Effects of Long-Term Nitrogen Fertilization and Ground Water Level Changes on Soil CO2 Fluxes from Oil Palm Plantation on Tropical Peatland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Measurement of Soil CO2 Flux and Environmental Variables
2.3. Peat Sampling
2.4. Statistical Analysis
3. Results
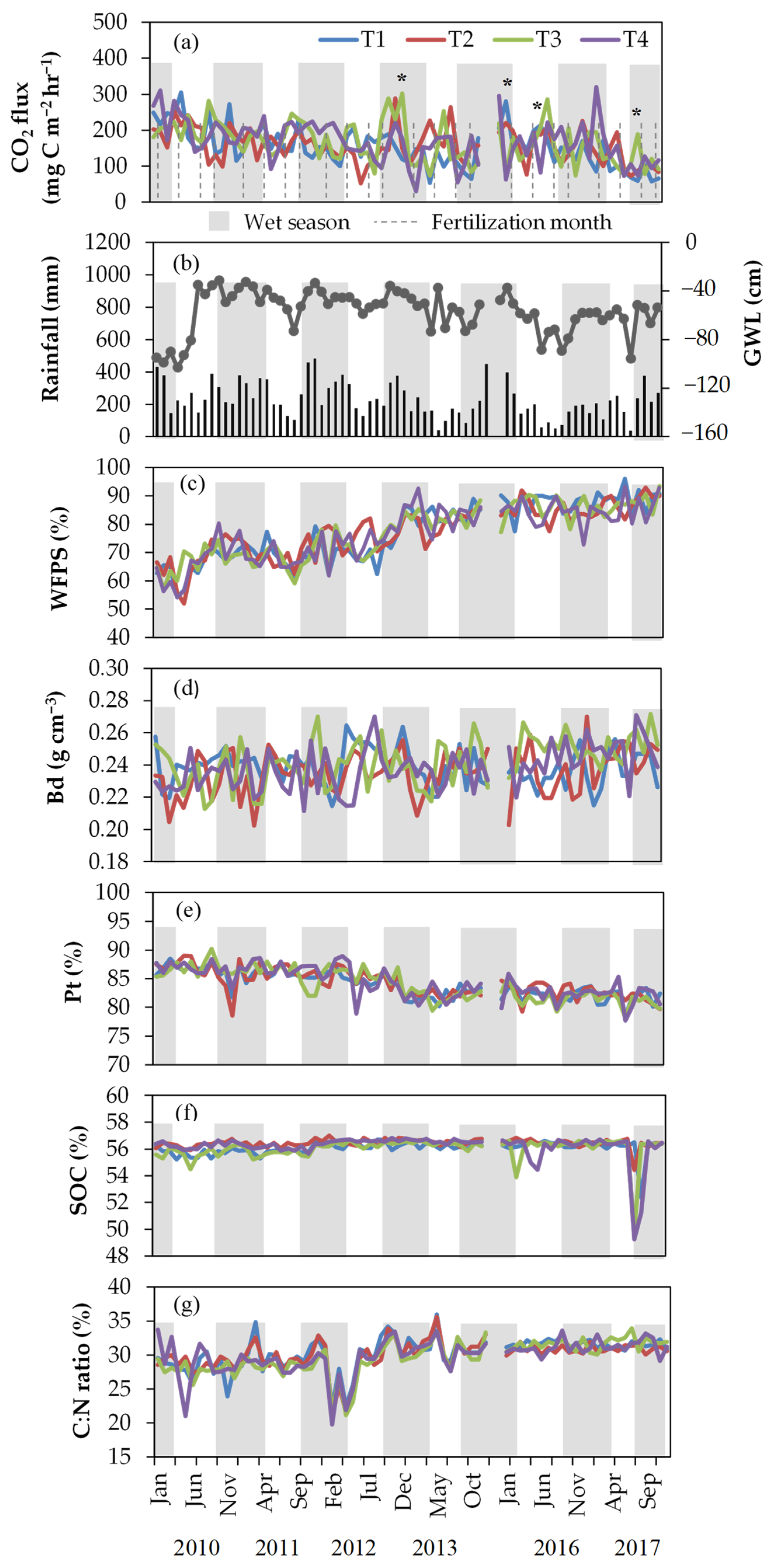
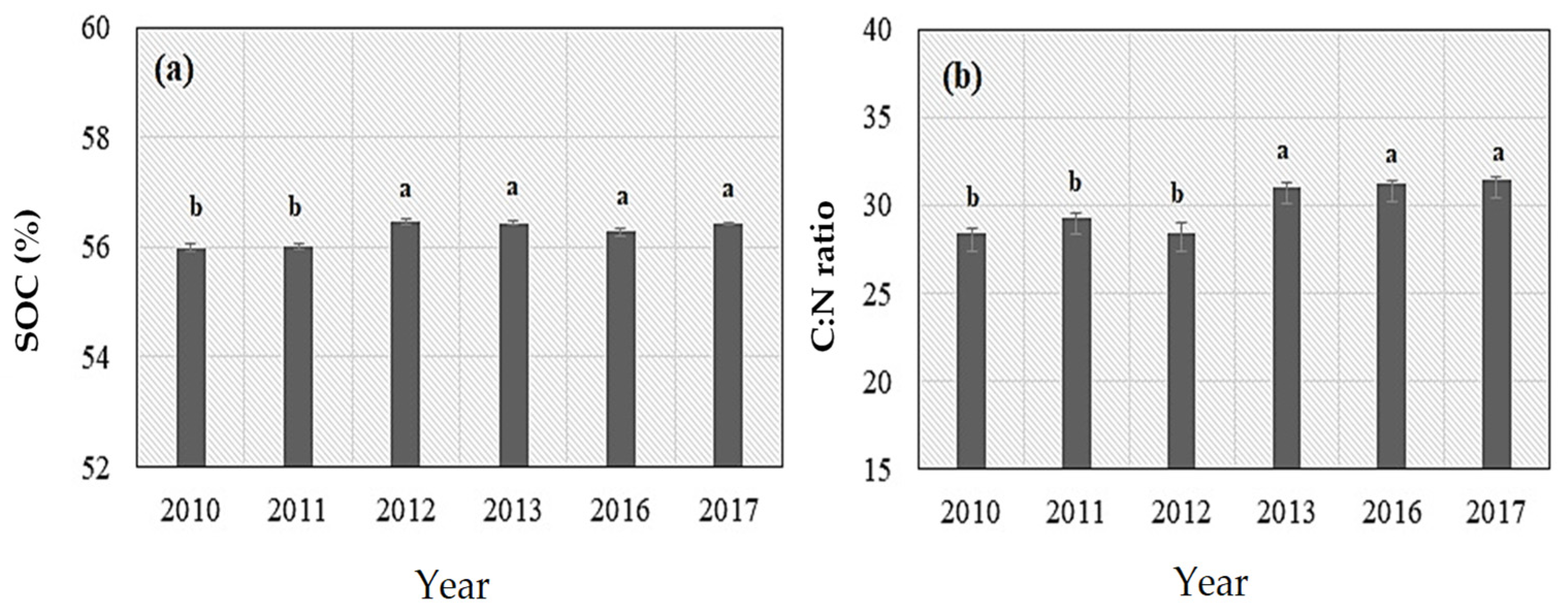
3.1. Soil CO2 Fluxes, Environmental Variables, and Soil Properties
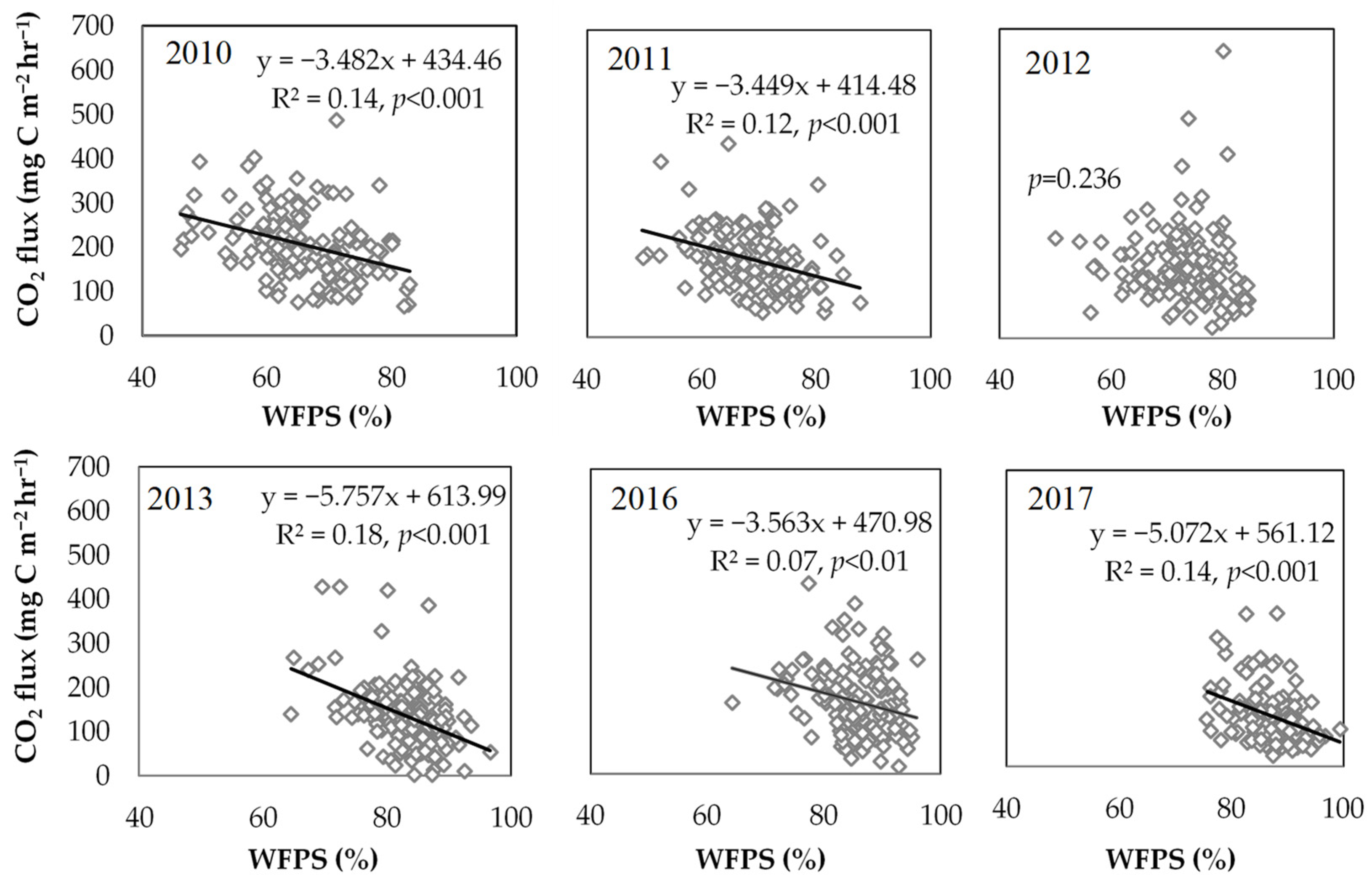
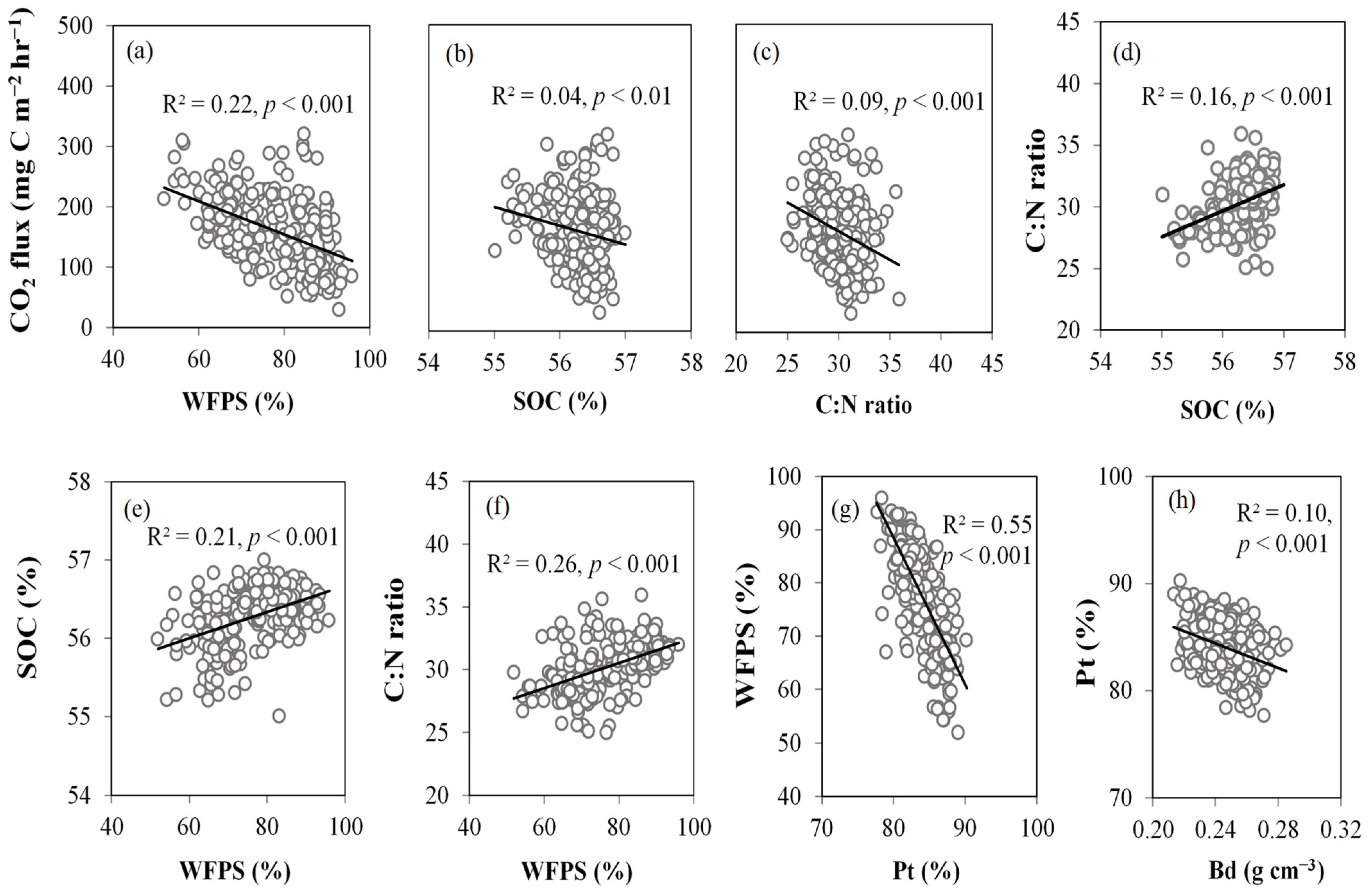
3.2. Factors Influencing CO2 Fluxes
4. Discussion
4.1. Effects of N Fertilization on Soil CO2 Emissions
4.2. Effects of WFPS and GWL on Soil CO2 Emissions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Page, S.E.; Morrison, R.; Malins, C.; Hooijer, A.; Rieley, J.O.; Jauhiainen, J. Review of Peat Surface Greenhouse Gas Emissions from Oil Palm Plantations in Southeast Asia; White Paper Number 15; International Council on Clean Transportation: Washington, DC, USA, 2011; pp. 1–78. Available online: https://theicct.org/sites/default/files/publications/ICCT_Peat-Emissions_Sept2011.pdf (accessed on 1 July 2021).
- Uning, R.; Latif, M.T.; Othman, M.; Juneng, L.; Mohd Hanif, N.; Nadzir, M.S.M.; Abdul Maulud, K.N.; Jaafar, W.S.W.M.; Said, N.F.S.; Ahamad, F.; et al. A Review of Southeast Asian Oil Palm and Its CO2 Fluxes. Sustainability 2020, 12, 5077. [Google Scholar] [CrossRef]
- Melling, L.; Hatano, R.; Goh, K.J. Soil CO2 flux from three ecosystems in tropical peatland of Sarawak, Malaysia. Tellus B Chem. Phys. Meteorol. 2005, 57, 1–11. [Google Scholar] [CrossRef]
- Hooijer, A.; Page, S.; Navratil, P.; Vernimmen, R.; van der Vat, M.; Tansey, K.; Konecny, K.; Siegert, F.; Ballhorn, U.; Mawdsley, N. Carbon Emissions from Drained and Degraded Peatland in Indonesia and Emission Factors for Measurement, Reporting and Verification (MRV) of Peatland Greenhouse Gas Emissions—A Summary of KFCP Research Results for Practitioners; IAFCP: Jakarta, Indonesia, 2014.
- Hirano, T.; Kusin, K.; Limin, S.; Osaki, M. Carbon dioxide emissions through oxidative peat decomposition on a burnt tropical peatland. Glob. Chang. Biol. 2014, 20, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Millard, P.; Midwood, A.J.; Hunt, J.E.; Whitehead, D.; Boutton, T.W. Partitioning soil surface CO2 efflux into autotrophic and heterotrophic components, using natural gradients in soil δ13C in an undisturbed savannah soil. Soil Biol. Biochem. 2008, 40, 1575–1582. [Google Scholar] [CrossRef]
- Toma, Y.; Takakai, F.; Darung, U.; Kuramochi, K.; Limin, S.H.; Dohong, S.; Hatano, R. Nitrous oxide emission derived from soil organic matter decomposition from tropical agricultural peat soil in central Kalimantan, Indonesia. Soil Sci. Plant Nutr. 2011, 57, 436–451. [Google Scholar] [CrossRef] [Green Version]
- Jauhiainen, J.; Hooijer, A.; Page, S.E. Carbon dioxide emissions from an Acacia plantation on peatland in Sumatra, Indonesia. Biogeosciences 2012, 8, 8269–8302. [Google Scholar] [CrossRef] [Green Version]
- Dariah, A.; Marwanto, S.; Agus, F. Root-and peat-based CO2 emissions from oil palm plantations. Mitig. Adapt. Strateg. Glob. Chang. 2014, 19, 831–843. [Google Scholar] [CrossRef]
- Comeau, L.P.; Hergoualc’h, K.; Hartill, J.; Smith, J.; Verchot, L.V.; Peak, D.; Salim, A.M. How do the heterotrophic and the total soil respiration of an oil palm plantation on peat respond to nitrogen fertilizer application? Geoderma 2016, 268, 41–51. [Google Scholar] [CrossRef]
- Ishikura, K.; Darung, U.; Inoue, T.; Hatano, R. Variation in soil properties regulate greenhouse gas fluxes and global warming potential in three land use types on tropical peat. Atmosphere 2018, 9, 465. [Google Scholar] [CrossRef] [Green Version]
- Klingenfuß, C.; Roßkopf, N.; Walter, J.; Heller, C.; Zeitz, J. Soil organic matter to soil organic carbon ratios of peatland soil substrates. Geoderma 2014, 235, 410–417. [Google Scholar] [CrossRef]
- Ishikura, K.; Yamada, H.; Toma, Y.; Takakai, F.; Morishita, T.; Darung, U.; Limin, A.; Limin, S.H.; Hatano, R. Effect of groundwater level fluctuation on soil respiration rate of tropical peatland in Central Kalimantan, Indonesia. Soil Sci. Plant Nutr. 2017, 63, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Itoh, M.; Okimoto, Y.; Hirano, T.; Kusin, K. Factors affecting oxidative peat decomposition due to land use in tropical peat swamp forests in Indonesia. Sci. Total Environ. 2017, 609, 906–915. [Google Scholar] [CrossRef] [Green Version]
- Könönen, M.; Jauhiainen, J.; Laiho, R.; Spetz, P.; Kusin, K.; Limin, S.; Vasander, H. Land use increases the recalcitrance of tropical peat. Wetl. Ecol. Manag. 2016, 24, 717–731. [Google Scholar] [CrossRef]
- Swails, E.; Jaye, D.; Verchot, L.; Hergoualc’h, K.; Schirrmann, M.; Borchard, N.; Wahyuni, N.; Lawrence, D. Will CO2 emissions from drained tropical peatlands decline over time? Links between soil organic matter quality, nutrients, and C mineralization rates. Ecosystems 2017, 21, 868–885. [Google Scholar] [CrossRef]
- Marwanto, S.; Sabiham, S.; Funakawa, S. Importance of CO2 production in subsoil layers of drained tropical peatland under mature oil palm plantation. Soil Tillage Res. 2019, 186, 206–213. [Google Scholar] [CrossRef]
- Könönen, M.; Jauhiainen, J.; Straková, P.; Heinonsalo, J.; Laiho, R.; Kusin, K.; Limin, S.; Vasander, H. Deforested and drained tropical peatland sites show poorer peat substrate quality and lower microbial biomass and activity than unmanaged swamp forest. Soil Biol. Biochem. 2018, 123, 229–241. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, M.; Pandey, R.; Zhi Guo, Y.; Cabral-Pinto, M. Forest soil nutrient stocks along altitudinal range of Uttarakhand Himalayas: An aid to Nature Based Climate Solutions. CATENA 2021, 207, 105667. [Google Scholar] [CrossRef]
- Kimura, S.D.; Melling, L.; Goh, K.J. Influence of soil aggregate size on greenhouse gas emission and uptake rate from tropical peat soil in forest and different oil palm development years. Geoderma 2012, 185, 1–5. [Google Scholar] [CrossRef]
- Mu, Z.; Huang, A.; Ni, J.; Xie, D. Linking annual N2O emission in organic soils to mineral nitrogen input as estimated by heterotrophic respiration and soil C/N ratio. PLoS ONE 2014, 9, e96572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, C.J.; Blackmer, A.M.; Horton, R. Nitrogen effects on conservation of carbon during corn residue decomposition in soil. Soil Sci. Soc. Am. J. 1995, 59, 453–459. [Google Scholar] [CrossRef]
- Lee, K.H.; Jose, S. Soil respiration and microbial biomass in a pecan—Cotton alley cropping system in southern USA. Agrofor. Syst. 2003, 58, 45–54. [Google Scholar] [CrossRef]
- Bowden, R.D.; Davidson, E.; Savage, K.; Arabia, C.; Steudler, P. Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. For. Ecol. Manag. 2004, 196, 43–56. [Google Scholar] [CrossRef]
- Watanabe, A.; Purwanto, B.H.; Ando, H.; Kakuda, K.I.; Jong, F.S. Methane and CO2 fluxes from an Indonesian peatland used for sago palm (Metroxylon sagu Rottb.) cultivation: Effects of fertilizer and groundwater level management. Agric. Ecosyst. Environ. 2009, 134, 14–18. [Google Scholar] [CrossRef]
- Sakata, R.; Shimada, S.; Arai, H.; Yoshioka, N.; Yoshioka, R.; Aoki, H.; Kimoto, N.; Sakamoto, A.; Melling, L.; Inubushi, K. Effect of soil types and nitrogen fertilizer on nitrous oxide and carbon dioxide emissions in oil palm plantations. Soil Sci. Plant Nutr. 2014, 61, 48–60. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Kerojoki, O.; Silvennoinen, H.; Limin, S.; Vasander, H. Heterotrophic respiration in drained tropical peat is greatly affected by temperature—A passive ecosystem cooling experiment. Environ. Res. Lett. 2014, 9, 105013. [Google Scholar] [CrossRef] [Green Version]
- Hatano, R. Impact of land use change on greenhouse gases emissions in peatland: A review. Int. Agrophys. 2019, 33, 167–173. [Google Scholar] [CrossRef]
- Ni, K.; Ding, W.X.; Cai, Z.C.; Wang, Y.F.; Zhang, X.L.; Zhou, B.K. Soil carbon dioxide emission from intensively cultivated black soil in Northeast China: Nitrogen fertilization effect. J. Soils Sediments 2012, 12, 1007–1018. [Google Scholar] [CrossRef]
- Tu, L.H.; Hu, T.X.; Zhang, J.; Li, X.W.; Hu, H.L.; Liu, L.; Xiao, Y.L. Nitrogen addition stimulates different components of soil respiration in a subtropical bamboo ecosystem. Soil Biol. Biochem. 2013, 58, 255–264. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Silvennoinen, H.; Könönen, M.; Limin, S.; Vasander, H. Management driven changes in carbon mineralization dynamics of tropical peat. Biogeochemistry 2016, 129, 115–132. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Chaddy, A.; Melling, L.; Ishikura, K.; Hatano, R. Soil N2O emissions under different N rates in an oil palm plantation on tropical peatland. Agriculture 2019, 9, 213. [Google Scholar] [CrossRef] [Green Version]
- Hasnol, O.; Farawahida, M.D.; Mohd, H.; Samsudin, A. Re-evaluation of nutrients requirements for oil palm planting on peat soil. Planter 2014, 90, 161–177. [Google Scholar]
- Nakano, T.; Sawamoto, T.; Morishita, T.; Inoue, G.; Hatano, R. A comparison of regression methods for estimating soil-atmosphere diffusion gas fluxes by a closed-chamber technique. Soil Biol. Biochem. 2004, 36, 107–113. [Google Scholar] [CrossRef]
- Agus, F.; Hairiah, K.; Mulyani, A. Pengukuran Cadangan Karbon Tanah Gambut; Petunjuk Praktis; World Agroforestry Centre-ICRAF, SEA Regional Office dan Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian (BBSDLP): Bogor, Indonesia, 2011; 58p. Available online: https://balittanah.litbang.pertanian.go.id/ind/dokumentasi/buku/2011%20Agus%20et%20al%20Pengukuran%20karbon%20tanah%20gambut.pdf (accessed on 1 July 2021).
- Kumar, A.; Sharma, M.P. Review of methodology for estimation of labile organic carbon in reservoirs and lakes for GHG emission. J. Mater. Environ. Sci. 2014, 5, 653–660. [Google Scholar]
- Kumar, A.; Sharma, M.P. Estimation of greenhouse gas emissions from Koteshwar hydropower reservoir, India. Environ. Monit. Assess. 2017, 189, 240. [Google Scholar] [CrossRef]
- Melling, L.; Chaddy, A.; Goh, K.J.; Hatano, R. Soil CO2 fluxes from different ages of oil palm in tropical peatland of Sarawak, Malaysia as influenced by environmental and soil properties. Acta Hortic. ISHS 2013, 982, 25–35. [Google Scholar] [CrossRef]
- Nelson, P.N.; Banabas, M.; Scotter, D.R.; Webb, M.J. Using soil water depletion to measure spatial distribution of root activity in oil palm (Elaeis guineensis Jacq.) plantations. Plant Soil. 2006, 286, 109–121. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, B.; Wang, Y.; Zheng, X. Field measures of the contribution of root respiration to soil respiration in an alder and cypress mixed plantation by two methods: Trenching method and root biomass regression method. Eur. J. For. Res. 2008, 127, 285–291. [Google Scholar] [CrossRef]
- Harianti, M.; Sutandi, A.; Saraswati, R.; Maswar, M.; Sabiham, S. Enzyme Activities in Relation to Total K, Ca, Mg, Fe, Cu, and Zn in Oil Palm Rhizosphere of Tropical Peatland in Riau, Indonesia. Biotropia 2018, 25, 202–213. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Z. Recalcitrant carbon controls the magnitude of soil organic matter mineralization in temperate forests of northern China. For. Ecosyst. 2018, 5, 17. [Google Scholar] [CrossRef]
- Cooper, H.V.; Vane, C.H.; Evers, S.; Aplin, P.; Girkin, N.T.; Sjögersten, S. From peat swamp forest to oil palm plantations: The stability of tropical peatland carbon. Geoderma 2019, 342, 109–117. [Google Scholar] [CrossRef]
- Linn, D.M.; Doran, J.W. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- van Lent, J.; Hergoualc’h, K.; Verchot, L.; Oenema, O.; van Groenigen, J.W. Greenhouse gas emissions along a peat swamp forest degradation gradient in the Peruvian Amazon: Soil moisture and palm roots effects. Mitig. Adapt. Strateg. Glob. Chang. 2019, 24, 625–643. [Google Scholar] [CrossRef] [Green Version]
- Couwenberg, J.; Dommain, R.; Joosten, H. Greenhouse gas fluxes from tropical peatlands in South-east Asia. Glob. Chang. Biol. 2010, 16, 1715–1732. [Google Scholar] [CrossRef]
- Hergoualc’h, K.; Hendry, D.T.; Murdiyarso, D.; Verchot, L.V. Total and heterotrophic soil respiration in a swamp forest and oil palm plantations on peat in Central Kalimantan, Indonesia. Biogeochemistry 2017, 135, 203–220. [Google Scholar] [CrossRef] [Green Version]
- Intara, Y.I.; Nusantara, A.D.; Supanjani, S.; Caniago, Z.; Ekawita, R. Oil palm roots architecture in response to soil humidity. Int. J. Oil Palm 2018, 1, 79–89. Available online: https://ijop.id/index.php/ijop/article/view/11/10 (accessed on 17 August 2021).
- Husnain, H.; Wigena, I.P.; Dariah, A.; Marwanto, S.; Setyanto, P.; Agus, F. CO2 emissions from tropical drained peat in Sumatra, Indonesia. Mitig. Adapt. Strateg. Glob. Chang. 2014, 19, 845–862. [Google Scholar] [CrossRef]
- Prananto, J.A.; Minasny, B.; Comeau, L.P.; Rudiyanto, R.; Grace, P. Drainage increases CO2 and N2O emissions from tropical peat soils. Glob. Chang. Biol. 2020, 26, 4583–4600. [Google Scholar] [CrossRef]
- Matysek, M.; Evers, S.; Samuel, M.K.; Sjogersten, S. High heterotrophic CO2 emissions from a Malaysian oil palm plantations during dry-season. Wetlands Ecol. Manag. 2018, 26, 415–424. [Google Scholar] [CrossRef] [Green Version]


| Year | T1 | T2 | T3 | T4 | Mean |
|---|---|---|---|---|---|
| 2010 | 16.6 ± 1.0 a A | 14.8 ± 1.4 a A | 16.8 ± 0.6 a A | 17.1 ± 3.9 a A | 11.5 ± 0.5 |
| 2011 | 13.5 ± 1.6 a B | 13.4 ± 0.5 a AB | 14.7 ± 2.4 a AB | 15.3 ± 3.5 a A | 10.5 ± 0.5 |
| 2012 | 12.9 ± 0.6 a B | 10.9 ± 1.5 a AB | 13.9 ± 3.7 a AB | 13.2 ± 2.5 a A | 9.7 ± 0.4 |
| 2013 | 8.9 ± 0.6 a C | 13.0 ± 2.4 a AB | 10.7 ± 1.0 a B | 10.4 ± 1.8 a A | 8.4 ± 0.3 |
| 2016 | 14.0 ± 1.2 a AB | 13.2 ± 2.6 a AB | 13.0 ± 1.5 a AB | 13.0 ± 0.2 a A | 10.4 ± 0.1 |
| 2017 | 7.7 ± 1.2 a C | 9.8 ± 0.5 a B | 10.5 ± 1.8 a B | 11.4 ± 3.7 a A | 9.1 ± 0.4 |
| Mean | 12.3 ± 1.0 a | 12.5 ± 1.5 a | 13.2 ± 1.8 a | 13.4 ± 2.6 a | |
| ANOVA | |||||
| Source | df | Mean Square | F | p-value | |
| N rates | 3 | 5.499 | 1.291 | 0.288 | |
| Year | 5 | 66.179 | 15.540 | 0.000 | |
| Year * N rates | 15 | 4.365 | 1.025 | 0.448 | |
| Error | 48 | 4.259 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaddy, A.; Melling, L.; Ishikura, K.; Goh, K.J.; Toma, Y.; Hatano, R. Effects of Long-Term Nitrogen Fertilization and Ground Water Level Changes on Soil CO2 Fluxes from Oil Palm Plantation on Tropical Peatland. Atmosphere 2021, 12, 1340. https://doi.org/10.3390/atmos12101340
Chaddy A, Melling L, Ishikura K, Goh KJ, Toma Y, Hatano R. Effects of Long-Term Nitrogen Fertilization and Ground Water Level Changes on Soil CO2 Fluxes from Oil Palm Plantation on Tropical Peatland. Atmosphere. 2021; 12(10):1340. https://doi.org/10.3390/atmos12101340
Chicago/Turabian StyleChaddy, Auldry, Lulie Melling, Kiwamu Ishikura, Kah Joo Goh, Yo Toma, and Ryusuke Hatano. 2021. "Effects of Long-Term Nitrogen Fertilization and Ground Water Level Changes on Soil CO2 Fluxes from Oil Palm Plantation on Tropical Peatland" Atmosphere 12, no. 10: 1340. https://doi.org/10.3390/atmos12101340
APA StyleChaddy, A., Melling, L., Ishikura, K., Goh, K. J., Toma, Y., & Hatano, R. (2021). Effects of Long-Term Nitrogen Fertilization and Ground Water Level Changes on Soil CO2 Fluxes from Oil Palm Plantation on Tropical Peatland. Atmosphere, 12(10), 1340. https://doi.org/10.3390/atmos12101340







