Bacterial Characteristics of Dust Particle Saltation in Gobi Dust Sites, Mongolia
Abstract
1. Introduction
2. Materials and Methods
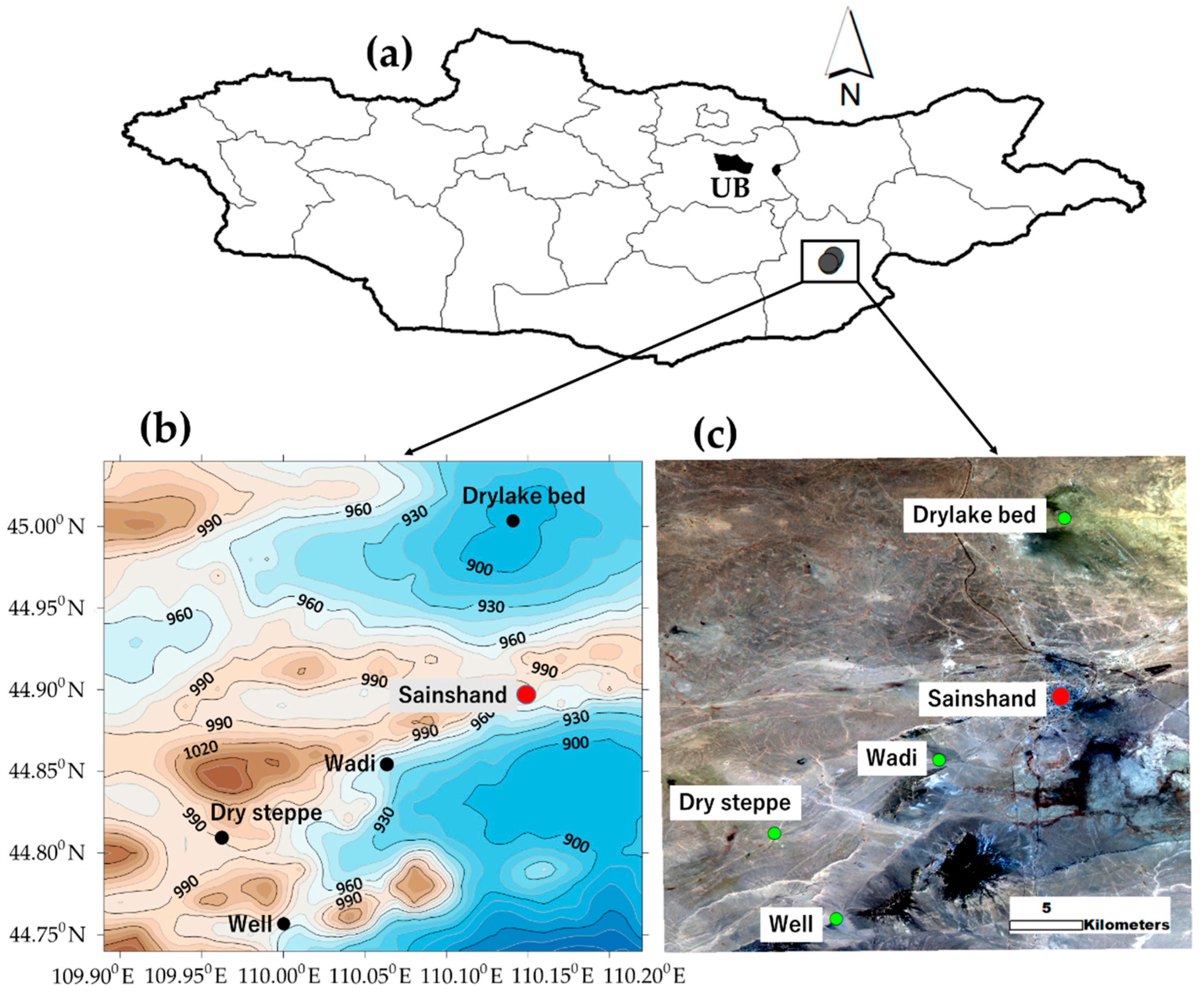
2.1. Sample Collection Sites
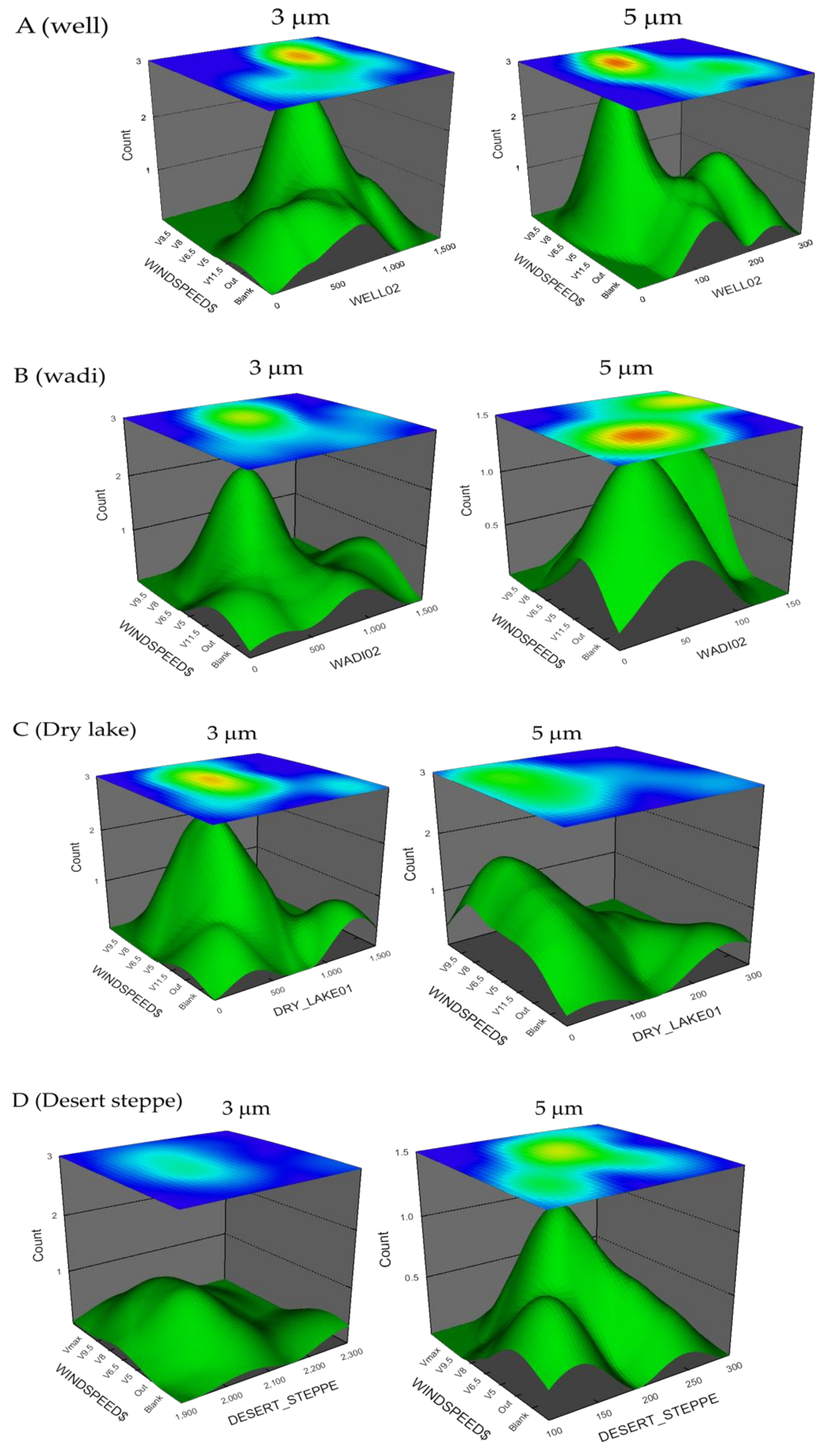
2.2. Dust Particle Saltation Experiments
2.3. Bacterial Isolation
Amplification of 16s rDNA and Determination of Gene Sequence
2.4. Sample Data Analysis
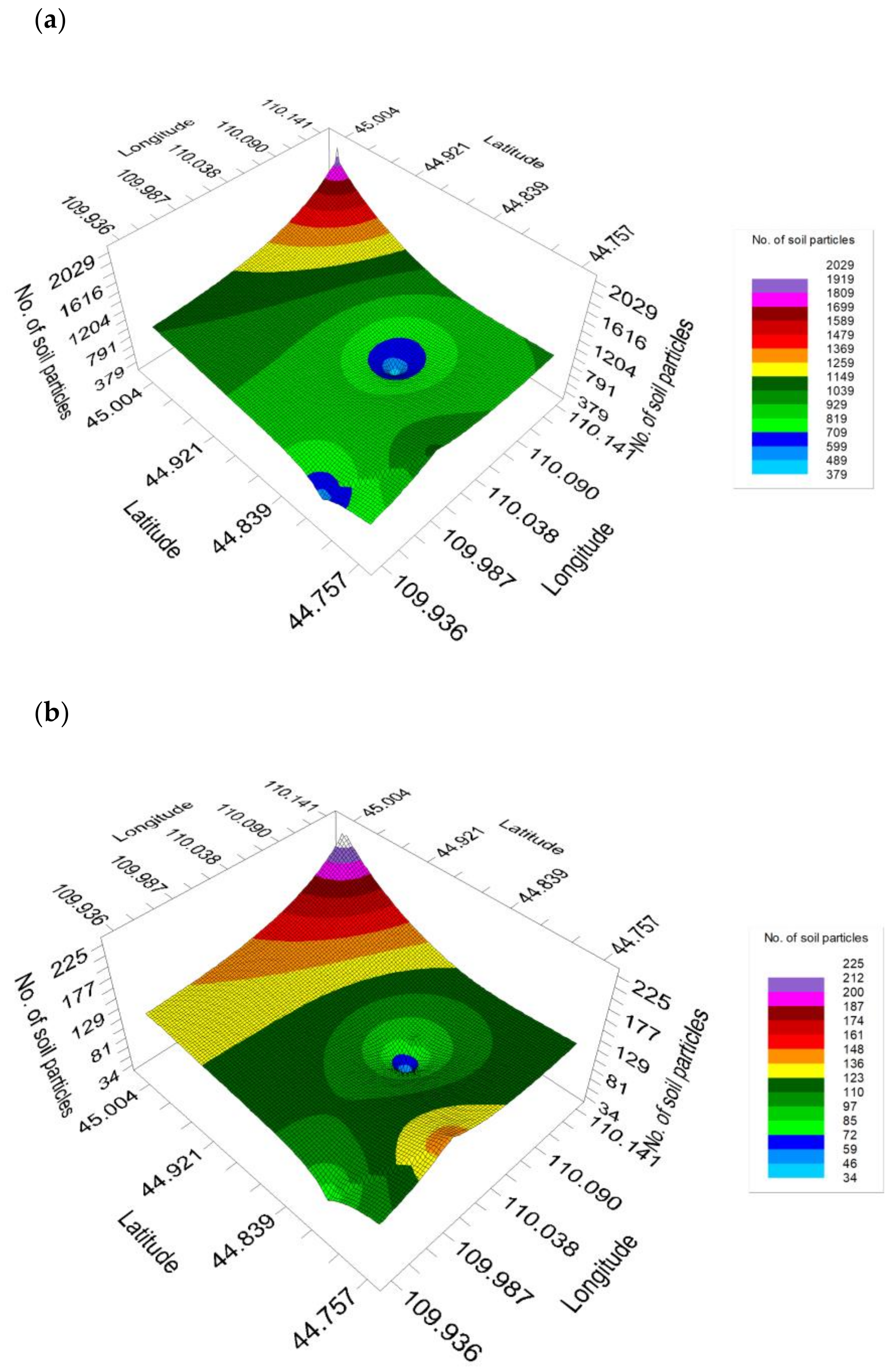
3. Results
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lelieveld, J.; Pöschl, U. Chemists Can Help to Solve the Air-Pollution Health Crisis. Nature 2017, 551, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.J.; Fooks, A.R.; van der Poel, W.H. Public Health Threat of New, Reemerging, and Neglected Zoonoses in the Industrialized World. Emerg. Infect. Dis. 2010, 16, 1–7. [Google Scholar] [CrossRef]
- Otte, J.; Pica-Ciamarra, U. Emerging Infectious Zoonotic Diseases: The Neglected Role of Food Animals. One Health 2021, 13, 100323. [Google Scholar] [CrossRef]
- Magouras, I.; Brookes, V.J.; Jori, F.; Martin, A.; Pfeiffer, D.U.; Dürr, S. Emerging Zoonotic Diseases: Should We Rethink the Animal-Human Interface? Front. Vet. Sci. 2020, 7, 582743. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.S.; Pepin, K.M. Board Invited Review: Prospects for Improving Management of Animal Disease Introductions Using Disease-Dynamic Models. J. Anim. Sci. 2019, 97, 2291–2307. [Google Scholar] [CrossRef]
- Tiwari, R.; Dhama, K.; Sharun, K.; Iqbal Yatoo, M.; Malik, Y.S.; Singh, R.; Michalak, I.; Sah, R.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. COVID-19: Animals, Veterinary and Zoonotic Links. Vet. Q. 2020, 40, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Torres-Velez, F.; Havas, K.A.; Spiegel, K.; Brown, C. Transboundary Animal Diseases as Re-Emerging Threats—Impact on One Health. Semin. Diagn. Pathol. 2019, 36, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.P.; Singh, R.K.; Malik, Y.S. Emerging and Transboundary Animal Viral Diseases: Perspectives and Preparedness. Livest. Dis. Manag. 2020, 1–25. [Google Scholar] [CrossRef]
- Jugder, D.; Shinoda, M.; Kimura, R.; Batbold, A.; Amarjargal, D. Quantitative Analysis on Windblown Dust Concentrations of PM10 (PM2.5) During Dust Events in Mongolia. Aeolian Res. 2014, 14, 3–13. [Google Scholar] [CrossRef]
- Shao, Y. Physics and Modelling of Wind Erosion; Springer Science and Business Media: New York, NY, USA, 2008. [Google Scholar]
- Hoshino, B.S.R.; Sofue, Y.; Demura, Y.; Purevsuren, T.; Hai, Y. Social Transition from Nomad to Settlement in the Mongolian Steppe and Its Impact on Japan; The Association for Kyosei Studies: Tokyo, Japan, 2015; Volume 9, pp. 1–27. [Google Scholar]
- Sugimoto, N.; Uno, I.; Nishikawa, M.; Shimizu, A.; Matsui, I.; Dong, X.; Chen, Y.; Quan, H. Record Heavy Asian Dust in Beijing in 2002: Observations and Model Analysis of Recent Events. Geophys. Res. Lett. 2003, 30, 1640. [Google Scholar] [CrossRef]
- Jugder, D.; Shinoda, M.; Sugimoto, N.; Matsui, I.; Nishikawa, M.; Park, S.-U.; Chun, Y.-S.; Park, M.-S. Spatial and Temporal Variations of Dust Concentrations in the Gobi Desert of Mongolia. Glob. Planet Chang. 2011, 78, 14–22. [Google Scholar] [CrossRef]
- Hoshino, B.; Sofue, Y.; Demura, Y.; Purevsuren, T.; Kuribayashi, M.; Baba, K.; Zoljargal, E.; Hagiwara, K.; Noda, J.; Kawano, K.; et al. Detection of Dry Lake Beds Formation and Estimate of Environmental Regime Shift in Semi-Arid Region. J. Arid Land Stud. 2018, 28, 109–113. [Google Scholar]
- Tsai, F.J.; Fang, Y.S.; Huang, S.J. Case Study of Asian Dust Event on March 19–25, 2010 and Its Impact on the Marginal Sea of China. J. Mar. Sci. Technol. 2013, 21, 353–360. [Google Scholar]
- Lin, C.Y.; Liu, S.C.; Chou, C.C.-K.; Liu, T.H.; Lee, C.-T.; Yuan, C.-S.; Shiu, C.-J.; Young, C.L. Long-Range Transport of Asian Dust and Air Pollutants to Taiwan. Terr. Atmos. Ocean. Sci. 2004, 15, 759–784. [Google Scholar] [CrossRef]
- Liu, G.R.; Lin, T.H. Application of Geostationary Satellite Observations for Monitoring Dust Storms of Asia. Terr. Atmos. Ocean. Sci. 2004, 15, 825–837. [Google Scholar] [CrossRef]
- Shaw, G.E. Transport of Asian Desert Aerosol to the Hawaiian Islands. J. Appl. Meteorol. 1980, 19, 1254–1259. [Google Scholar] [CrossRef]
- Parrington, J.R.; Zoller, W.H.; Aras, N.K. Asian Dust: Seasonal Transport to the Hawaiian Islands. Science 1983, 220, 195–197. [Google Scholar] [CrossRef]
- Uematsu, M.; Duce, R.A.; Prospero, J.M.; Chen, L.; Merrill, J.T.; McDonald, R.L. Transport of Mineral Aerosol from Asia Over the North Pacific Ocean. J. Geophys. Res. 1983, 88, 5343–5352. [Google Scholar] [CrossRef]
- Merrill, J.T.; Uematsu, M.; Bleck, R. Meteorological Analysis of Long Range Transport of Mineral Aerosols Over the North Pacific. J. Geophys. Res. 1989, 94, 8584–8598. [Google Scholar] [CrossRef]
- Ishizuka, M.; Mikami, M.; Yamada, Y.; Zeng, F. Threshold Friction Velocities of Saltation Sand Particles for Different Soil Moisture Conditions in the Taklimakan Desert. Sola 2009, 5, 184–187. [Google Scholar] [CrossRef]
- Shao, Y.; Dong, C.H. A review on East Asian Dust storm climate, modelling and monitoring. Glob. Planet. Chang. 2006, 52, 1–22. [Google Scholar] [CrossRef]
- Greeley, R.; White, B.R.; Pollack, J.B.; Iversen, J.D.; Leach, R.N. Dust Storms on Mars: Considerations and Simulations. NASA Technical Memorandum 78423. ID: 19780006043. 1977, pp. 1–30. Available online: https://research.engineering.ucdavis.edu/wind/wp-content/uploads/sites/17/2014/03/Greeley_1977_NASATM_Dust_Storms_on.pdf (accessed on 20 August 2020).
- Greeley, R.; White, B.R.; Pollack, J.B.; Iversen, J.D.; Leach, R.N. Dust storms on Mars: Considerations and simulations. In Desert Dust: Origin, Characteristics, and Effect on Man; Geological Society of America: Boulder, CO, USA, 1981; Volume 186, pp. 101–121. [Google Scholar]
- Greeley, R.; Iversen, J.D. Wind as a Geologic Process on Earth, Mars, Venus and Titan; Cambridge University Press: New York, NY, USA, 1985. [Google Scholar]
- Greeley, R. Toward an understanding of the Martian dust cycle, Dust on Mars ZZ. LPI Tech. Rept. 1986, 86-09, 29–31. [Google Scholar]
- Bagnold, R.A. The Physics of Blown Sand and Desert Dunes; Methuen: London, UK, 1941; p. 265. [Google Scholar]
- Greeley, R.; Lancaster, N.; Lee, S.; Thomas, P. Martian aeolian processes, sediments, and features. In Mars; Kieffer, H.H., Jakosky, B.M., Synder, C.W., Matthews, M.S., Eds.; University of Arizona Press: Tucson, AZ, USA, 1992; pp. 730–766. [Google Scholar]
- Greeley, R.; Kraft, M.; Sullivan, R.; Wilson, G.; Bridges, N.; Herkenhoff, K.; Kuzmin, O.; Malin, M.; Ward, W. Aeolian features and processes at the Mars Pathfinder landing site. J. Geophys. Res. Planets 1999, 104, 8573–8584. [Google Scholar] [CrossRef]
- NIES Research Booklet; Nishikawa, M. No. 8; National Institute for Environmental Studies: Tsukuba, Japan, 2003; p. 5. ISSN 1346-776X. (In Japanese)
- Greeley, R.; Bridges, N.T.; Kuzmin, R.O.; Laity, J.E. Terrestrial analogs to wind-related features at the Viking and Pathfinder landing sites on Mars. J. Geophys. Res. Planets 2002, 107, 5-1–5-22. [Google Scholar] [CrossRef]
- Greeley, R.; Balme, M.R.; Iversen, J.D.; Metzger, S.; Mickelson, R.; Phoreman, J.; White, B. Martian dust devils: Laboratory simulations of particle threshold. J. Geophys. Res. Planets 2003, 108. [Google Scholar] [CrossRef]
- Hagiwara, K.; Matsumoto, T.; Tsedendamba, P.; Baba, K.; Hoshino, B. Distribution of Viable Bacteria in the Dust-Generating Natural Source Area of the Gobi Region, Mongolia. Atmosphere 2020, 11, 893. [Google Scholar] [CrossRef]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef]
- Ichinose, T.; Nishikawa, M.; Takano, H.; Sera, N.; Sadakane, K.; Mori, I.; Yanagisawa, R.; Oda, T.; Tamura, H.; Hiyoshi, K.; et al. Pulmonary Toxicity Induced by Intratracheal Instillation of Asian Yellow Dust (Kosa) in Mice. Environ. Toxicol. Pharmacol. 2005, 20, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Baba, T.; Ichijo, T.; Himezawa, Y.; Enoki, K.; Saraya, M.; Li, P.F.; Nasu, M. Abundance and Community Structure of Bacteria on Asian Dust Particles Collected in Beijing, China, During the Asian Dust Season. Biol. Pharm. Bull. 2016, 39, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Kojima, H.; Saito, I.; Jin, K.; Kobayashi, S.; Tanaka-Kagawa, T.; Jinno, H. Detection of 34 Plasticizers and 25 Flame Retardants in Indoor Air from Houses in Sapporo, Japan. Sci. Total Environ. 2014, 491–492, 28–33. [Google Scholar] [CrossRef]
- Tang, K.; Huang, Z.; Huang, J.; Maki, T.; Zhang, S.; Shimizu, A.; Ma, X.; Shi, J.; Bi, J.; Zhou, T.; et al. Characterization of Atmospheric Bioaerosols Along the Transport Pathway of Asian Dust During the Dust-Bioaerosol 2016 Campaign. Atmos. Chem. Phys. 2018, 18, 7131–7148. [Google Scholar] [CrossRef]
- Martiny, J.B.; Bohannan, B.J.; Brown, J.H.; Colwell, R.K.; Fuhrman, J.A.; Green, J.L.; Horner-Devine, M.C.; Kane, M.; Krumins, J.A.; Kuske, C.R.; et al. Microbial Biogeography: Putting Microorganisms on the Map. Nat. Rev. Microbiol. 2006, 4, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.W. Atmospheric Movement of Microorganisms in Clouds of Desert Dust and Implications for Human Health. Clin. Microbiol. Rev. 2007, 20, 459–477. [Google Scholar] [CrossRef]
- Higashi, T.; Kambayashi, Y.; Ohkura, N.; Fujimura, M.; Nakanishi, S.; Yoshizaki, T.; Saijoh, K.; Hayakawa, K.; Kobayashi, F.; Michigami, Y.; et al. Exacerbation of Daily Cough and Allergic Symptoms in Adult Patients with Chronic Cough by Asian Dust: A Hospital-Based Study in Kanazawa. Atmos. Environ. 2014, 97, 537–543. [Google Scholar] [CrossRef]
- Demura, Y.; Hoshino, B.; Baba, K.; McCarthy, C.; Sofue, Y.; Kai, K.; Purevsuren, T.; Hagiwara, K.; Noda, J. Determining the Frequency of Dry Lake Bed Formation in Semi-Arid Mongolia From Satellite Data. Land 2017, 6, 88. [Google Scholar] [CrossRef]
- Sofue, Y.; Hoshino, B.; Demura, Y.; Kai, K.; Baba, K.; Nduati, E.; Kondoh, A.; Sternberg, T. Satellite Monitoring of Vegetation Response to Precipitation and Dust Storm Outbreaks in Gobi Desert Regions. Land 2018, 7, 19. [Google Scholar] [CrossRef]
- Wieringa, J. Updating the Davenport Roughness Classification. J. Wind Eng. Ind. Aér. 1992, 41, 357–368. [Google Scholar] [CrossRef]
- Davenport, A.G. Rationale for Determining Design Wind Velocities. J. Struct. Div. 1960, 86, 39–68. [Google Scholar] [CrossRef]
- Garrity, G.M.; Bell, J.A.; Lilburn, T.G. Taxonomic Outline of the Prokaryotes. In Bergey’s Manual of Systematic Bacteriology; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Bio Edit (a Biological Sequence Alignment Editor, Version 7.0.9). Available online: https://bioedit.software.informer.com (accessed on 20 August 2020).
- Mega, X. (Molecular Evolutionary Genetics Analysis across Computing Platforms, Version 10). Available online: https://www.megasoftware.net (accessed on 20 August 2020).
- Kenzaka, T.; Sueyoshi, A.; Baba, T.; Li, P.; Tani, K.; Yamaguchi, N.; Nasu, M. Soil Microbial Community Structure in an Asian Dust Source Region (Loess Plateau). Microbes Environ. 2010, 25, 53–57. [Google Scholar] [CrossRef]
- Yang, Y.; Dou, Y.; An, S. Testing Association Between Soil Bacterial Diversity and Soil Carbon Storage on the Loess Plateau. Sci. Total Environ. 2018, 626, 48–58. [Google Scholar] [CrossRef]
- Becker, K.; Ballhausen, B.; Köck, R.; Kriegeskorte, A. Methicillin Resistance in Staphylococcus Isolates: The “mec Alphabet” with Specific Consideration of mecC, a mec Homolog Associated with Zoonotic S. aureus Lineages. Int. J. Med. Microbiol. 2014, 304, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Vishnupriya, S.; Antony, P.X.; Mukhopadhyay, H.K.; Pillai, R.M.; Thanislass, J.; Vivek Srinivas, V.M.; Sumanth Kumar, R. Methicillin Resistant Staphylococci Associated with Bovine Mastitis and Their Zoonotic Importance. Vet. World 2014, 7, 422–427. [Google Scholar] [CrossRef]
- Becker, K. Methicillin-Resistant Staphylococci and Macrococci at the Interface of Human and Animal Health. Toxins 2021, 13, 61. [Google Scholar] [CrossRef]
- Clauß, M. Emission of Bioaerosols from Livestock Facilities: Methods and Results from Available Bioaerosol Investigations in and Around Agricultural Livestock Farming; Thünen Working Paper: Braunschweig, Germany, 2020; Volume 138a. [Google Scholar]
- Yang, X.; Yang, F.; Zhou, C.; Mamtimin, A.; Huo, W.; He, Q. Improved Parameterization for Effect of Soil Moisture on Threshold Friction Velocity for Saltation Activity Based on Observations in the Taklimakan Desert. Geoderma 2020, 369, 114322. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, J.; Ishizuka, M.; Mikami, M.; Leys, J.; Huang, N. Dependency of Particle Size Distribution at Dust Emission on Friction Velocity and Atmospheric Boundary-Layer Stability. Atmos. Chem. Phys. 2020, 20, 12939–12953. [Google Scholar] [CrossRef]
- Takahashi, Y. Exploitation of New Microbial Resources for Bioactive Compounds and Discovery of New Actinomycetes. Actinomycetologica 2004, 18, 54–61. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Zhou, Y.H. Reconstructing the Electrical Structure of Dust Storms from Locally Observed Electric Field Data. Nat. Commun. 2020, 11, 5072. [Google Scholar] [CrossRef]
- Goudarzi, G.; Daryanoosh, S.M.; Godini, H.; Hopke, P.K.; Sicard, P.; De Marco, A.; Rad, H.D.; Harbizadeh, A.; Jahedi, F.; Mohammadi, M.J.; et al. Health Risk Assessment of Exposure to the Middle-Eastern Dust Storms in the Iranian Megacity of Kermanshah. Public Health 2017, 148, 109–116. [Google Scholar] [CrossRef]
- Soleimani, Z.; Teymouri, P.; Darvishi Boloorani, A.D.; Mesdaghinia, A.; Middleton, N.; Griffin, D.W. An Overview of Bioaerosol Load and Health Impacts Associated with Dust Storms: A Focus on the Middle East. Atmos. Environ. 2020, 223, 117187. [Google Scholar] [CrossRef]

| Location | Ground Surface Temperature (°C) | Soil Moisture (%) | Temperature (°C) | Humidity (%) |
|---|---|---|---|---|
| Well | 40.2 | 3.0 | 26 | 14 |
| Wadi | 37.8 | 6.8 | 19.7 | 19 |
| Dry lake bed | 41.4 | 6.5 | 22.5 | 20 |
| Desert Steppe | 37.8 | 6.8 | 19.7 | 19 |
| Location | Surface Bacteria | Saltation Bacteria | ||
|---|---|---|---|---|
| Total (log CFU/g) | Total (log CFU/g) | Family | log CFU/g | |
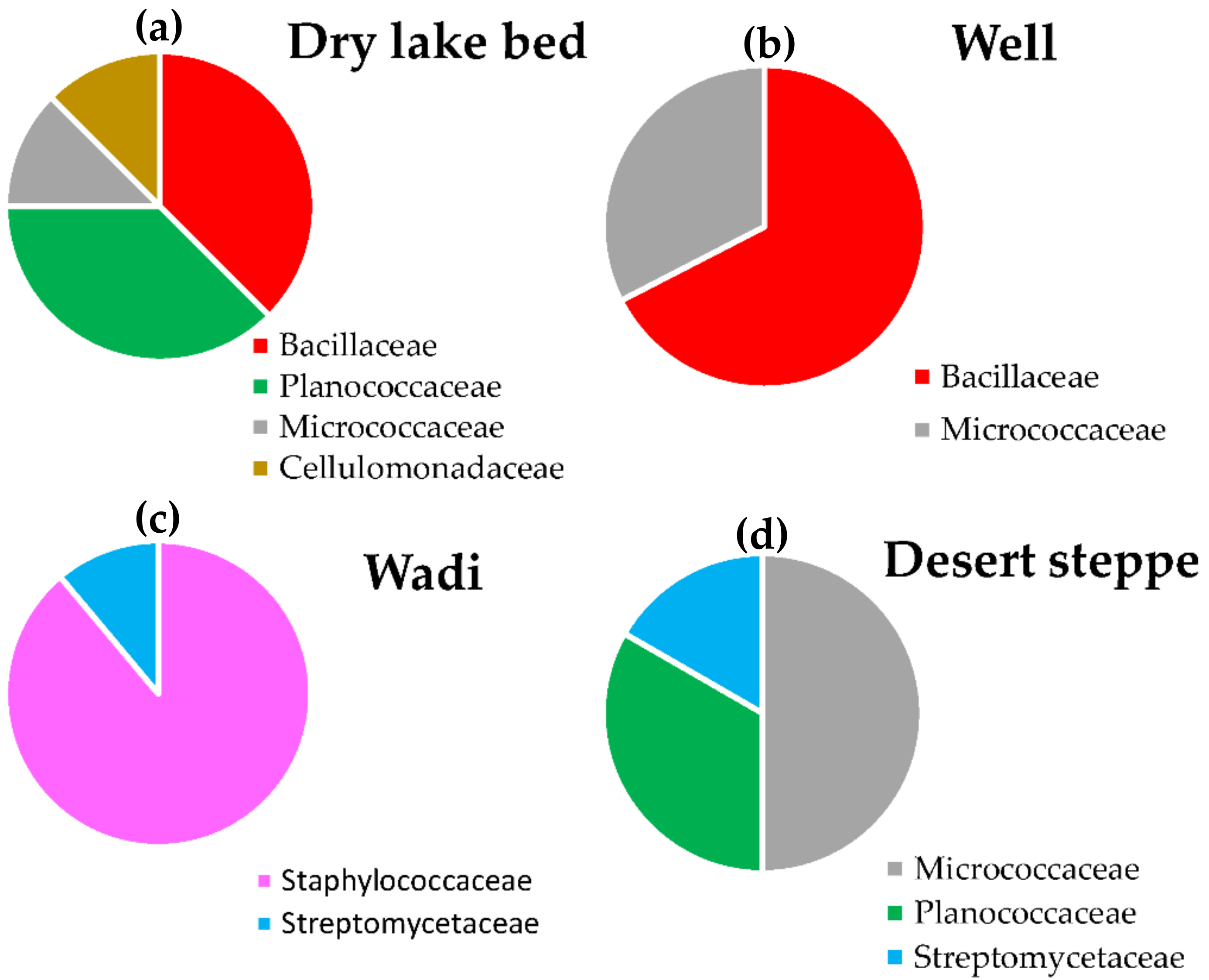
| Dry lake bed | 7.0 | 4.1 | Bacillaceae | 3.7 |
| Planococcaceae | 3.7 | |||
| Micrococcaceae | 3.2 | |||
| Cellulomonadaceae | 3.2 | |||
| Wadi | 6.5 | 4.5 | Staphylococcaceae | 4.4 |
| Streptomycetaceae | 3.5 | |||
| Well | 7.3 | 4.9 | Bacillaceae | 4.7 |
| Micrococcaceae | 4.4 | |||
| Desert Steppe | 6.4 | 4.0 | Micrococcaceae | 3.7 |
| Planococcaceae | 3.5 | |||
| Streptomycetaceae | 3.2 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagiwara, K.; Matsumoto, T.; Tsedendamba, P.; Baba, K.; Hoshino, B. Bacterial Characteristics of Dust Particle Saltation in Gobi Dust Sites, Mongolia. Atmosphere 2021, 12, 1456. https://doi.org/10.3390/atmos12111456
Hagiwara K, Matsumoto T, Tsedendamba P, Baba K, Hoshino B. Bacterial Characteristics of Dust Particle Saltation in Gobi Dust Sites, Mongolia. Atmosphere. 2021; 12(11):1456. https://doi.org/10.3390/atmos12111456
Chicago/Turabian StyleHagiwara, Katsuro, Tamaki Matsumoto, Purevsuren Tsedendamba, Kenji Baba, and Buho Hoshino. 2021. "Bacterial Characteristics of Dust Particle Saltation in Gobi Dust Sites, Mongolia" Atmosphere 12, no. 11: 1456. https://doi.org/10.3390/atmos12111456
APA StyleHagiwara, K., Matsumoto, T., Tsedendamba, P., Baba, K., & Hoshino, B. (2021). Bacterial Characteristics of Dust Particle Saltation in Gobi Dust Sites, Mongolia. Atmosphere, 12(11), 1456. https://doi.org/10.3390/atmos12111456






