Microscopic Fungi in Big Cities: Biodiversity, Source, and Relation to Pollution by Potentially Toxic Metals
Abstract
:1. Introduction
2. Materials and Methods
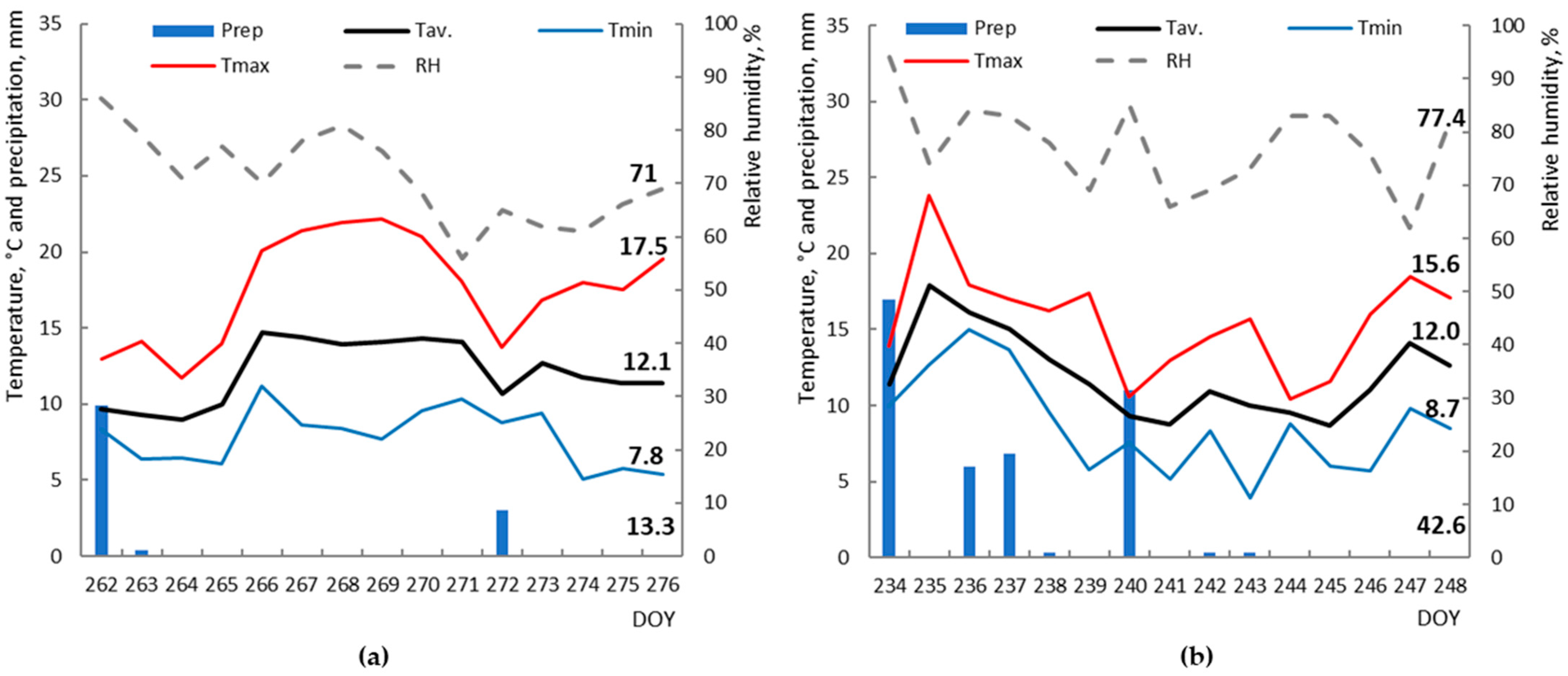
2.1. Climatic Characteristics of Research Areas
2.2. Site Description
2.3. Sampling
2.4. Preparation of a Suspension for Analysis
2.5. Chemical Analysis
2.6. Microbiological Analysis
2.7. Statistical Analysis
3. Results
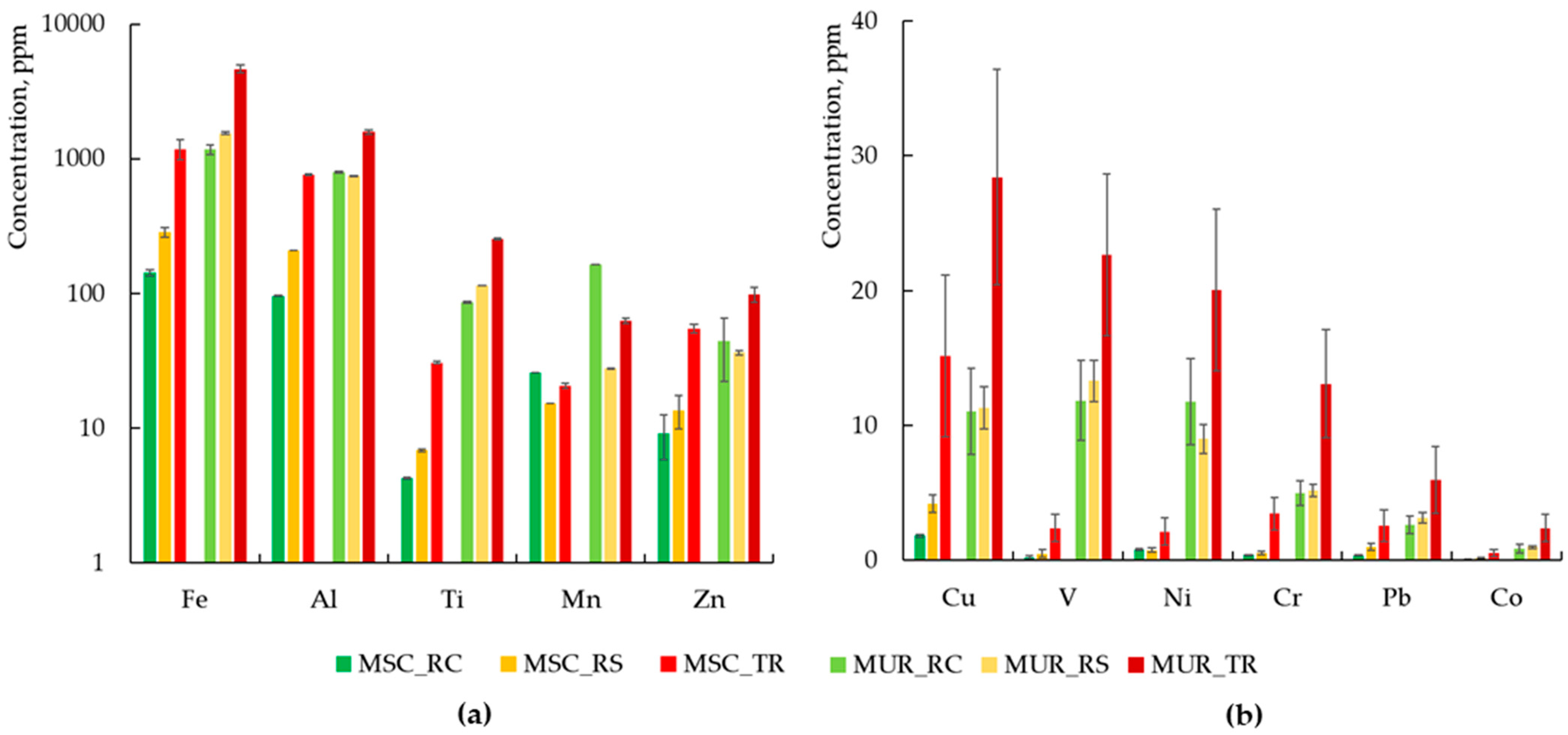
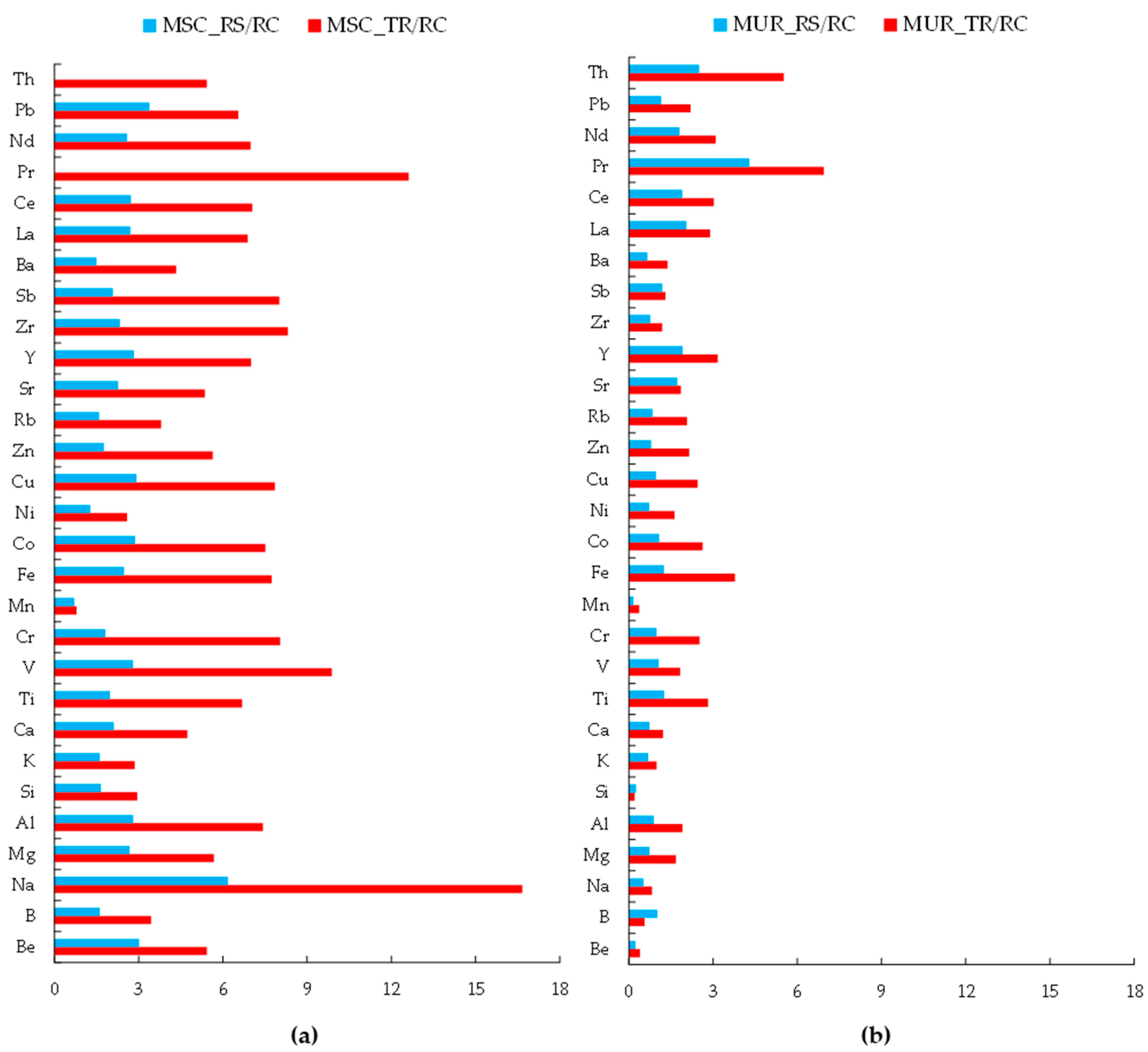
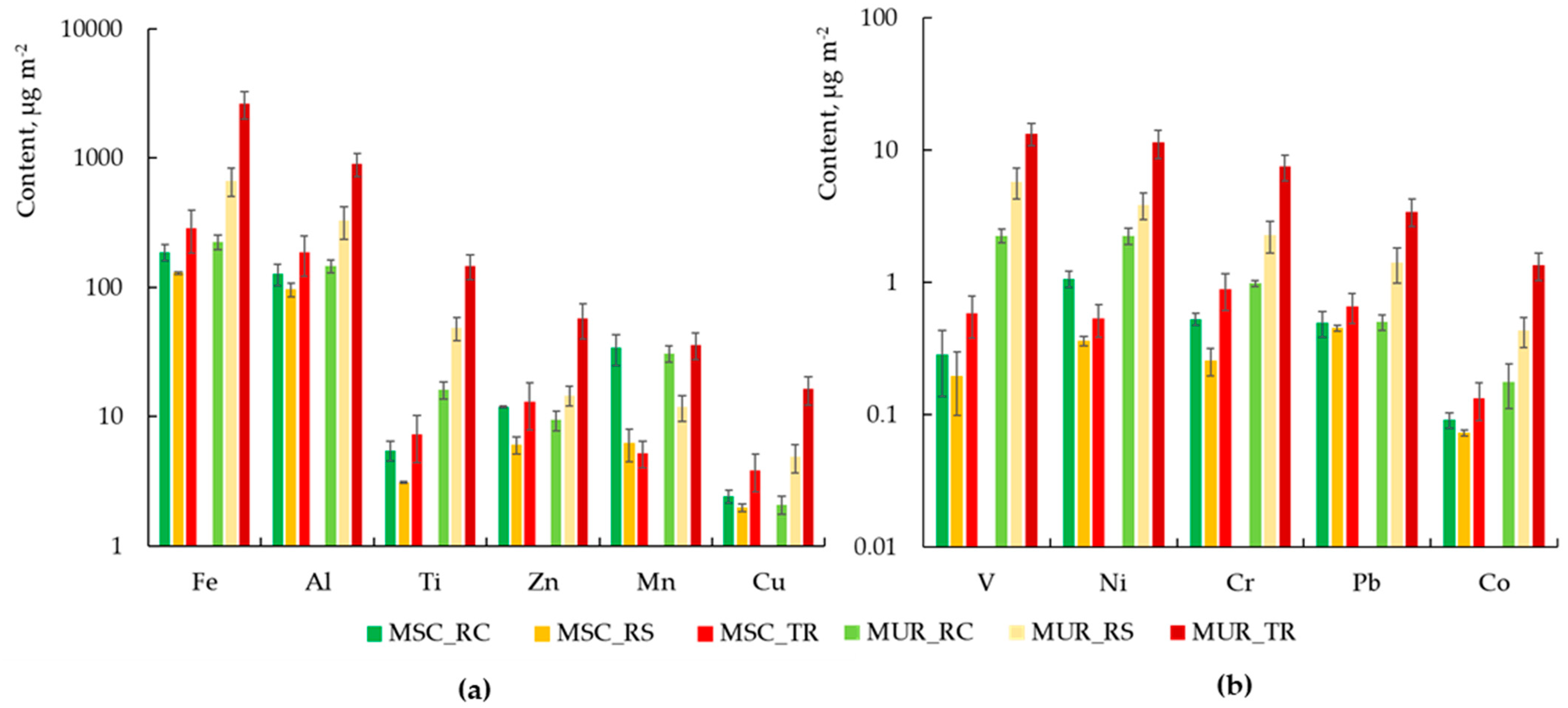
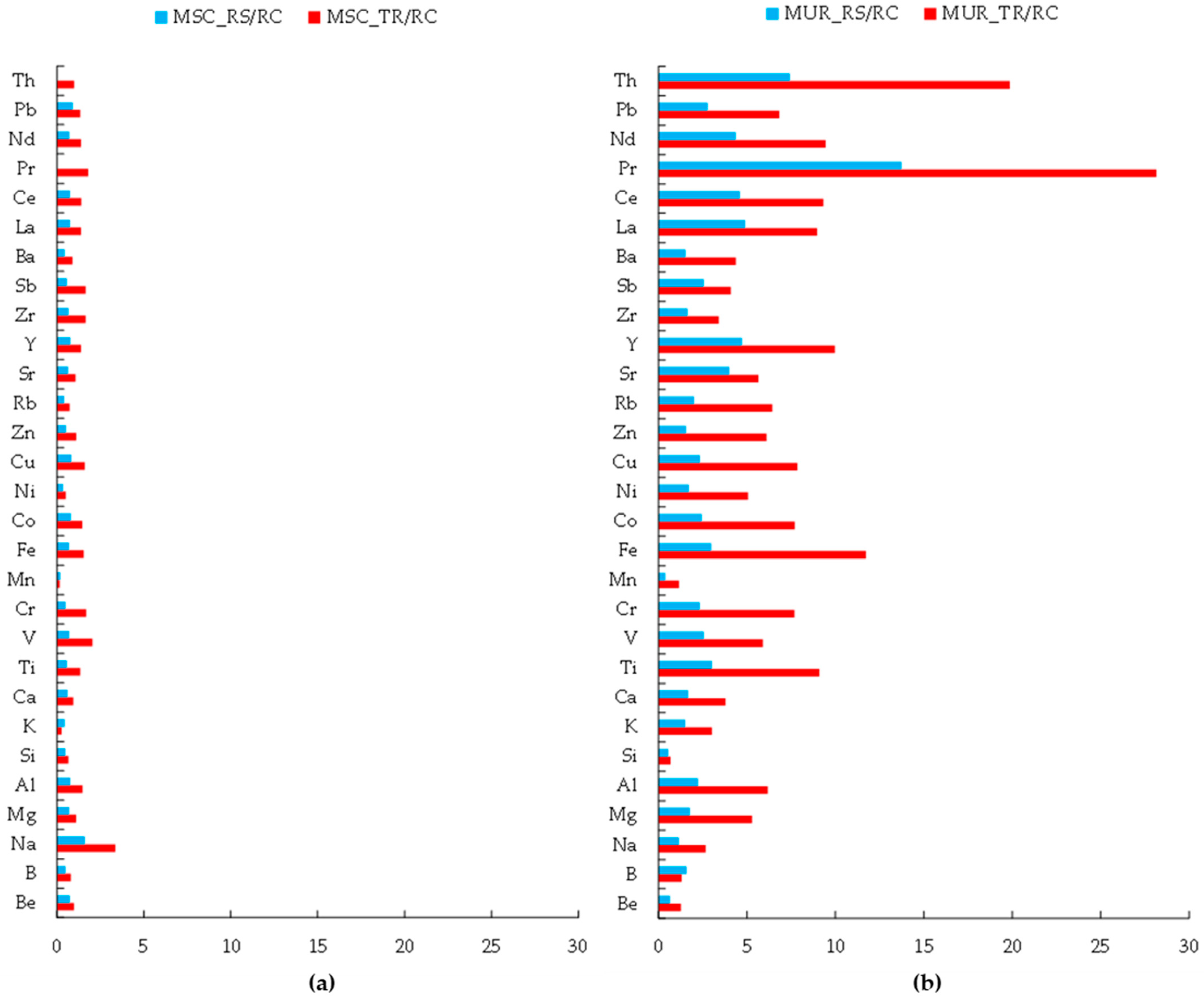
3.1. Chemical Composition of Dust in Two Cities
- Qualitative Contamination
- Quantitative analysis
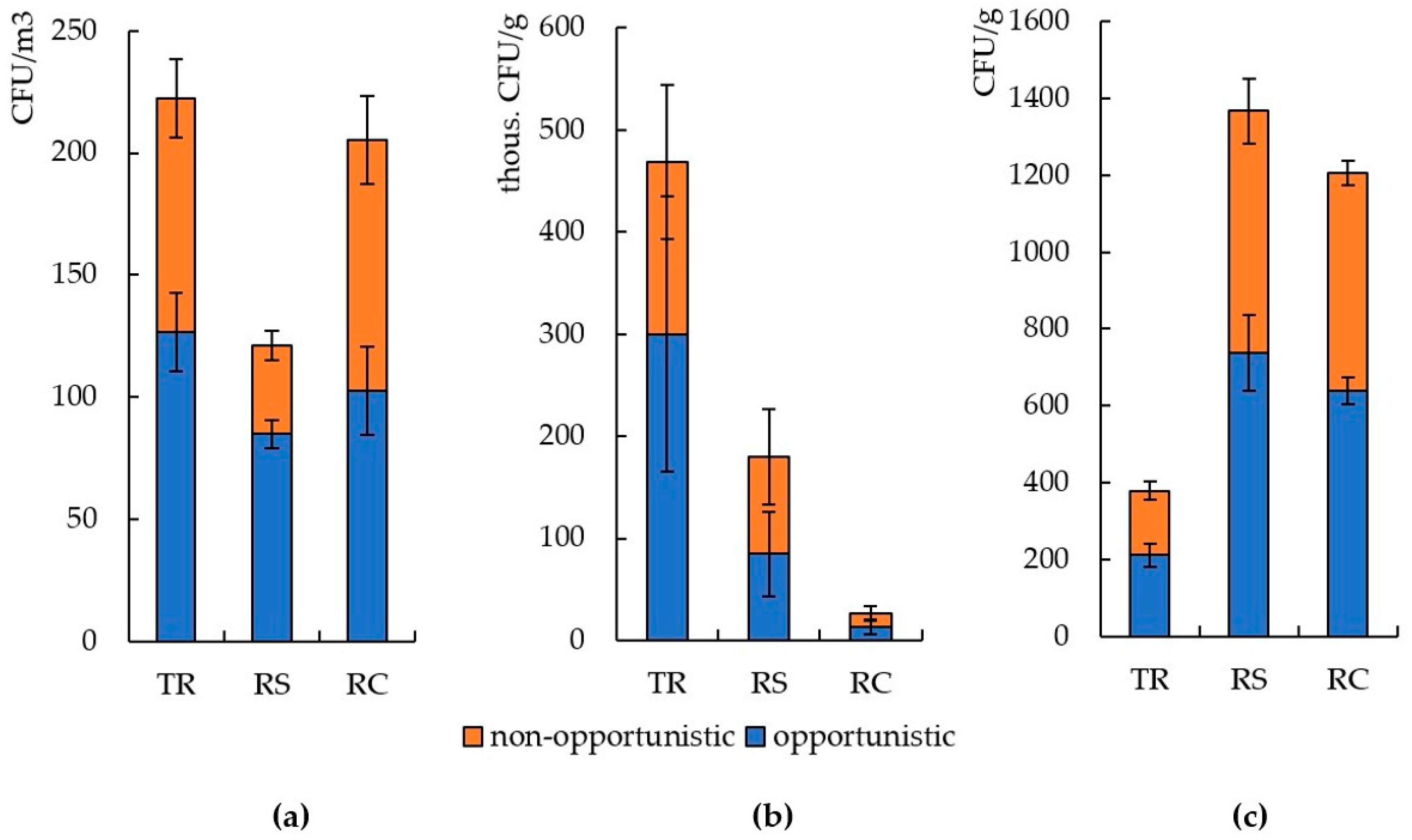
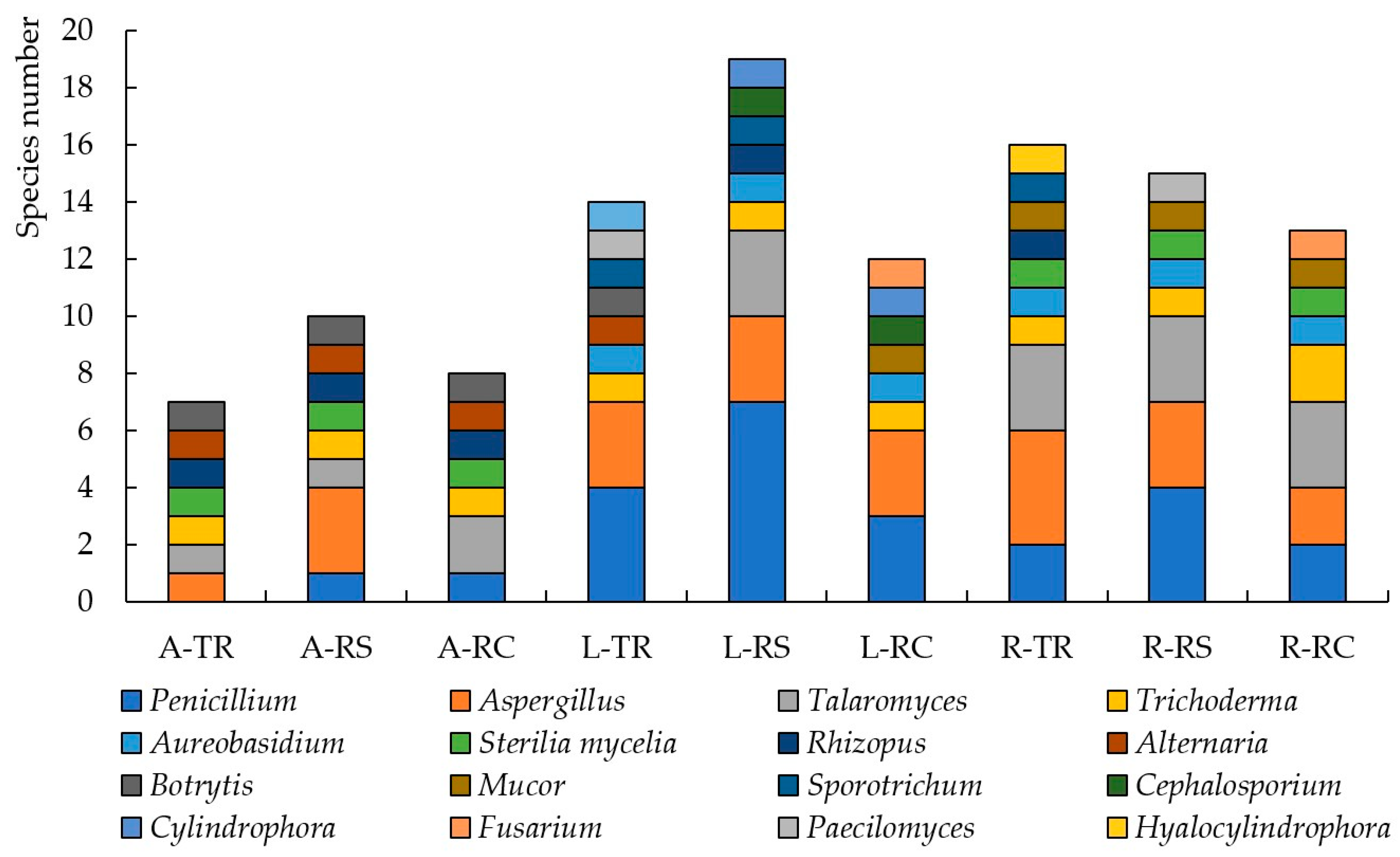
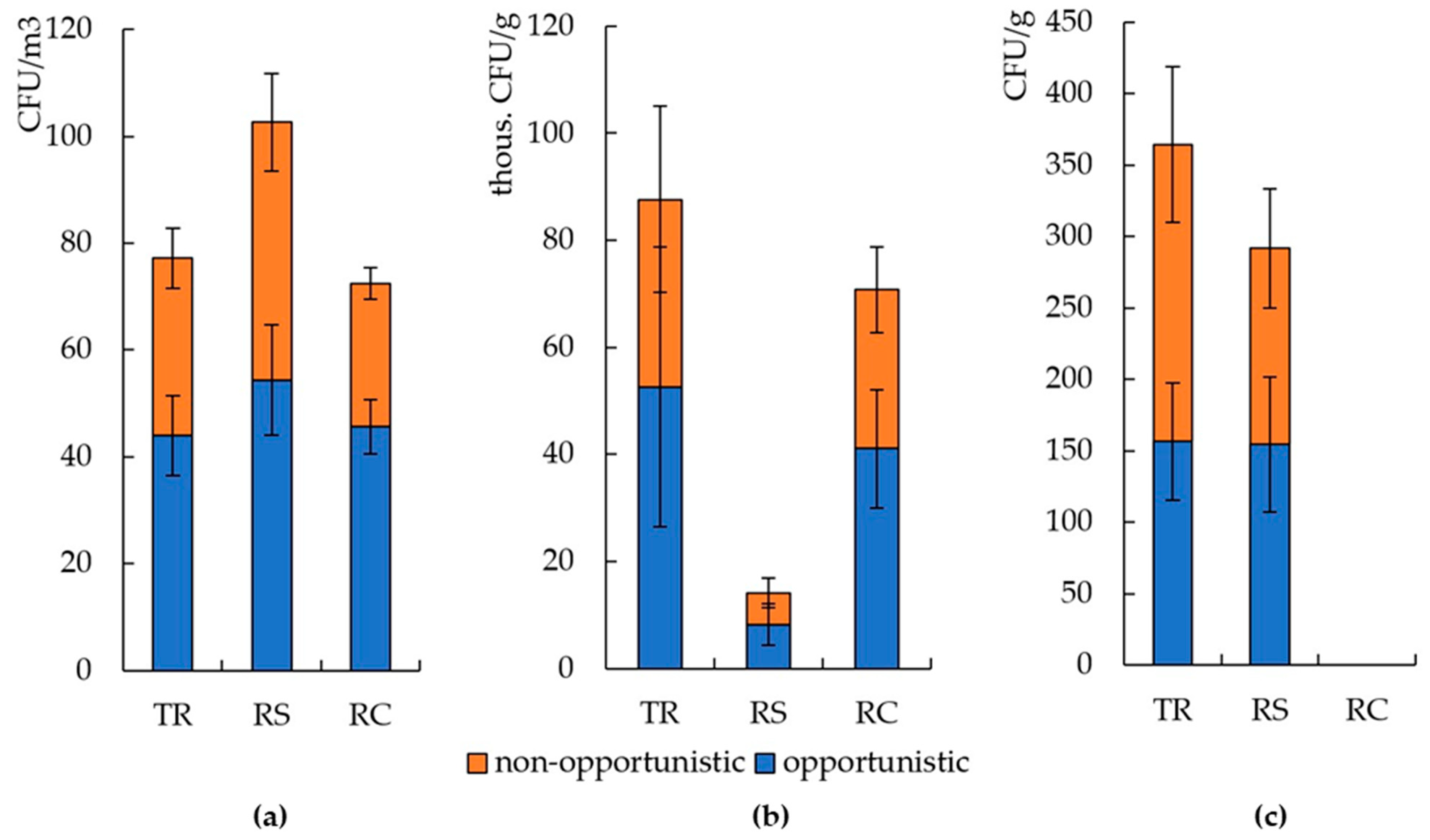
3.2. The Number and Diversity of Microscopic Fungi in Moscow
3.3. The Number and Diversity of Microscopic Fungi in Murmansk
3.4. Correlations and Multivariate Analyses
4. Discussion
4.1. Effect of Climate and Pollution across Cities
4.2. Functional Zones
4.3. Species Composition Clustering
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morel, J.L.; Chenu, C.; Lorenz, K. Ecosystems services provided by soil of urban, industrial, traffic, mining, and military areas (SUITMAs). J. Soils Sediments 2015, 15, 1659–1666. [Google Scholar] [CrossRef]
- Steffan, J.J.; Brevik, E.C.; Burgess, L.C.; Cerdà, A. The effect of soil on human health: An overview. Eur. J. Soil Sci. 2018, 69, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Vasenev, V.; Kuzyakov, Y. Urban soils as hot spots of anthropogenic carbon accumulation: Review of stocks, mechanisms and driving factors. Land. Degrad. Dev. 2018, 29, 1607–1622. [Google Scholar] [CrossRef]
- WHO. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Deseas; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Ivashchenko, K.V.; Ananyeva, N.; Vasenev, V.I.; Kudeyarov, V.; Valentini, R. Biomass and respiration activity of soil microorganisms in anthropogenically transformed ecosystems (Moscow region). Eurasian Soil Sci. 2014, 47, 892–903. [Google Scholar] [CrossRef]
- Sarzhanov, D.A.; Vasenev, V.I.; Sotnikova, Y.L.; Tembo, A.; Vasenev, I.; Valentini, R. Short-term dynamics and spatial heterogeneity of CO2 emission from the soils of natural and urban ecosystems in the Central Chernozemic Region. Eurasian Soil Sci. 2015, 48, 416–424. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Kroeger, T.; Wagner, J.E. Urban forests and pollution mitigation: Analyzing ecosystem services and disservices. Environ. Pollut. 2011, 159, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Baldacchini, C.; Castanheiro, A.; Maghakyan, N.; Sgrigna, G.; Verhelst, J.; Alonso, R.; Amorim, J.H.; Bellan, P.; Bojović, D.; Breuste, J.; et al. How Does the Amount and Composition of PM Deposited on Platanus acerifolia Leaves Change Across Different Cities in Europe? Environ. Sci. Technol. 2017, 51, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Baldacchini, C.; Sgrigna, G.; Clarke, W.; Tallis, M.; Calfapietra, C. An ultra-spatially resolved method to quali-quantitative monitor particulate matter in urban environment. Environ. Sci. Pollut. Res. 2019, 26, 18719–18729. [Google Scholar] [CrossRef]
- Adams, R.I.; Bateman, A.C.; Bik, H.M.; Meadow, J.F. Microbiota of the indoor environment: A meta-analysis. Microbiome 2015, 3, 1–18. [Google Scholar] [CrossRef] [Green Version]
- O’Gorman, C.M.; Fuller, H.T. Prevalence of culturable airborne spores of selected allergenic and pathogenic fungi in outdoor air. Atmos. Environ. 2008, 42, 4355–4368. [Google Scholar] [CrossRef]
- Chakrabarti, H.S.; Das, S.; Gupta-Bhattacharya, S. Outdoor airborne fungal spora load in a suburb of Kolkata, India: Its variation, meteorological determinants and health impact. Int. J. Environ. Health Res. 2012, 22, 37–50. [Google Scholar] [CrossRef]
- Korneykova, M.V.; Lebedeva, E.V. Opportunistic Fungi in the Polluted Soils of Kola Peninsula. Geogr. Environ. Sustain. 2018, 11, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Salem, H.; Gardner, D.E. Health aspects of bioaerosols. In Atmospheric Microbial Aerosols; Lighthart, B., Mohr, A.J., Eds.; Springer: Boston, MA, USA, 1994; pp. 304–330. [Google Scholar] [CrossRef]
- Beasley, R. The burden of asthma with specific reference to the United States. J. Allergy Clin. Immunol. 2002, 109, S482–S489. [Google Scholar] [CrossRef]
- Isolauri, E.; Huurre, A.; Salminen, S.; Impivaara, O. The allergy epidemic extends beyond the past few decades. Clin. Exp. Allergy 2004, 34, 1007–1010. [Google Scholar] [CrossRef]
- Burge, H.A.; Rogers, C.A. Outdoor allergens. Environ. Health Perspect. 2000, 108, 653–659. [Google Scholar] [CrossRef] [Green Version]
- Ross, M.A.; Curtis, L.; Scheff, P.A.; Hryhorczuk, D.O.; Ramakrishnan, V.; Wadden, R.A.; Persky, V.W. Association of asthma symptoms and severity with indoor bioaerosols. Allergy 2000, 55, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Innocente, E.; Squizzato, S.; Visin, F.; Facca, C.; Rampazzo, G.; Bertolini, V.; Gandolfi, I.; Franzetti, A.; Ambrosini, R.; Bestetti, G. Influence of seasonality, air mass origin and particulate matter chemical composition on airborne bacterial community structure in the Po Valley, Italy. Sci. Total Environ. 2017, 593–594, 677–687. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhang, H.; Yao, X.; Zhou, M.; Wang, J.; He, Z.; Zhang, H.; Lou, L.; Mao, W.; et al. Effect of air pollution on the total bacteria and pathogenic bacteria in different sizes of particulate matter. Environ. Pollut. 2018, 233, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Li, X.; Wang, T.; Wang, B.; Li, C.; Zeng, G. A review on airborne microorganisms in particulate matters: Composition, characteristics and influence factors. Environ. Int. 2018, 113, 74–90. [Google Scholar] [CrossRef]
- Salawu-Rotimi, A.; Lebre, P.H.; Vos, H.C.; Fister, W.; Kuhn, N.; Eckardt, F.D.; Cowan, D.A. Gone with the Wind: Microbial Communities Associated with Dust from Emissive Farmlands. Microb. Ecol. 2021, 82, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Antropova, A.B.; Mokeeva, V.L.; Bilanenko, E.N.; Chekunova, L.N.; Zheltikova, T.M.; Petrova-Nikitina, A.D. Aeromycota of Moscow dwellings. Mikol. Fitopatol. 2003, 37, 1–11. (In Russian) [Google Scholar]
- Yegorova, L.N.; Klimova, Y.A. Saprotrophic micromycetes in the air of various rooms in Vladivostok. Uspekhi Meditsinskoy Mikol. 2005, 5, 64–67. (In Russian) [Google Scholar]
- Abbasi, F.; Samaei, M.R. The effect of temperature on airborne filamentous fungi in the indoor and outdoor space of a hospital. Environ. Sci. Pollut. Res. 2018, 26, 16868–16876. [Google Scholar] [CrossRef] [PubMed]
- Chegini, F.M.; Baghani, A.N.; Hassanvand, M.S.; Sorooshian, A.; Golbaz, S.; Bakhtiari, R.; Ashouri, A.; Joubani, M.N.; Alimohammadi, M. Indoor and outdoor airborne bacterial and fungal air quality in kindergartens: Seasonal distribution, genera, levels, and factors influencing their concentration. Build. Environ. 2020, 175, 106690. [Google Scholar] [CrossRef]
- Guo, K.; Qian, H.; Zhao, D.; Ye, J.; Zhang, Y.; Kan, H.; Zhao, Z.; Deng, F.; Huang, C.; Zhao, B.; et al. Indoor exposure levels of bacteria and fungi in residences, schools, and offices in China: A systematic review. Indoor Air 2020, 30, 1147–1165. [Google Scholar] [CrossRef] [PubMed]
- Pyrri, I.; Tripyla, E.; Zalachori, A.; Chrysopoulou, M.; Parmakelis, A.; Kapsanaki-Gotsi, E. Fungal contaminants of indoor air in the National Library of Greece. Aerobiologia 2020, 36, 387–400. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Tian, Y.; Li, A.; Li, Y.; Xiong, J.; Gao, R. On-site investigation of the concentration and size distribution characteristics of airborne fungi in a university library. Environ. Pollut. 2020, 261, 114138. [Google Scholar] [CrossRef] [PubMed]
- Bowers, R.M.; Clements, N.; Emerson, J.; Wiedinmyer, C.; Hannigan, M.; Fierer, N. Seasonal Variability in Bacterial and Fungal Diversity of the Near-Surface Atmosphere. Environ. Sci. Technol. 2013, 47, 12097–12106. [Google Scholar] [CrossRef] [PubMed]
- Be, N.A.; Thissen, J.; Fofanov, V.Y.; Allen, J.; Rojas, M.; Golovko, G.; Fofanov, Y.; Koshinsky, H.; Jaing, C.J. Metagenomic Analysis of the Airborne Environment in Urban Spaces. Microb. Ecol. 2014, 69, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Gandolfi, I.; Canedoli, C.; Imperato, V.; Tagliaferri, I.; Gkorezis, P.; Vangronsveld, J.; Schioppa, E.P.; Papacchini, M.; Bestetti, G.; Franzetti, A. Diversity and hydrocarbon-degrading potential of epiphytic microbial communities on Platanus x acerifolia leaves in an urban area. Environ. Pollut. 2016, 220, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Sazonova, O.I.; Sokolov, S.; Prisyazhnaya, N.V.; Izmalkova, T.Y.; Kosheleva, I.A.; Boronin, A.M. Epiphytic microorganisms degrading aromatic hydrocarbons from the phyllosphere of urban woody plants. Microbiology 2017, 86, 82–88. [Google Scholar] [CrossRef]
- Franzetti, A.; Gandolfi, I.; Bestetti, G.; Schioppa, E.P.; Canedoli, C.; Brambilla, D.; Cappelletti, D.; Sebastiani, B.; Federici, E.; Papacchini, M.; et al. Plant-microorganisms interaction promotes removal of air pollutants in Milan (Italy) urban area. J. Hazard. Mater. 2020, 384, 121021. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, T.A.; Tkachenko, M.A. Micromycetes of Phylloplane of Woody Plants in the Urban Environment in Autumn. Proc. Young Sci. Spec. Samara Univ. 2018, 1, 41–45. [Google Scholar]
- Setti, L.; Passarini, F.; De Gennaro, G.; Barbieri, P.; Perrone, M.G.; Piazzalunga, A.; Borelli, M.; Palmisani, J.; Di Gilio, A.; Piscitelli, P.; et al. The potential role of particulate matter in the spreading of COVID-19 in Northern Italy: First evidence-based research hypotheses. MedRxiv 2020. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, J.; Wang, W.; Liu, Z.; Kan, H.; Qiu, Y.; Meng, X.; Wang, W. Association of particulate matter pollution and case fatality rate of COVID-19 in 49 Chinese cities. Sci. Total Environ. 2020, 741, 140396. [Google Scholar] [CrossRef]
- Yair, Y.; Yair, Y.; Rubin, B.; Confino-Cohen, R.; Rosman, Y.; Shachar, E.; Rottem, M. First reported case of thunderstorm asthma in Israel. Nat. Hazards Earth Syst. Sci. 2019, 19, 2715–2725. [Google Scholar] [CrossRef] [Green Version]
- Barberán, A.; Ladau, J.; Leff, J.W.; Pollard, K.S.; Menninger, H.L.; Dunn, R.; Fierer, N. Continental-scale distributions of dust-associated bacteria and fungi. Proc. Natl. Acad. Sci. USA 2015, 112, 5756–5761. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Park, E.H.; Lee, S.; Ko, G.; Honda, Y.; Hashizume, M.; Deng, F.; Yi, S.M.; Kim, H. Airborne bacterial communities in three east Asian cities of China, South Korea, and Japan. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dietzel, K.; Valle, D.; Fierer, N.; U’Ren, J.M.; Barberán, A. Geographical Distribution of Fungal Plant Pathogens in Dust Across the United States. Front. Ecol. Evol. 2019, 7, 304. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, A.M.; Kirtsideli, I.Y. Microfungi from air at St.-Petersburg. Nov. Sist. Nizshikh Rasteniy 2005, 39, 136–141. (In Russian) [Google Scholar]
- Wuyts, K.; Smets, W.; Lebeer, S.; Samson, R. Green infrastructure and atmospheric pollution shape diversity and composition of phyllosphere bacterial communities in an urban landscape. FEMS Microbiol. Ecol. 2020, 96, fiz173. [Google Scholar] [CrossRef]
- Marfenina, O.Y.; Makarova, N.V.; Ivanova, A.Y. Opportunistic moulds in soils and upper soil air layers in megalopolis (on an example of region Tushino, Moscow). Mikol. Fitopatol. 2011, 45, 397–407. (In Russian) [Google Scholar]
- Fernández-Rodríguez, S.; Tormo-Molina, R.; Maya-Manzano, J.M.; Silva-Palacios, I.; Gonzalo-Garijo, Á. Outdoor airborne fungi captured by viable and non-viable methods. Fungal Ecol. 2014, 7, 16–26. [Google Scholar] [CrossRef]
- Marfenina, O.Y.; Kolosova, Y.D.; Glagolev, M.V. Number of fungal diaspores deposited from surface air layers at the areas with different vegetation cover in Moscow City. Mikol. I Fitopatol. 2016, 50, 379–385. (In Russian) [Google Scholar]
- Korneykova, M.V.; Evdokimova, G.A. Microbiota of the ground air layers in natural and industrial zones of the Kola Arctic. J. Environ. Sci. Health Part A 2017, 53, 271–277. [Google Scholar] [CrossRef]
- Elmassry, M.M.; Ray, N.; Sorge, S.; Webster, J.; Merry, K.; Caserio, A.; Vecellio, D.J.; Kruczek, C.; Dowd, S.; Ardon-Dryer, K.; et al. Investigating the culturable atmospheric fungal and bacterial microbiome in West Texas: Implication of dust storms and origins of the air parcels. FEMS Microbes 2020, 1, xtaa009. [Google Scholar] [CrossRef]
- Perrino, C.; Marcovecchio, F. A new method for assessing the contribution of Primary Biological Atmospheric Particles to the mass concentration of the atmospheric aerosol. Environ. Int. 2016, 87, 108–115. [Google Scholar] [CrossRef]
- Fierer, N.; Liu, Z.; Rodríguez-Hernández, M.; Knight, R.; Henn, M.; Hernandez, M.T. Short-Term Temporal Variability in Airborne Bacterial and Fungal Populations. Appl. Environ. Microbiol. 2008, 74, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Frohlich-Nowoisky, J.; Pickersgill, D.A.; Despres, V.R.; Poschl, U. High diversity of fungi in air particulate matter. Proc. Natl. Acad. Sci. USA 2009, 106, 12814–12819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrego, N.; Norros, V.; Halme, P.; Somervuo, P.; Ali-Kovero, H.; Ovaskainen, O. Give me a sample of air and I will tell which species are found from your region: Molecular identification of fungi from airborne spore samples. Mol. Ecol. Resour. 2018, 18, 511–524. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Hu, Z.; Zhou, M.; Hu, J.; Yao, X.; Zhang, H.; Li, Z.; Lou, L.; Xi, C.; Qian, H.; et al. The distribution variance of airborne microorganisms in urban and rural environments. Environ. Pollut. 2019, 247, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Stephens, B.; Adams, R.I.; Bhangar, S.; Bibby, K.; Waring, M.S. From commensalism to mutualism: Integrating the microbial ecology, building science, and indoor air communities to advance research on the indoor microbiome. Indoor Air 2015, 25, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Climate-Data.org. Climate Data for Cities Worldwide. 2021. Available online: https://ru.climate-data.org/ (accessed on 15 February 2021).
- Weather Online Ltd. Meteorological Services. 2021. Available online: https://www.weatheronline.co.uk/ (accessed on 15 February 2021).
- Mosecomonitoring Site. 2020. Available online: https://mosecom.mos.ru/home-page/ (accessed on 15 August 2020).
- Zvyagintsev, D.G. Methods of Soil Microbiology and Biochemistry; P.C. MGU: Moscow, Russia, 1991. (In Russian) [Google Scholar]
- Klich, M.A. Identification of Common Aspergillus Species; CBS Fungal Biodiversity Centre: Utrecht, The Netherlands, 2002. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi, 2nd ed.; IHW–Verlag: Eching, Germany, 2007. [Google Scholar]
- Seifert, K.; Morgan-Jones, G.; Gams, W.; Kendrick, B. The Genera of Hyphomycetes; CBS, Reus: Utrecht, The Netherlands, 2011. [Google Scholar]
- Index Fungorum. A Nomenclatural Database. 2021. Available online: http://www.indexfungorum.org (accessed on 3 March 2021).
- de Hoog, G.S.; Guarro, J.; Gené, J.; Figueras, M.J. Atlas of Clinical Fungi, 3rd ed.; Reus: Utrecht, The Netherlands, 2019. [Google Scholar]
- Еvdokimova, G.A. Ecological-Microbiological Foundations of Soil Protection in the Far North; P.C. The Kola Science Centre of the Russian Academy of Sciences: Apatity, Russia, 1995. (In Russian) [Google Scholar]
- Kireyeva, N.A.; Miftakhova, A.M.; Bakayeva, M.D.; Vodopyanov, V.V. Complexes of Soil Microscopic Fungi under Technogenic Conditions; P.C. Gilem: Ufa, Russia, 2005. (In Russian) [Google Scholar]
- Marfenina, O.Y. Anthropogenic Ecology of Soil Fungi; P.C. Meditsina dlya vsekh: Moscow, Russia, 2005. (In Russian) [Google Scholar]
- Khabibullina, F.M. Soil Mycobiota of Natural and Anthropogenically Disturbed Ecosystems in the North-East of the European Part of Russia. Ph.D. Dissertation, P.C. Komi Scientific Center (Ural Branch of the Russian Academy of Sciences), Syktyvkar, Russia, 2009. (In Russian). [Google Scholar]
- Korneykova, M.V.; Еvdokimova, G.A.; Lebedeva, Y.V. The complexes of potentially pathogenic microscopic fungi in anthropogenic polluted soils of Kola North. Mikol. Fitopatol. 2012, 46, 322–328. (In Russian) [Google Scholar]
- Bogomolova, Y.V.; Velikova, T.D.; Goryayeva, A.G.; Ivanova, A.M.; Kirtsideli, I.Y.; Lebedeva, Y.V.; Mamayeva, N.Y.; Panina, L.K.; Popikhina, Y.A.; Smolyanitskaya, O.L.; et al. Microfungi in the Air of Saint Petersburg; P.C. Khimizdat: Saint Peterburg, Russia, 2012. (In Russian) [Google Scholar]
- Novikov, A.I.; Shirokaya, A.A.; Drogobuzhskaya, S.V.; Gaponenkov, I.A. Elemental analysis of suspended atmospheric particles in the city by inductively coupled plasma atomic emission spectrometry. In Arkticheskiye Issledovaniya: Ot Ekstensivnogo Osvoyeniya k Kompleksnomu Razvitiyu [Arctic Research: From Extensive Development to Integrated Development]: Materialy II Mezhdunarodnoy Nauchno-Prakticheskoy Konferentsii; Publishing Center A3 +: Arkhangelsk, Russia, 2020; pp. 391–395. (In Russian) [Google Scholar]
- Xie, W.-Y.; Su, J.-Q.; Zhu, Y.-G. Arsenite Oxidation by the Phyllosphere Bacterial Community Associated withWolffia australiana. Environ. Sci. Technol. 2014, 48, 9668–9674. [Google Scholar] [CrossRef] [PubMed]
- Sangthong, S.; Suksabye, P.; Thiravetyan, P. Air-borne xylene degradation by Bougainvillea buttiana and the role of epiphytic bacteria in the degradation. Ecotoxicol. Environ. Saf. 2016, 126, 273–280. [Google Scholar] [CrossRef]
- Gilbert, K.J.; Renner, T. Acid or base? How do plants regulate the ecology of their phylloplane? AoB Plants 2021, 13, plab032. [Google Scholar] [CrossRef]
- Xiao, L.; Yu, Z.; Liu, H.; Tan, T.; Yao, J.; Zhang, Y.; Wu, J. Effects of Cd and Pb on diversity of microbial community and enzyme activity in soil. Ecotoxicology 2020, 29, 551–558. [Google Scholar] [CrossRef]
- Mourato, M.; Pinto, F.; Moreira, I.; Sales, J.; Leitão, I.; Martins, L.L. The effect of Cd stress in mineral nutrient uptake in plants. In Cadmium Toxicity and Tolerance in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 327–348. [Google Scholar] [CrossRef]
- Fan, D.; Sun, J.; Liu, C.; Wang, S.; Han, J.; Agathokleous, E.; Zhu, Y. Measurement and modeling of hormesis in soil bacteria and fungi under single and combined treatments of Cd and Pb. Sci. Total Environ. 2021, 783, 147494. [Google Scholar] [CrossRef] [PubMed]
- Marfenina, O.Y.; Fomicheva, G.M. Potentially pathogenic filamentous fungi in the human environment. Modern tendencies. In Mikologiya Segodnya; Dyakov, Y.T., Sergeyev, Y.V., Eds.; Natsionalnaya akademiya: Moscow, Russia, 2007; pp. 235–266. (In Russian) [Google Scholar]
- Nagaki, M.; Narita, T.; Ichikawa, H.; Kawakami, J.; Nakane, A. Antibacterial and antifungal activities of isoprenoids. Trans. Mater. Res. Soc. Jpn. 2011, 36, 55–58. [Google Scholar] [CrossRef] [Green Version]
- Kurkina, Y.; Esina, E.; Barskova, A. Phytoncidal activity of essential oils of medicinal plants to some strains of mold fungi. J. Agric. Environ. 2020, 1, 1–4. [Google Scholar] [CrossRef]
- Hui, N.; Jumpponen, A.; Francini, G.; Kotze, D.J.; Liu, X.; Romantschuk, M.; Strömmer, R.; Setälä, H. Soil microbial communities are shaped by vegetation type and park age in cities under cold climate. Environ. Microbiol. 2017, 19, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Resolution of 09.11.1999 No. 1018 On Approval of the Rules for the Sanitary Maintenance of Territories, the Organization of Cleaning and Ensuring Cleanliness and Right in Moscow (as Amended on 2 February 2017); The Government of Moscow: Moscow, Russia, 1999. (In Russian)
- Zelenskaya, M.S.; Sidelnikova, M.V.; Panova, Y.G.; Pautov, A.A.; Krylova, E.G.; Pagoda, Y.O.; Vlasov, D.Y. Phylloplane fungi in the urban environment. Biosfera 2017, 9, 136–151. (In Russian) [Google Scholar]
- Carlile, M.J.; Watkinson, S.C.; Gooday, G.W. The Fungi, 2nd ed.; Academic Press: San Diego, CA, USA; San Francisco, CA, USA; New York, NY, USA; Boston, MA, USA, 2001. [Google Scholar]

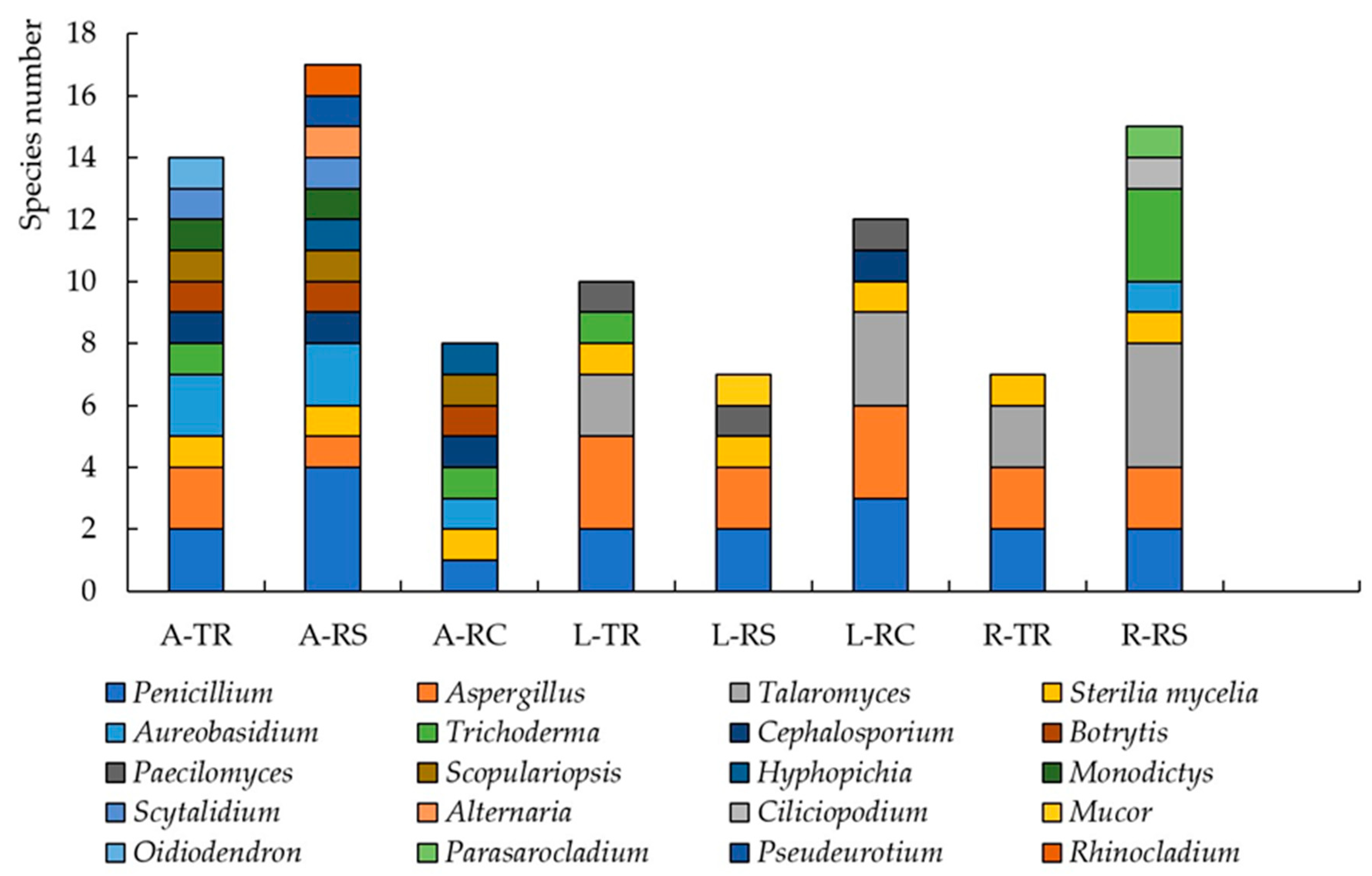
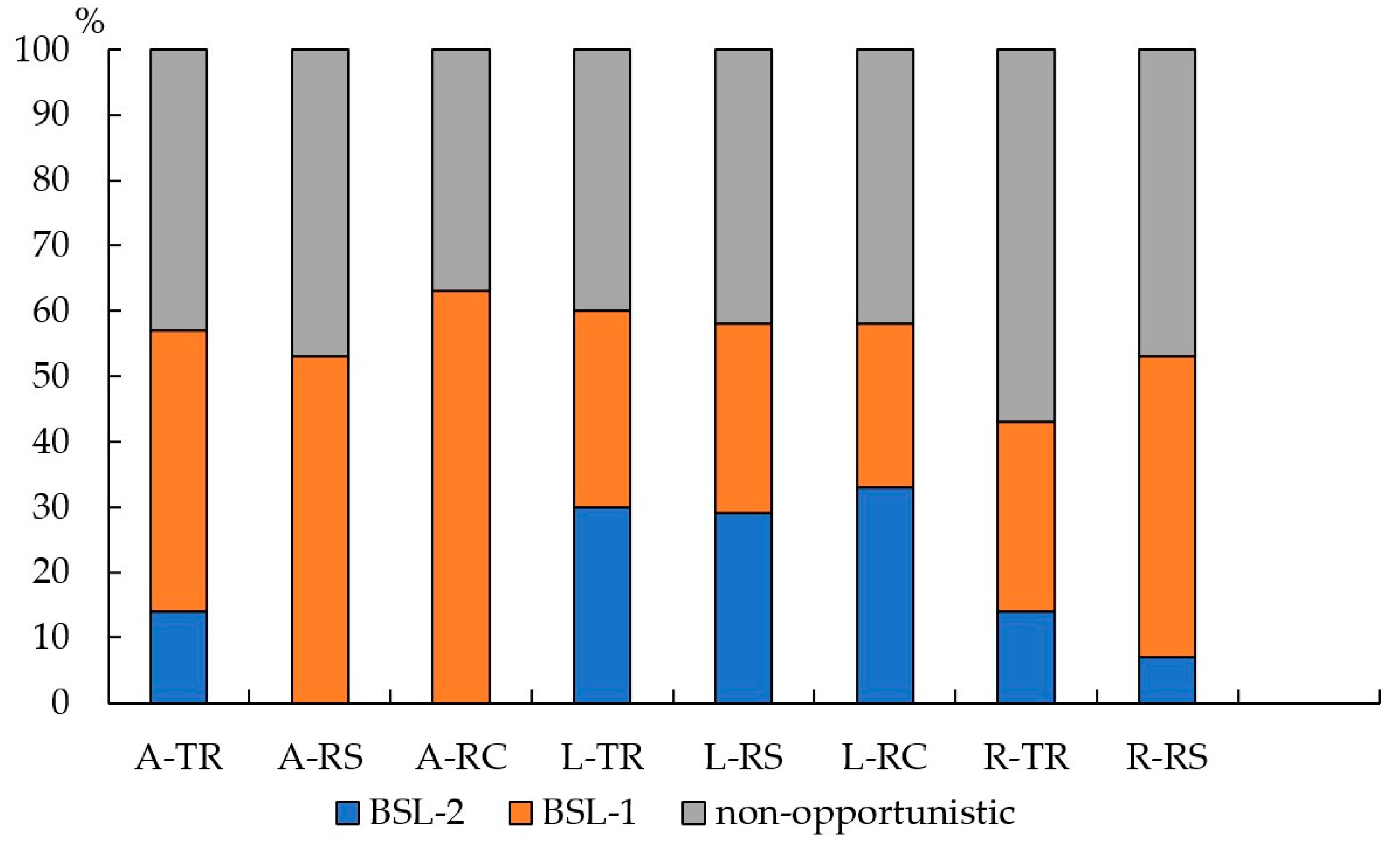
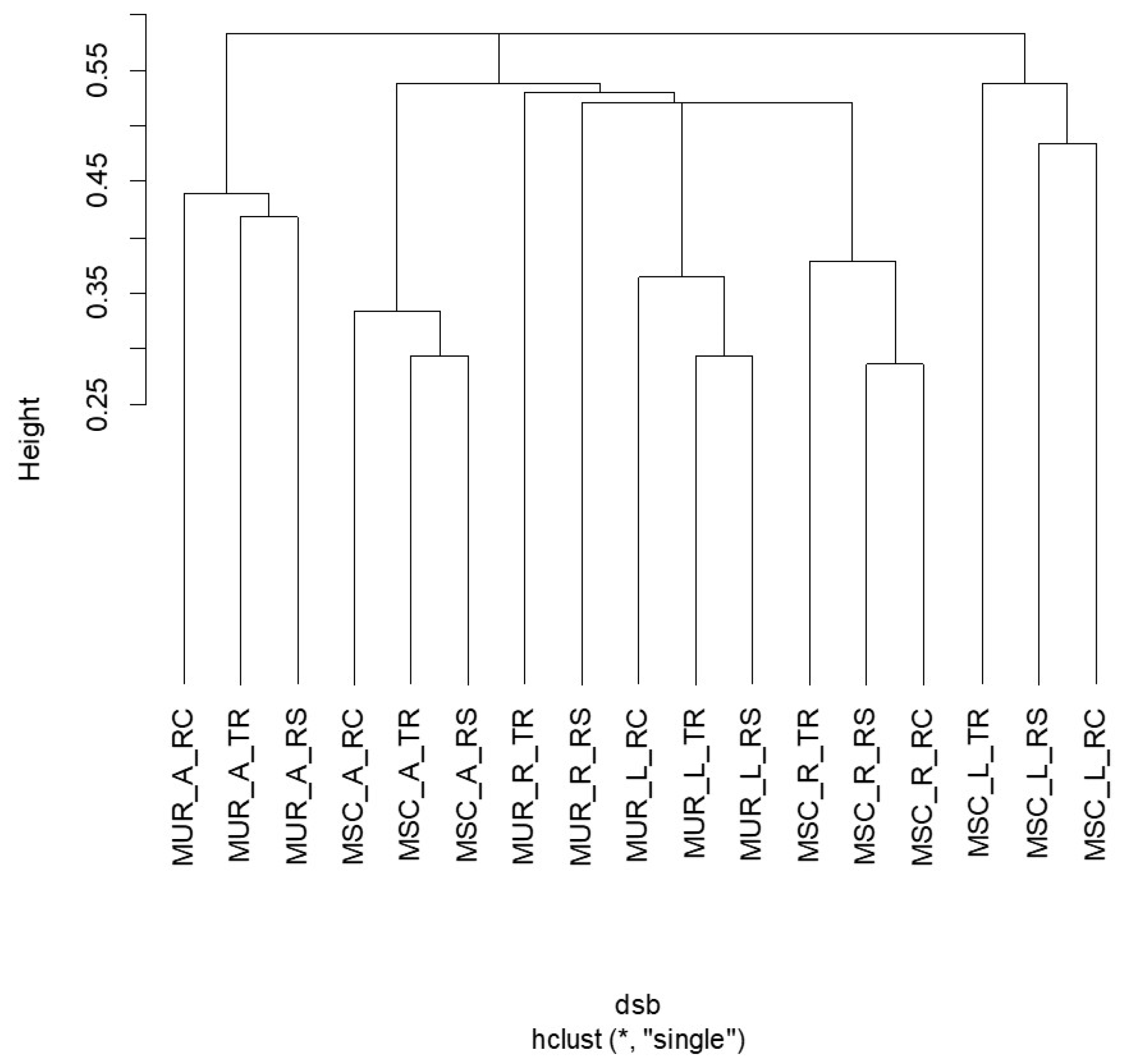
| Index | Traffic | Residential | Recreational |
|---|---|---|---|
| Murmansk | |||
| Coordinates | 68°57′36.0″ N 33°03′50.1″ E | 68°58′44.2″ N 33°05′36.8″ E | 68°57′36.0″ N 33°03′50.1″ E |
| Description | Crossing major highways; Proximity of the railway track; Proximity of a combined heat and power plant running on heavy fuel oil | Inter-house territory; Road transport—parking, carriageway; Houses: 5-storey, 2-storey | The territory of the ski sports complex with a closed passage for motor transport; Remoteness from major highways |
| Location of trees | Protective strip between the highway and the pedestrian sidewalk | House greening | Background forest |
| Dominating tree species | Betula pubescens Ehrh. Sorbus aucuparia L. | Betula pubescens Ehrh. Populus tremula L. Sorbus aucuparia L. | Betula pubescens Ehrh. Salix spp. |
| Moscow | |||
| Coordinates | 55°71′86.5″ N, 37°57′01.4″ E | 55°71′54.4″ N, 37°60′70.2″ E | 55°70′93.0″ N 37°52′82.0″ E |
| Description | - Crossing major highways; -Proximity of fuel stations | - Inter-house territory; - Road transport—parking, carriageway; - Houses: 4-storey | - Territories close to the university botanical garden - Remoteness from major highways |
| Location of trees | Protective strip between the highway and the pedestrian sidewalk | House greening | Urban park area |
| Dominating tree species | Betula pendula Roth. Betula pubescens Ehrh Acer platanoides L. Tilia cordata Mill. Picea abies L. | Betula pendula Roth Betula pubescens Ehrh. Acer platanoides L. Acer negundo L. Tilia cordata Mill. Picea abies L. | Betula pendula Roth Betula pubescens Ehrh. Malus sylvestris Acer platanoides L. Acer negundo L. Picea abies L. |
| Species | Moscow | Murmansk | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Air | Leaves | Road | Air | Leaves | Road | ||||||||||||
| TR | RS | RC | TR | RS | RC | TR | RS | RC | TR | RS | RC | TR | RS | RC | TR | RS | |
| Ascomycota Pezizomycotina Dothideomycetes Dothideomycetidae Dothideales Saccotheciaceae | |||||||||||||||||
| Aureobasidium melanogenum (Herm.–Nijh.) Zalar, Gostinčar, and Gunde–Cim. | 2 | 4 | |||||||||||||||
| A. pullulans (de Bary and Löwenthal) G. Arnaud | 87 | 63 | 23 | 5 | 1 | 18 | 5 | 2 | 22 | 2 | |||||||
| Pleosporomycetidae Pleosporales Pleosporaceae | |||||||||||||||||
| Alternaria atra (Preuss) Woudenb. and Crous | 8 | ||||||||||||||||
| A. tenuissima (Kunze) Wiltshire | 25 | 7 | 34 | <1 | |||||||||||||
| Eurotiomycetes Eurotiomycetidae Eurotiales Aspergillaceae | |||||||||||||||||
| Aspergillus candidus Link | 3 | 7 | |||||||||||||||
| A. flavipes (Bainier and R. Sartory) Thom and Church | 4 | ||||||||||||||||
| A. flavus Link | <1 | 5 | 2 | 8 | 23 | 7 | 7 | 2 | 7 | 13 | 12 | 2 | |||||
| A. fumigatus Fresen. | 21 | <1 | 2 | 11 | 14 | 11 | 3 | 2 | 4 | 4 | 2 | ||||||
| A. niger Tiegh. | 16 | 1 | 3 | 1 | 17 | 23 | 6 | 4 | 2 | ||||||||
| A. terreus Thom | 2 | ||||||||||||||||
| A. versicolor (Vuill.) Tirab. | 1 | ||||||||||||||||
| Paecilomyces variotii Bainier | 1 | 1 | 3 | 3 | 4 | ||||||||||||
| Penicillium aurantiogriseum Dierckx | 1 | 1 | 1 | ||||||||||||||
| P. chrysogenum Thom | 14 | 1 | |||||||||||||||
| P. expansum Link | <1 | ||||||||||||||||
| P. griseofulvum Dierckx | <1 | 1 | |||||||||||||||
| P. sp | 32 | 2 | 6 | ||||||||||||||
| P. spinulosum Thom | 1 | 7 | 2 | ||||||||||||||
| Talaromyces helicus (Raper and Fennell) C.R. Benj. | <1 | 2 | 6 | 12 | 4 | 5 | |||||||||||
| T. purpureogenus Samson, N. Yilmaz, Houbraken, Spierenb., Seifert, Peterson, Varga, and Frisvad | 3 | 2 | 5 | ||||||||||||||
| T. rugulosus (Thom) Samson, N. Yilmaz, Frisvad, and Seifert | 4 | 1 | |||||||||||||||
| Sordariomycetes Hypocreomycetidae Hypocreales Hypocreaceae | |||||||||||||||||
| Trichoderma aureoviride Rifai | 1 | 8 | |||||||||||||||
| T. glaucum E.V. Abbott | 15 | ||||||||||||||||
| T. koningii Oudem. | 2 | 2 | 1 | 22 | 10 | 29 | 6 | 22 | |||||||||
| T. viride Pers. | <1 | <1 | 8 | 5 | |||||||||||||
| Nectriaceae | |||||||||||||||||
| Fusarium sp. | 2 | 12 | |||||||||||||||
| Microascales Microascaceae | |||||||||||||||||
| Scopulariopsis candida Vuill. | 16 | 25 | 18 | ||||||||||||||
| Saccharomycotina Saccharomycetes Saccharomycetidae Saccharomycetales Incertae sedis | |||||||||||||||||
| Hyphopichia burtonii (Boidin, Pignal, Lehodey, Vey & Abadie) Arx and Van der Walt | 1 | 1 | 1 | ||||||||||||||
| Mucoromycota Mucoromycotina Mucoromycetes Incertae sedis Mucorales Mucoraceae | |||||||||||||||||
| Mucor hiemalis Wehmer | 7 | ||||||||||||||||
| Rhizopodaceae | |||||||||||||||||
| Rhizopus stolonifer (Ehrenb.) Vuill. | 1 | 14 | 1 | 2 | 1 | ||||||||||||
| Element Concentration µg g−1 | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subsrate | Parameters | Dust Weight | N. of Elements | Weight of Elements | Li | Be | B | Cr | Fe | Co | Ni | Cu | Zn | Ga | Rb | Sr | Y | Zr | Nb | Mo | Cd | Sn | Sb | Cs | Ba | Lanthanide | Hf | W | Pb | Th | U | Ti | V | Mn | Na | Mg | Al | Si | K | Ca |
| Air | Quantity total | 0.5 | 0.6 | 0.6 | 0.6 | 0.6 | 0.5 | 0.6 | 0.6 | 0.5 | 0.5 | 0.6 | 0.6 | 0.5 | 0.5 | |||||||||||||||||||||||||
| Quantity opportunistic | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.7 | 0.7 | 0.7 | 0.6 | 0.5 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.7 | 0.5 | ||||||||||||||||||
| Species richness | 0.5 | |||||||||||||||||||||||||||||||||||||||
| Fraction BSL-2 | ||||||||||||||||||||||||||||||||||||||||
| Fraction BSL-1 | 0.5 | 0.6 | 0.6 | 0.6 | ||||||||||||||||||||||||||||||||||||
| Leaves | Quantity total | |||||||||||||||||||||||||||||||||||||||
| Quantity opportunistic | ||||||||||||||||||||||||||||||||||||||||
| Species richness | 0.5 | 0.5 | 0.5 | 0.5 | ||||||||||||||||||||||||||||||||||||
| Fraction BSL-2 | 0.5 | |||||||||||||||||||||||||||||||||||||||
| Fraction BSL-1 | ||||||||||||||||||||||||||||||||||||||||
| Road | Quantity total | 0.7 | 0.6 | 0.6 | 0.5 | 0.6 | 0.5 | 0.6 | 0.5 | 0.5 | 0.6 | 0.7 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | ||||||||||||||
| Quantity opportunistic | 0.6 | 0.6 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | ||||||||||||||||||||||||||||||||
| Species richness | 0.6 | 0.6 | 0.5 | 0.6 | 0.6 | 0.6 | 0.5 | 0.6 | 0.5 | 0.6 | ||||||||||||||||||||||||||||||
| Fraction BSL-2 | 0.6 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.6 | 0.6 | 0.6 | 0.5 | 0.6 | 0.6 | 0.6 | 0.6 | 0.5 | 0.6 | 0.5 | 0.5 | ||||||||||||||||||||||
| Fraction BSL-1 | 0.7 | |||||||||||||||||||||||||||||||||||||||
| Element Concentration µg m−2 | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subsrate | Parameters | Dust Weight | N. of Elements | Weight of Elements | Li | Be | B | Cr | Fe | Co | Ni | Cu | Zn | Ga | Rb | Sr | Y | Zr | Nb | Mo | Cd | Sn | Sb | Cs | Ba | Lanthanide | Hf | W | Pb | Th | U | Ti | V | Mn | Na | Mg | Al | Si | K | Ca |
| Air | Quantity total | |||||||||||||||||||||||||||||||||||||||
| Quantity opportunistic | 0.6 | 0.6 | 0.5 | 0.6 | 0.5 | 0.5 | 0.5 | 0.6 | 0.5 | 0.5 | ||||||||||||||||||||||||||||||
| Species richness | 0.5 | |||||||||||||||||||||||||||||||||||||||
| Fraction BSL-2 | ||||||||||||||||||||||||||||||||||||||||
| Fraction BSL-1 | 0.6 | 0.6 | 0.6 | 0.7 | 0.7 | 0.6 | 0.6 | |||||||||||||||||||||||||||||||||
| Leaves | Quantity total | |||||||||||||||||||||||||||||||||||||||
| Quantity opportunistic | ||||||||||||||||||||||||||||||||||||||||
| Species richness | ||||||||||||||||||||||||||||||||||||||||
| Fraction BSL-2 | ||||||||||||||||||||||||||||||||||||||||
| Fraction BSL-1 | ||||||||||||||||||||||||||||||||||||||||
| Road | Quantity total | 0.7 | 0.6 | 0.6 | 0.6 | 0.5 | 0.7 | 0.6 | 0.5 | 0.5 | 0.5 | 0.6 | 0.5 | |||||||||||||||||||||||||||
| Quantity opportunistic | 0.6 | 0.5 | 0.5 | 0.6 | ||||||||||||||||||||||||||||||||||||
| Species richness | 0.6 | 0.6 | 0.7 | 0.6 | 0.6 | 0.6 | 0.6 | 0.5 | 0.7 | 0.6 | 0.7 | 0.6 | 0.7 | 0.6 | 0.6 | 0.7 | 0.7 | 0.6 | 0.6 | 0.6 | 0.7 | 0.6 | 0.6 | |||||||||||||||||
| Fraction BSL-2 | 0.6 | 0.5 | 0.5 | 0.5 | 0.5 | 0.6 | 0.6 | 0.5 | 0.6 | 0.5 | 0.6 | 0.6 | 0.6 | 0.6 | 0.5 | 0.6 | 0.6 | 0.5 | 0.5 | |||||||||||||||||||||
| Fraction BSL-1 | 0.7 | |||||||||||||||||||||||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korneykova, M.V.; Soshina, A.S.; Novikov, A.I.; Ivashchenko, K.V.; Sazonova, O.I.; Slukovskaya, M.V.; Shirokaya, A.A.; Vasenev, V.I.; Vetrova, A.A.; Gavrichkova, O. Microscopic Fungi in Big Cities: Biodiversity, Source, and Relation to Pollution by Potentially Toxic Metals. Atmosphere 2021, 12, 1471. https://doi.org/10.3390/atmos12111471
Korneykova MV, Soshina AS, Novikov AI, Ivashchenko KV, Sazonova OI, Slukovskaya MV, Shirokaya AA, Vasenev VI, Vetrova AA, Gavrichkova O. Microscopic Fungi in Big Cities: Biodiversity, Source, and Relation to Pollution by Potentially Toxic Metals. Atmosphere. 2021; 12(11):1471. https://doi.org/10.3390/atmos12111471
Chicago/Turabian StyleKorneykova, Maria V., Anastasia S. Soshina, Andrey I. Novikov, Kristina V. Ivashchenko, Olesya I. Sazonova, Marina V. Slukovskaya, Anna A. Shirokaya, Viacheslav I. Vasenev, Anna A. Vetrova, and Olga Gavrichkova. 2021. "Microscopic Fungi in Big Cities: Biodiversity, Source, and Relation to Pollution by Potentially Toxic Metals" Atmosphere 12, no. 11: 1471. https://doi.org/10.3390/atmos12111471
APA StyleKorneykova, M. V., Soshina, A. S., Novikov, A. I., Ivashchenko, K. V., Sazonova, O. I., Slukovskaya, M. V., Shirokaya, A. A., Vasenev, V. I., Vetrova, A. A., & Gavrichkova, O. (2021). Microscopic Fungi in Big Cities: Biodiversity, Source, and Relation to Pollution by Potentially Toxic Metals. Atmosphere, 12(11), 1471. https://doi.org/10.3390/atmos12111471







