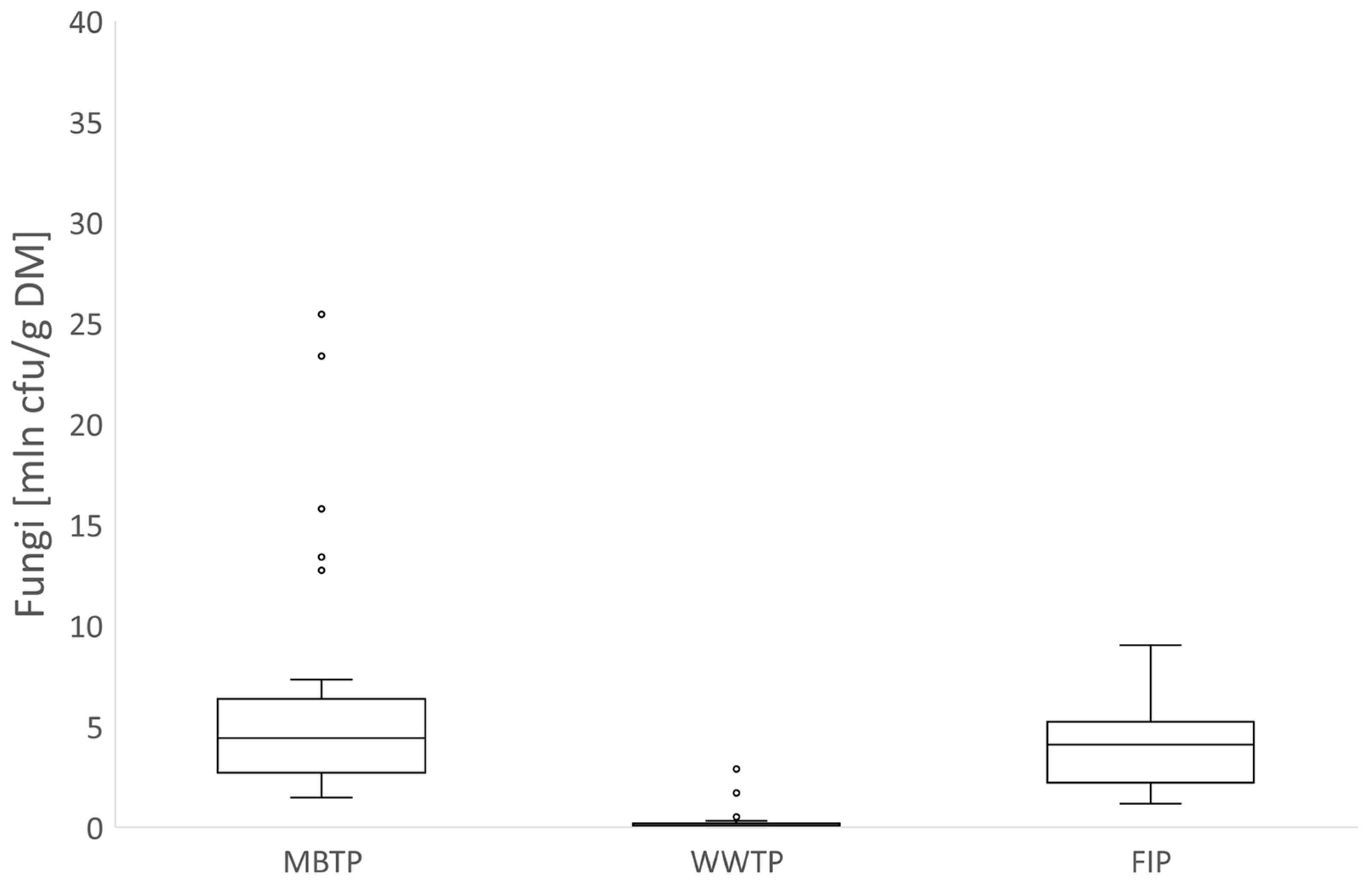
3.1. Microbial Abundance in Gases
3.1.1. CB Beds
3.1.2. CBC Beds
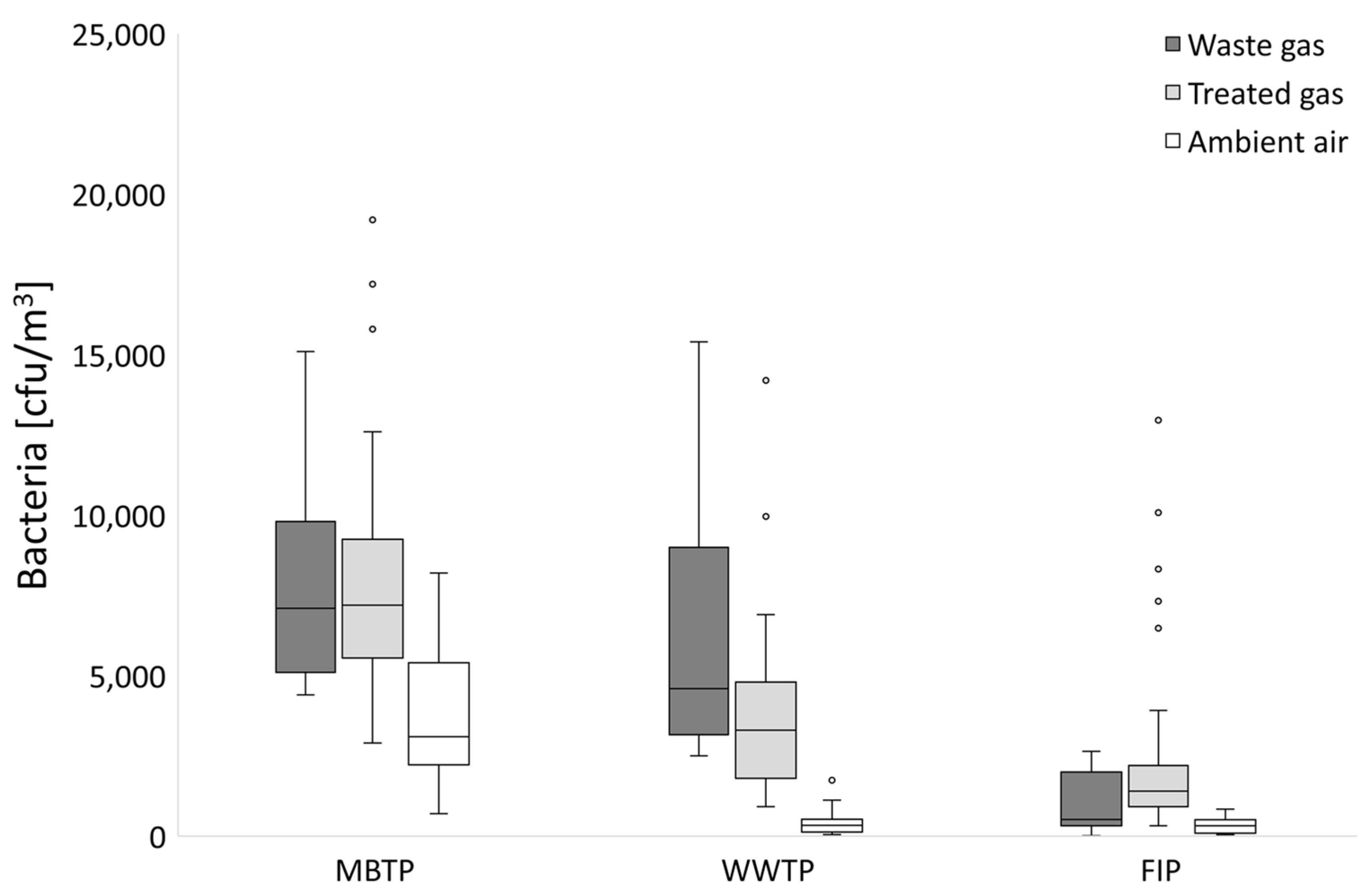
3.2. Microbial Abundance in Biofilters’ Bed Materials
3.2.1. CB Beds
3.2.2. CBC Beds
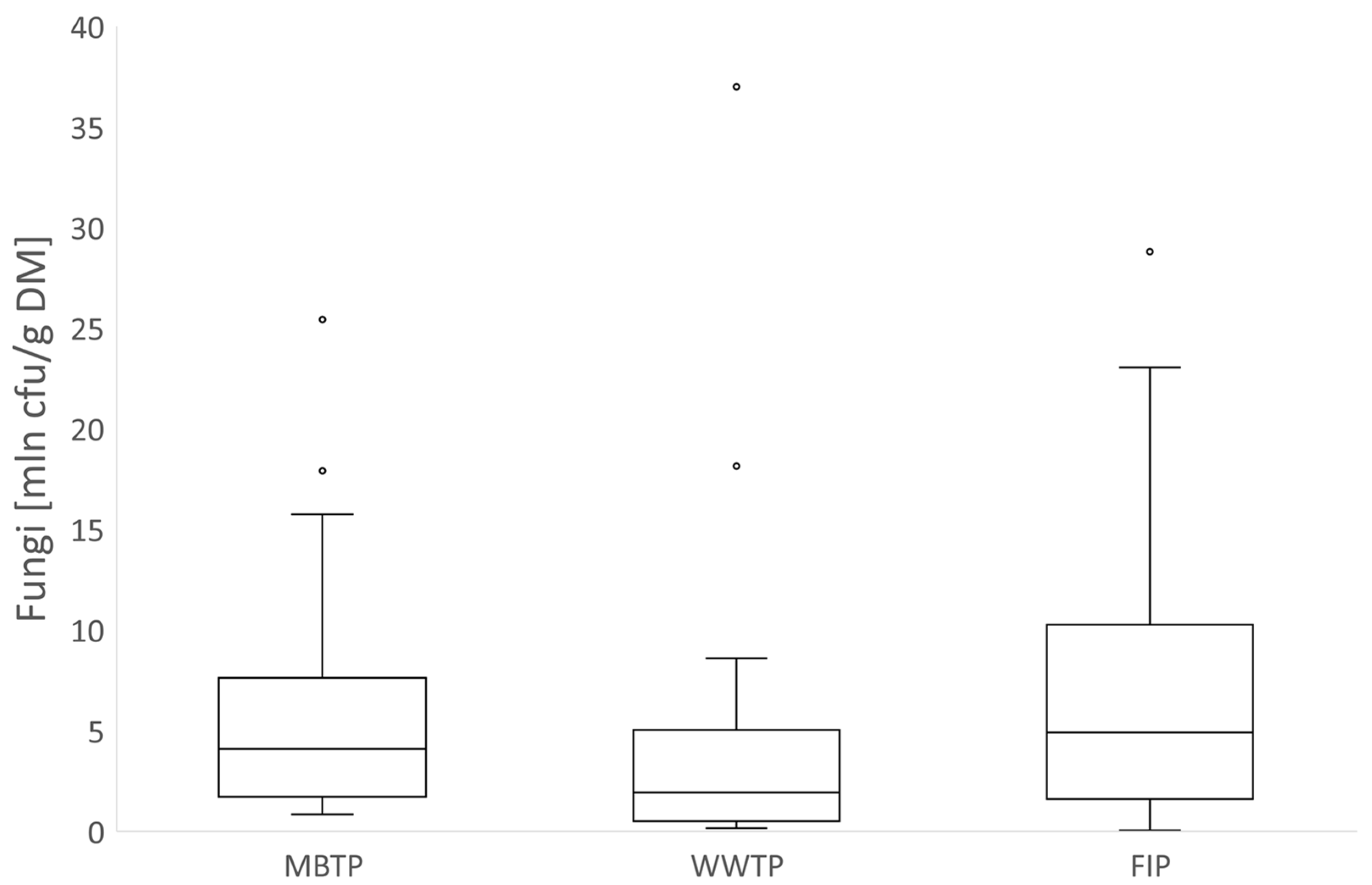
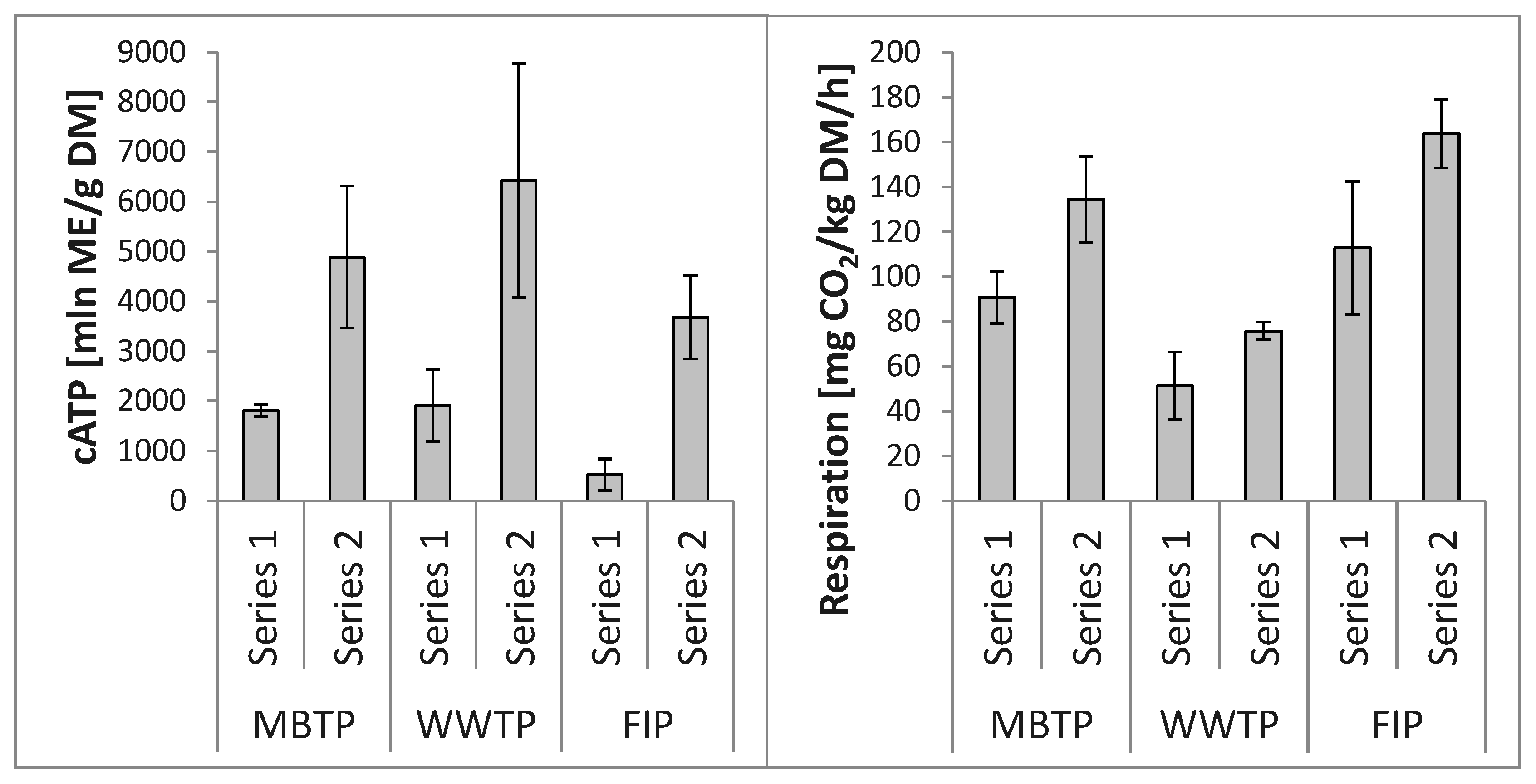
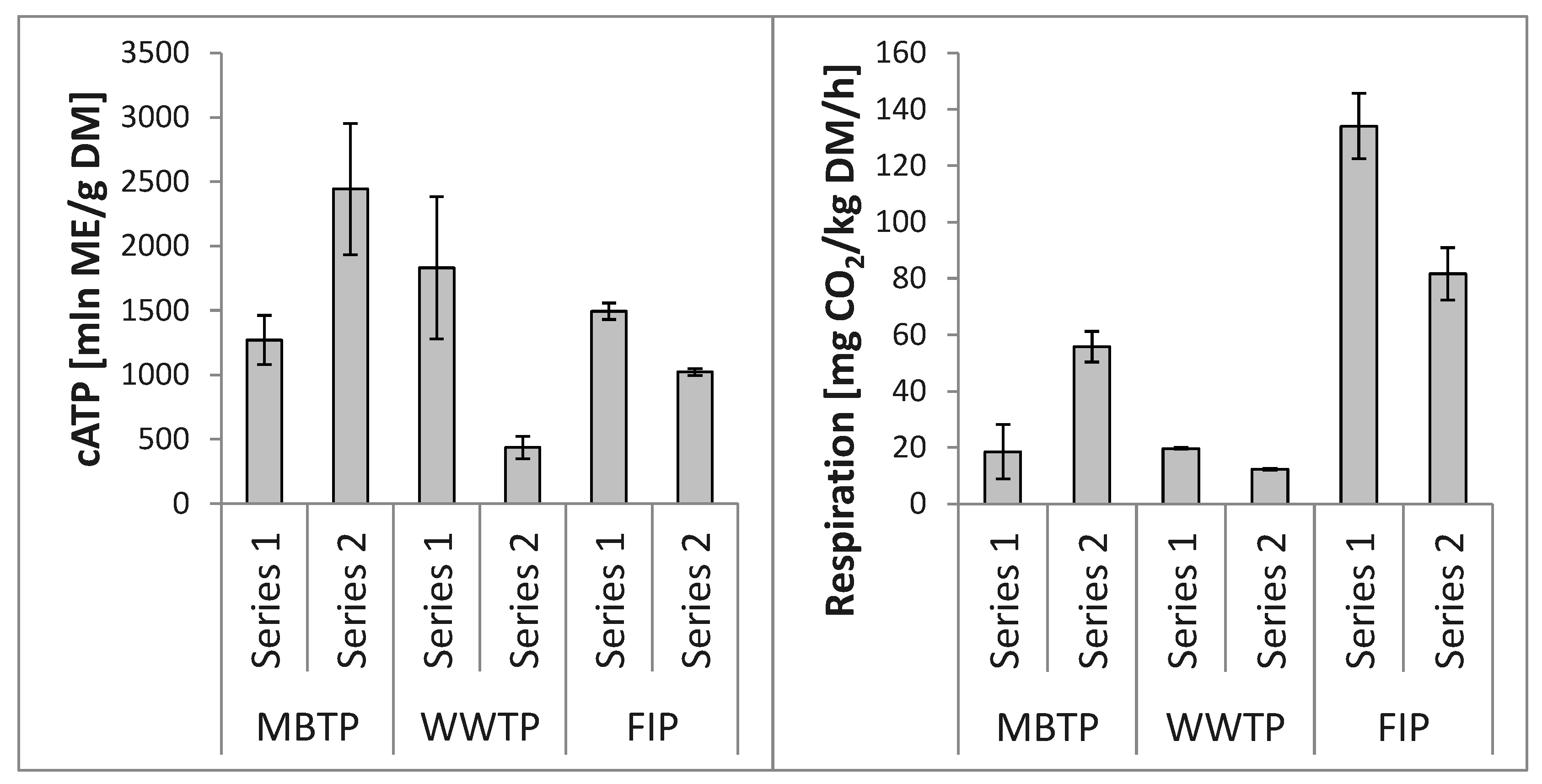
3.3. Relationships between Microbial Abundance and Microbial Respiration Activity
4. Discussion
4.1. Relationships between Microbial Abundance in Gases and Biofilters’ Bed Materials
4.2. Relationships between Microbial Abundance and Microbial Respiration Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maia, G.D.N.; Sales, G.T.; Day, G.B.V.; Gates, R.S.; Taraba, J.L. Characterizing physical properties of gas-phase biofilter media. Trans. ASABE 2012, 55, 1939–1950. [Google Scholar] [CrossRef]
- Wéry, N. Bioaerosols from composting facilities–A review. Front. Cell. Infect. Microbiol. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Ralebitso-Senior, T.K.; Senior, E.; Di Felice, R.; Jarvis, K. Waste gas biofiltration: Advances and limitations of current approaches in microbiology. Environ. Sci. Technol. 2012, 46, 8542–8573. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, L.A.; Jones, N.; Warren, L.; Stentiford, E.I. Understanding Biofilter Performance and Determining Emission Concentrations under Operational Conditions. 2014. Available online: http://organics-recycling.org.uk/uploads/article2834/ER36%20Final%20Report%20for%20publication.pdf (accessed on 23 October 2021).
- Tang, H.M.; Hwang, S.J.; Hwang, S.C. Waste Gas Treatment in Biofilters. J. Air Waste Manag. Assoc. 1996, 46, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Omri, I.; Aouidi, F.; Bouallagui, H.; Godon, J.J.; Hamdi, M. Performance study of biofilter developed to treat H2S from wastewater odour. Saudi J. Biol. Sci. 2013, 20, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Le Borgne, S.; Baquerizo, G. Microbial ecology of biofiltration units used for the desulfurization of biogas. Chem. Eng. 2019, 3, 72. [Google Scholar] [CrossRef] [Green Version]
- Runye, Z.; Kennes, C.; Zhuowei, C.; Lichao, L.; Jianming, Y.; Jianmeng, C. Styrene removal in a biotrickling filter and a combined UV-biotrickling filter: Steady- and transient-state performance and microbial analysis. Chem. Eng. J. 2015, 275, 168–178. [Google Scholar] [CrossRef]
- Pandey, R.A.; Padoley, K.V.; Mukherji, S.S.; Mudliar, S.N.; Vaidya, A.N.; Rajvaidya, A.S.; Subbarao, T.V. Biotreatment of waste gas containing pyridine in a biofilter. Bioresour. Technol. 2007, 98, 2258–2267. [Google Scholar] [CrossRef]
- Ghasemi, R.; Golbabaei, F.; Rezaei, S.; Pourmand, M.R.; Nabizadeh, R.; Jafari, M.J.; Masoorian, E. A comparison of biofiltration performance based on bacteria and fungi for treating toluene vapors from airflow. AMB Express 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lian, J.; Han, Y.; Liu, J. A biofilter integrated with gas membrane separation unit for the treatment of fluctuating styrene loads. Bioresour. Technol. 2012, 111, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Rene, E.R.; Kar, S.; Krishnan, J.; Pakshirajan, K.; López, M.E.; Murthy, D.V.S.; Swaminathan, T. Start-up, performance and optimization of a compost biofilter treating gas-phase mixture of benzene and toluene. Bioresour. Technol. 2015, 190, 529–535. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Y.; Wang, C.; Wen, N. Dissimilar emission characteristics between bioaerosol and suspended particles from gaseous biofilters and bioaerosol health risk evaluation. Aerosol Air Qual. Res. 2018, 18, 1874–1885. [Google Scholar] [CrossRef]
- Ibanga, I.E.; Fletcher, L.A.; Noakes, C.J.; King, M.F.; Steinberg, D. Pilot-scale biofiltration at a materials recovery facility: The impact on bioaerosol control. Waste Manag. 2018, 80, 154–167. [Google Scholar] [CrossRef]
- Hu, X.R.; Han, M.F.; Wang, C.; Yang, N.Y.; Wang, Y.C.; Duan, E.H.; Hsi, H.C.; Deng, J.G. A short review of bioaerosol emissions from gas bioreactors: Health threats, influencing factors and control technologies. Chemosphere 2020, 253, 126737. [Google Scholar] [CrossRef]
- Rolewicz-Kalińska, A.; Lelicińska-Serafin, K.; Manczarski, P. Volatile organic compounds, ammonia and hydrogen sulphide removal using a two-stage membrane biofiltration process. Chem. Eng. Res. Des. 2021, 165, 69–80. [Google Scholar] [CrossRef]
- Lelicińska-Serafin, K.; Rolewicz-Kalińska, A.; Manczarski, P. VOC removal performance of a joint process coupling biofiltration and membrane-filtration treating food industry waste gas. Int. J. Environ. Res. Public Health 2019, 16, 3009. [Google Scholar] [CrossRef] [Green Version]
- Muszyński, A.; Tabernacka, A.; Załęska-Radziwiłł, M. How to reduce the emission of microorganisms from a biofilter used to treat waste gas from a food industry plant. Atmosphere 2021, 12, 673. [Google Scholar] [CrossRef]
- ISO 16072:2011. Soil Quality—Laboratory Methods for Determining Respiration of Soil Microorganisms. Technical Committee CEN TC 444. 2011. Available online: https://standards.iteh.ai/catalog/standards/cen/d89f3b14-4f89-49da-82ee-c479553931be/en-iso-16072-2011 (accessed on 23 October 2021).
- Mirskaya, E.; Agranovski, I.E. Sources and mechanisms of bioaerosol generation in occupational environments. Crit. Rev. Microbiol. 2018, 44, 739–758. [Google Scholar] [CrossRef]
- Millner, P.D. Bioaerosols associated with animal production operations. Bioresour. Technol. 2009, 100, 5379–5385. [Google Scholar] [CrossRef] [PubMed]
- Lues, J.F.R.; Theron, M.M.; Venter, P.; Rasephei, M.H.R. Microbial composition in bioaerosols of a high-throughput chicken-slaughtering facility. Poult. Sci. 2007, 86, 142–149. [Google Scholar] [CrossRef]
- Bauer, H.; Fuerhacker, M.; Zibuschka, F.; Schmid, H.; Puxbaum, H. Bacteria and fungi in aerosols generated by two different types of wastewater treatment plants. Water Res. 2002, 36, 3965–3970. [Google Scholar] [CrossRef]
- Korzeniewska, E.; Filipkowska, Z.; Gotkowska-Płachta, A.; Janczukowicz, W.; Dixon, B.; Czułowska, M. Determination of emitted airborne microorganisms from a BIO-PAK wastewater treatment plant. Water Res. 2009, 43, 2841–2851. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.; Zhang, X.; Xu, C.; Dong, L.; Yao, M. Bioaerosol emissions and detection of airborne antibiotic resistance genes from a wastewater treatment plant. Atmos. Environ. 2016, 124, 402–412. [Google Scholar] [CrossRef]
- Vijatov, T.; Dražić, G.; Jovanović, F. Environmental aspects of biological wastewater treatment by different methods and microorganisms. Sustain. For. Collect. 2020, 81–82, 133–147. [Google Scholar] [CrossRef]
- Pearson, C.; Littlewood, E.; Douglas, P.; Robertson, S.; Gant, T.W.; Hansell, A.L. Exposures and health outcomes in relation to bioaerosol emissions from composting facilities: A systematic review of occupational and community studies. J. Toxicol. Environ. Health 2015, 18, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Pahari, A.K.; Dasgupta, D.; Patil, R.S.; Mukherji, S. Emission of bacterial bioaerosols from a composting facility in Maharashtra, India. Waste Manag. 2016, 53, 22–31. [Google Scholar] [CrossRef]
- Schlegelmilch, M.; Herold, T.; Streese, J.; Hensel, A.; Stegmann, R. The potential to reduce emissions of airborne microorganisms by means of biological waste gas treatment systems. Waste Manag. 2005, 25, 955–964. [Google Scholar] [CrossRef]
- Esquivel-Gonzalez, S.; Aizpuru, A.; Patrón-Soberano, A.; Arriaga, S. Characterization of bioaerosol emissions from two biofilters during treatment of toluene vapours using epifluorescence microscopy. Int. Biodeter. Biodegr. 2017, 123, 78–86. [Google Scholar] [CrossRef]
- Frederickson, J.; Boardman, C.P.; Gladding, T.L.; Simpson, A.E.; Howell, G.; Sgouridis, F. Evidence: Biofilter Performance and Operation as Related to Commercial Composting; Environment Agency: Bristol, UK, 2013.
- Ottengraf, S.P.P.; Konings, J.H.G. Emission of microorganisms from biofilters. Bioprocess Eng. 1991, 7, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Chmielowiec-Korzeniowska, A.; Tymczyna, L.; Skórska, C.; Sitkowska, J.; Cholewa, G.; Dutkiewicz, J. Efficacy of a novel biofilter in hatchery sanitation: I. Removal of airborne bacteria, dust and endotoxin. Ann. Agric. Environ. Med. 2007, 14, 141–150. [Google Scholar] [PubMed]
- Zilli, M.; Guarinoa, C.; Daffonchio, D.; Borin, S.; Converti, A. Laboratory–scale experiments with a powdered compost biofilter treating benzene-polluted air. Proc. Biochem. 2005, 40, 2035–2043. [Google Scholar] [CrossRef]
- Valdez-Castillo, M.; Saucedo-Lucero, J.O.; Arriaga, S. Photocatalytic inactivation of airborne microorganisms in continuous flow using perlite-supported ZnO and TiO2. Chem. Eng. J. 2019, 374, 914–923. [Google Scholar] [CrossRef]
- Valdez-Castillo, M.; Saucedo-Lucero, J.O.; Villalobos-Romero, K.L.; Péréz-Rodriguez, F.; Arriaga, S. Steady-state operation of a biofilter coupled with photocatalytic control of bacterial bioaerosol emissions. Environ. Sci. Pollut. Res. 2021, 28, 13970–13980. [Google Scholar] [CrossRef]
- Flores-Barbosa, A.B.; Aizpuru, A.; Quijano, G.; Arriaga, S. Evaluation of bioaerosols by flow cytometry and removal performance in a biofilter treating toluene/ethyl acetate vapors. Chemosphere 2020, 251, 126404. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Galera, M.M.; Lorenzana, A.; Chung, W.-J. Ethylbenzene, o-xylene, and BTEX removal by Sphingomonas sp. D3K1 in rock wool-compost biofilters. Environ. Eng. Sci. 2009, 26, 45–52. [Google Scholar] [CrossRef]
- Cho, K.-S.; Yoo, S.-K.; Ryu, H.W. Thermophilic biofiltration of benzene and toluene. J. Microbiol. Biotechnol. 2007, 17, 1976–1982. [Google Scholar] [PubMed]
- Sakuma, T.; Hattori, T.; Deshusses, M.A. Comparison of different packing materials for the biofiltration of air toxics. J. Air Waste Manage. Assoc. 2006, 56, 1567–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada, J.M.; Hernández, S.; Muñoz, R.; Revah, S. A comparative study of fungal and bacterial biofiltration treating a VOC mixture. J. Hazard. Mater. 2013, 250, 190–197. [Google Scholar] [CrossRef]
- Yang, K.; Li, L.; Ding, W.; Liu, J.; Xue, S. A full-scale thermophilic biofilter in the treatment of sludge drying exhaust: Performance, microbial characteristics and bioaerosol emission. J. Chem. Technol. Biotechnol. 2018, 93, 2216–2225. [Google Scholar] [CrossRef]
- Wikuats, C.F.H.; Duarte, E.H.; Prates, K.V.M.C.; Janiaski, L.L.L.; Gabriel, D.O.; Molina, A.C.; Martins, L.D. Assessment of airborne particles and bioaerosols concentrations in a waste recycling environment in Brazil. Sci. Rep. 2020, 10, 14812. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, L.; Xue, S.; Wang, Y.; Liu, J.; Yang, T. Influence factors and health risk assessment of bioaerosols emitted from an industrial scale thermophilic biofilter for off gas treatment. Process. Saf. Environ. Prot. 2019, 129, 55–62. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.W.; Liu, H. Microwave-induced release and degradation of airborne endotoxins from Escherichia coli bioaerosol. J. Hazard Mater. 2019, 366, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xi, J.Y.; Hu, H.Y. Reduction of toxic products and bioaerosol emission of a combined ultraviolet-biofilter process for chlorobenzene treatment. J. Air Waste Manag. Assoc. 2009, 59, 405–410. [Google Scholar] [CrossRef]
- Saucedo-Lucero, J.O.; Quijano, G.; Arriaga, S.; Muňoza, R. Hexane abatement and spore emission control in a fungal biofilter-photoreactor hybrid unit. J. Hazard Mater. 2014, 276, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Andrès, Y.; Dumant, E.; Gerente, C. Characterization of packing material colonization in gas biofiltration processes. Can. J. Civ. Eng. 2009, 36, 1895–1902. [Google Scholar] [CrossRef]
- Anet, B.; Couriol, C.; Lendormi, T.; Amrane, A.; Le Cloirec, P.; Cogny, G.; Fillières, R. Characterization and selection of packing materials for biofiltration of rendering odourous emissions. Water Air Soil Pollut. 2013, 224, 1–13. [Google Scholar] [CrossRef]
- Martin, F.J.; Loehr, R.C. Effect of periods of non-use on biofilter performance. J. Air Waste Manag. Assoc. 1996, 46, 539–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada, J.M.; Rodríguez, E.; Quijano, G.; Muñoz, R. Influence of gaseous VOC concentration on the diversity and biodegradation performance of microbial communities. Bioprocess Biosys. Eng. 2012, 35, 1477–1488. [Google Scholar] [CrossRef] [PubMed]




| Test Site | Bed Material | Water Content [%] | Average Inlet Concentrations ± SD (Range) | Average Flow Rate ± SD [m3/h] | Average Surface Load ± SD [m3/(m2 × h)] | Average Volumetric Load ± SD [m3/(m3 × h)] | Average Pressure Drop ± SD [Pa] | ||
|---|---|---|---|---|---|---|---|---|---|
| VOCs [ppb] | NH3 [ppm] | H2S [ppm] | |||||||
| MBTP | CB | 55 ± 4 | 938 ± 372 | 5 ± 1 | 1.5 ± 0.4 | 379 ± 14 | 95.8 ± 3.5 | 83.2 ± 2.9 | 372 ± 9 |
| CBC | 27 ± 2 | (405–1820) | (4–6) | (0.9–2.3) | 381 ± 12 | 96.1 ± 3.0 | 83.6 ± 2.6 | 542 ± 9 | |
| WWTP | CB | 62 ± 5 | 2258 ± 955 | 26 ± 14 | 4.8 ± 0.1 | 380 ± 11 | 96.1 ± 2.8 | 83.5 ± 2.4 | 375 ± 15 |
| CBC | 45 ± 4 | (140–3980) | (6–59) | (4.6–5.0) | 385 ± 16 | 97.1 ± 1.6 | 84.4 ± 1.4 | 510 ± 26 | |
| FIP | CB | 61 ± 5 | 3478 ± 3119 | 10 ± 4 | 7.5 ± 3.9 | 382 ± 24 | 96.5 ± 6.1 | 84.0 ± 5.3 | 379 ± 13 |
| CBC | 51 ± 5 | (480–9400) | (4–15) | (2.1–14.3) | 322 ± 86 | 81.3 ± 21.7 | 70.7 ± 18.9 | 596 ± 84 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Affek, K.; Tabernacka, A.; Załęska-Radziwiłł, M.; Doskocz, N.; Muszyński, A. Bioaerosol Emission from Biofilters: Impact of Bed Material Type and Waste Gas Origin. Atmosphere 2021, 12, 1574. https://doi.org/10.3390/atmos12121574
Affek K, Tabernacka A, Załęska-Radziwiłł M, Doskocz N, Muszyński A. Bioaerosol Emission from Biofilters: Impact of Bed Material Type and Waste Gas Origin. Atmosphere. 2021; 12(12):1574. https://doi.org/10.3390/atmos12121574
Chicago/Turabian StyleAffek, Katarzyna, Agnieszka Tabernacka, Monika Załęska-Radziwiłł, Nina Doskocz, and Adam Muszyński. 2021. "Bioaerosol Emission from Biofilters: Impact of Bed Material Type and Waste Gas Origin" Atmosphere 12, no. 12: 1574. https://doi.org/10.3390/atmos12121574






