Abstract
Sub-Saharan Africa is seeing rapid urbanization, with the population of cities such as Lagos and Nairobi growing at a rate of 3–4% a year. The region is extremely under-sampled for all air pollutants, particularly VOCs, which are useful markers for source apportionment as well as toxic in their own right. There are many contributors to air pollution in the region, and studies examining fine particulate pollution implicate traffic as the primary source in urban areas. In this pilot study, VOCs were analysed at a selection of roadside and urban background locations in Nairobi and Lagos, and 74 VOCs were quantified. GC×GC–MS/FID analysis revealed all locations were dominated by hydrocarbons typical of vehicle emissions, with the aromatic hydrocarbons benzene and toluene among the most abundant VOCs. Typical personal exposure scenarios for citizens of the cities were calculated to far exceed those of a resident in a city in Europe/US. Finally, the calculated ozone forming potential of the VOCs measured was found to be similarly high to other large cities studied with similar air pollution problems. Further study is therefore essential to determine the full extent of VOC pollution in the region and its impact on tropospheric chemistry.
1. Introduction
There are a number of contributors to poor air quality in sub-Saharan Africa (SSA), of both biogenic and anthropogenic origin. Firstly, seasonal biomass burning in Africa contributes to approximately 40% of global biomass burning [1,2]. Secondly, Saharan dust can contribute to the global burden of aerosol and can be transported worldwide [3,4]. Finally, local traffic emissions can have a major detrimental impact on air quality [5], particularly in cities, through the emission of primary particulate matter (PM), oxides of nitrogen (NOx, i.e., NO and NO2) and volatile organic compounds (VOCs). In addition, windblown and traffic re-suspended dust as well as waste burning are also well-known contributors to the urban pollution mix [6,7,8,9]. Increasing urban populations, poorly maintained vehicles, inadequate air quality regulations and air pollution control polices have resulted in increased air pollution in major African cities [10]. Added to this, urbanization rates in Africa are among the highest in the world, where more than half the population is expected to reside in urban areas by 2035 [11]. Landrigan et al., (2018) [12] in their review of pollution and health observed air pollution as an important causative agent of many non-communicable diseases including cancer, asthma and chronic obstructive pulmonary disease, thus posing a major human health problem and sustainable socio-economic and economic development issue. Exposure to ambient air pollution (AAP) is a major threat to human health in Africa, with data indicating that deaths owing to AAP exposure increased by 57% over the period 1990 to 2017. Exposure to air pollutants is also reported to decrease the life expectancy of African children by 2 years [13]. According to the WHO, in 2012, exposure to AAP in the SSA regions resulted in as many as 176,000 deaths [14]. Finally, according to a recent study on the Global Burden of Disease, air pollution has an attributable burden percentage of 10–15% in SSA countries [15].
On a local scale, in densely populated SSA cities like Nairobi in Kenya and Lagos in Nigeria the major sources of air pollution are industry, burning of waste and traffic [16,17,18,19,20,21], with as much as 90% of air pollution attributable to motor vehicle emissions [22]. Currently there are no official urban air quality monitoring sites in Kenya producing validated, publicly accessible data [10,23]. The full extent of the problem is therefore poorly understood, making safeguarding the population from poor air quality almost impossible. From the information that is available, there are numerous locations with raised pollution, particularly roadside locations [9]. Whilst some air quality monitoring exists in Nigeria [24,25,26] infrastructure is limited, and the problem of increasing urbanization and a lack of regulation of pollutant levels has resulted in extremely poor air quality [27].
Previous studies on air quality in SSA have mostly focused on particulate matter [8,28,29,30]. There have also been some studies on black carbon and NOx [8,18,31,32]. Ambient NOx in urban environments primarily results from traffic processes; however, a wide range of VOCs are also co-emitted from motor vehicle exhaust, and despite the importance of their contribution to air quality in urban environments they have only been measured in a handful of studies within the region [33,34].
VOCs emitted from vehicles comprise a complex mixture of hydrocarbons, including benzene, toluene, ethyl benzene and o,m,p-xylene, known collectively as BTEX compounds, which are particularly hazardous to human health [35,36,37,38]. Huang et al., (2020) [39] identified 102 different VOCs from both diesel and gasoline fuelled vehicles in Wuhan, China, including acetylene, alkenes, olefins, aromatic hydrocarbons and oxygenated VOCs. The study demonstrated differing proportions of the same VOCs between the two fuel types with higher concentrations of aromatic hydrocarbons in gasoline vehicles in comparison to diesel vehicles, with the higher proportion of aromatic hydrocarbons being the main contributor to the increase in octane in gasoline. The identity of specific emitted VOCs can also provide information on their source; for example, benzene is a key indicator of vehicle emissions [40]. In addition to the emissions of VOCs, vehicles also emit semi-volatile organic compounds (SVOCs), including polycyclic aromatic hydrocarbons (PAHs), e.g., naphthalene, which also have numerous detrimental health affects [41,42].
VOCs also originate from other anthropogenic sources, notably industrial emissions and biomass burning. Liu et al., (2019) [43] measured VOC profiles at an industrial park in China and found that aromatic VOCs contributed to the highest percentage of the total VOCs (46%), followed by oxygenated VOCs (41%), alkenes (10%) and nitrogen containing VOCs (3%). In contrast Huang et al., (2020) [39] found that the contributors of total VOCs from diesel vehicles were alkenes (29%), OVOCs (27%), aromatics (18%), halo-hydrocarbons (14%), olefins (8%) and ethylene (1%). VOC emissions from biomass burning have been monitored by Wang et al., (2014) [44] in China, with the dominant VOCs being attributed to OVOCs (49%), alkenes (21%), aromatics (13%), alkenes (10%) and halogenated VOCs (5%). In addition to having a range of primary impacts on human health and the environment, VOCs can also be oxidized via reactions with OH radicals, NO3 and Cl atoms to form aerosol particles, which also have a range of negative impacts on human health and the natural and built environments. Such secondary products include excess ground level ozone (O3) and other condensable organic species that can contribute to ultrafine (UFP) and fine particulate matter [45]. This process is not well understood in tropical Africa, where photolysis can be expected to drive tropospheric chemistry, due to scarce information on gaseous pollutants. Whilst there have been a small number of studies on roadside VOCs in Nigeria, there is little information on VOC levels or identity in Kenya. Furthermore, those studies that have been conducted on ambient VOCs in Lagos are limited in their scope and report a wide range of concentrations. Baumbach et al., (1995) [33] measured mean benzene and toluene concentrations of 250 µgm−3 and 750 µgm−3, respectively, at a roadside location in Lagos, and Olajire and Azeez (2014) [34] observed benzene and toluene concentrations of 3 µgm−3 and 1.9 µgm−3, respectively, at an industrial estate in Lagos. In the same study, Olajire and Azeez (2014) [34] measured concentrations of only 3.4 µgm−3 of benzene and 1.5 µgm−3 of toluene in an area where the authors have stated has large amounts of congested traffic.
Many commonly occurring VOCs are contributors to adverse health effects in humans [46], causing symptoms ranging from irritation of the eyes and skin (e.g., formaldehyde, acetaldehyde, toluene, limonene, styrene and alpha-pinene), the exacerbation of chronic diseases such as asthma and cardiovascular disease (e.g., various aromatics) and the development of cancer (e.g., trichloroethylene, benzene and naphthalene) [47]. Exposure to BTEX VOCs can be particularly harmful, causing damage to lung, kidney and liver tissue [48]. For example, Rumchev et al., (2004) [49] determined that the risk of children developing asthma increases with benzene and toluene concentrations in the indoor environment. With respect to health risks associated with human exposure to BTEX VOCs from vehicle emissions, Dehghani et al., (2018) [50] calculated an inhalation lifetime cancer risk for benzene at a bus terminal in Iran, and their results showed that exposure levels exceeded the levels recommended by the US EPA and the WHO. This elevated cancer risk owing to human exposure to benzene in air was also determined in another study by Zhou et al., (2011) [51]. In addition, Hoxha et al., (2009) [52] indicated a link between the shortening of leukocyte telomere and the exposure of traffic related VOCs, and Sørensen et al., (2003) [53] indicated that the exposure to benzene can result in DNA base oxidation, thus implying that hot spots where concentrations of BTEX VOCs are high can result in increased health problems.
Owing to the lack of available data regarding VOC concentrations in SSA cities, the aim of this pilot study was to examine the VOC composition of air in the cities of Nairobi and Lagos, both roadside and in residential/background areas. Multi-dimensional chromatography was applied to the analysis, offering easier identification and quantitation of VOCs from such complex mixtures by virtue of its increased peak capacity [54,55]. Whilst the technique has been increasingly applied to atmospheric studies, this is the first time this powerful analytical tool has been applied to examine the complex mixture of VOCs present in the urban air of African megacities.
Using the concentrations of VOCs determined, the personal exposure potential of residents of both cities to the health relevant BTEX compounds is investigated and compared to that experienced in other cities, and the potential for ozone formation from the quantified VOCs is also examined.
2. Materials and Methods
2.1. Sample Collection Sites
Nairobi is situated south of the equator at 1.3° S, 36.9° E and at approximately 1600 m above sea level. It experiences frequent temperature inversions and mild pressure variations at around 826 hPa [32]. Nairobi is the capital of Kenya and is typical of the fast-growing cities of sub-Saharan Africa. The City of Nairobi had a resident population of 3.1 million in 2009 which increased to 4.4 million in 2019 [56] representing a growth of 4.2% per year. VOC samples were collected at four sites (three roadside and one urban background) northwest of the centre of Nairobi and at the outskirts of the Nairobi Central Business District (CBD) (see Table 1). Samples were collected during a one-week period in June 2019 to avoid the two rainy seasons (March to May and October to December) at two sampling time points per day, i.e., one between 07:30 and 08:30 and one between 16:30 and 17:30. Thus, a total of 12 samples were taken per day.

Table 1.
Description and locations of sampling sites in Nairobi CBD, and Lagos.
Lagos is the commercial capital of Nigeria and one of the most densely populated and fast-growing cities in the world, with an estimated population of over 14 million [57] and an estimated growth rate of 3.3% [11]. Eleven sampling locations were selected to represent roadside and commercial environments (i.e., those areas with a high concentration of businesses dealing in the sale of goods and/or services) as well as urban background/residential areas. In Lagos, there are two main seasons: the rainy season from April to October and the dry season from October to March. The sampling period was between November and December 2019 in the dry season, and a total of 22 samples from 11 locations were taken (see Table 1).
2.2. Sample Collection
One litre of air was actively sampled onto pre-conditioned (for 2.5 h at 330 °C in 50 mL/min CP grade N2, BOC) Tenax/TA with Carbograph 1TD sorbent tubes (Hydrophobic, Markes International Ltd., Llantrisant, UK) using a battery-operated pump (Escort Pump, Sigma Aldrich), operated at 250 mL/min at a height of 1.5 m above the ground. Sampled tubes, along with field control blank tubes, were immediately capped after sampling (brass caps, Markes International Ltd., Llantrisant, UK) and were shipped back to the UK with cool packs for analyses by GC–MS within 2 months of collection (field blanks stored over this time showed total integrated peak areas within 10% of the total peak areas of newly conditioned tubes). Nairobi samples were run in a single batch over one week in July, and the Lagos samples in a single batch in January 2020.
2.3. Internal Standard Addition
An internal standard solution was prepared from toluene-d8 and phenanthrene-d10 certified reference solutions (Sigma Aldrich, Dorset, UK) and n-octane-d18 (D, 99% Cambridge Isotope Laboratories, Tewksbury, MA, USA) to give a final concentration of 20 μg/mL (in methanol) per analyte. Before analysis, the samples were loaded with the internal standard solution using the calibration solution loading rig (CSLR, Markes International Ltd., Llantrisant, UK). A 0.6 μL aliquot of internal standard solution was injected onto the tube in a stream of nitrogen at a flow rate of 100 mL/min for 2 min, purging the excess solvent.
2.4. TD-GC×GC–MS Quality Control
A reference solution used to monitor retention behaviour was prepared in methanol (SupraSolv grade, Sigma Aldrich, Dorset, UK) to give final concentrations of 10 μg/mL and 20 μg/mL for L C8–C20 saturated alkanes certified reference material (Sigma Aldrich, Dorset, UK) and aromatic calibration standard (NJDEP EPH 10/08 Rev.2, Thames Restek, Saunderton, UK), respectively.
The multi-component air standard mix used for calibration was also used as a performance monitoring solution (1 μL at 10 μg/mL). A sample of retention monitoring solution was run at the beginning of each ten-sample sequence (loaded onto sorbent tubes as described above) alongside a trap blank, system blank and field blank to monitor potential interference, and the final sample of the sequence was the multi-component standard mix.
2.5. Calibration Standards and Compound Identification
A 100 μg/mL multi component air standard (47537-U Sigma Aldrich, Dorset, UK, Certified Reference Material (CRM) produced and certified in accordance with ISO 17034 and ISO/IEC 17025) was used for quantitation and identification of 52 components (to Metabolomics Standards Initiative (MSI) level 1) [58]. This consisted of a mixture of alkanes, aromatic hydrocarbons, halogenated compounds, carbonyls, alcohols, esters and terpenes commonly found in air. The solution was diluted in methanol to give final concentrations of 100, 50, 25, 10, 5, 2.5, 1 and 0.5 μg/mL. Standards (1 μL) were loaded into sorbent tubes into a stream of N2 (zero grade, BOC) at 100 mL/min and purged for 2 min. FID data was used for compound quantitation of the 52 VOCs.
The retention monitoring mixture was also used to confirm the identities (to MSI level 1) of the alkanes and aromatic hydrocarbon absent in the multicomponent air standard (acenaphthene, acenaphthylene, eicosane, fluorene, heptadecane, naphthalene, nonadecane, octadecane, phenanthrene, pyrene). For these compounds, a response factor from the retention monitoring solution was used.
The additional compounds quantified that were not present in either standard (benzophenone; butane, 2,2-dimethyl-; butane, 2-methyl-; cyclohexane, methyl-; cyclopentane, methyl-; cyclopropane; 1,2-dimethyl-, trans-; dibenzofuran; hexane, 2 methyl-; hexane, 3-methyl-; naphthalene, 1-methyl-; naphthalene, 2-methyl-; pentane; pentane, 2-methyl- and pentane, 3-methyl-) were identified using library matching (MSI level 2) and their chromatographic location. Quantitation was carried out using the calibration data for the closest eluting proxy.
2.6. TD-GC–MS Analysis
Analysis by two-dimensional gas chromatography was carried out on an Agilent 7890 A gas chromatogram, with a G3486 A CFT flow modulator and three-way splitter plate coupled to a flame ionization detector and a HES 5977B quadrupole mass spectrometer with election ionization (Agilent Technologies Ltd., Stockport, UK). Full details of the method and performance are given in Wilde et al., (2019) [59]. The column configuration was an Rxi-5Sil MS 30 m × 0.25 mm × 0.25 μm primary column (Thames Restek Ltd., Saunderton, UK) and a DB-WAX 4 m × 0.25 mm × 0.25 μm as the secondary column (Agilent Technologies Ltd., Stockport, UK). Helium (N6.0 BOC) was used as the carrier gas with the primary and secondary column flow rates of 0.6 and 23 mL/min, respectively. The modulation period was set to 3 s, with a fill and flush time of 2.799 s and 0.201 s, respectively. The restrictor from the first outlet port of the splitter plate to the flame ionization detector (FID) was 1.2 m × 0.25 mm deactivated fused silica, with a constant flow of 23 mL/min, and the restrictor from the splitter plate to the qMS was 0.76 m × 0.10 mm deactivated fused silica. The FID heater was set to 250 °C, and the make-up gas was nitrogen at 25 mL/min, with an air flow rate of 400 mL/min and 35 mL/min of hydrogen from a Peak Scientific hydrogen generator (Trace hydrogen, Peak Scientific Instruments Ltd., Inchinnan, UK). The FID collected data at 100 Hz. The transfer line to the qMS was kept at 250 °C and the ion source and quadrupole at 230 °C and at 150 °C, respectively. The mass scan range was m/z 40–300 at 10,000 µs−1, giving an acquisition rate of 21.5 Hz. The oven was programmed from 30 °C, held for 5 min, then heated to 80 °C at 3 °C/min, then at 5 °C/min to 250 °C and held for 10 min. Between each run a bake-out method was performed, the primary column flow was increased to 1.5 mL/min and the oven was held at 250 °C for 30 min.
The GCxGC was interfaced with a Markes TD-100xr thermal desorption autosampler (Markes International Ltd., Llantrisant, UK). Tubes were pre-purged with carrier gas for 1 min at 50 mL/min and then desorbed at 300 °C for 5 min with a flow of 50 mL/min onto a “hydrophobic general” trap (Markes International Ltd., Llantrisant, UK) held at −10 °C. The trap was then purged for 2 min at 2 mL/min before being heated at the maximum heating rate to 300°C for 5 min with a split flow rate of 2 mL/min. Between each sample tube, a bake-out method was performed, which involved an empty tube (no sorbent) being loaded by the autosampler. Data was acquired in MassHunter GC–MS Acquisition B.07.04.2260 (Agilent Technologies Ltd., Stockport, UK) and the data processed using GC Image™ v2.6 along with Project and Image Investigator (JSB Ltd., Horsham, UK).
3. Results
3.1. Urban Air Visualization
Initial observation of the chromatograms obtained showed an extremely complex and chemically diverse mixture of VOCs for all locations, particularly in the roadside samples, an example of which can be seen in Figure 1 showing a GC×GC chromatogram of a roadside location in Nairobi. Multi-dimensional chromatography facilitates easier identification and quantitation of VOCs from such complex mixtures by virtue of its increased peak capacity [54,55]. The nature of GC×GC allows characterization of a sample by the regions in which compounds are eluting, as each region has a defined boiling point and polarity. The samples collected were dominated by the presence of large concentrations of aromatic compounds including several PAHs, and in particularly high concentrations, toluene and benzene followed by the C8 and C9 aromatics hydrocarbons. A complex mixture of many aliphatic hydrocarbons was also detected, with a clear n-alkane series present alongside a multitude of other unsaturated and branched hydrocarbons. This profile is typical of that seen at roadside locations [60,61] that are dominated by fresh vehicle emissions. Urban background sites within both cities were also dominated by the same VOCs despite the sampling sites being ≥20 m away from the roadside.
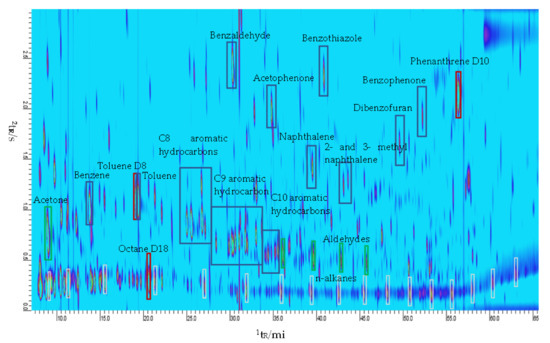
Figure 1.
GCxGC-FID chromatogram of a roadside sample from Nairobi highlighting the dominant compounds observed in all samples. Added deuterated internal standards are shown in red boxes.
3.2. Total VOC by Site
Total VOC (TVOC) is a useful measurement to assess the overall contribution of VOCs to air quality across locations [62] and is defined in this study as the sum of the 74 definitively identified and quantified VOCs. An increasing TVOC was observed across the sites in Nairobi corresponding to the proximity to increasing traffic volumes (Figure 2). The roundabout on the Waiyaki Way and University Way is a particular VOC hotspot in the area; multiple lanes of traffic slow and queues form approaching the junction before vehicles accelerate away, and in doing so emit high levels of VOCs. TVOC levels drop significantly at the Institute of Nuclear Science and Technology (INS) building site measured 500 m from the junction, with average roadside levels being over a factor of 3 or higher.
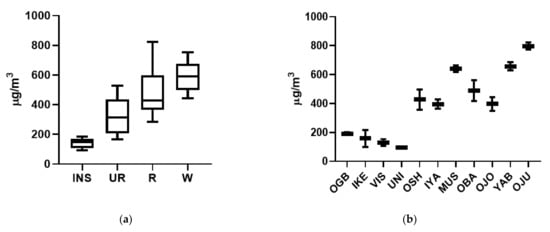
Figure 2.
Concentration of TVOCs measured at individual sites in (a) Nairobi and (b) Lagos. Location name key can be found in Table 1.
Similarly, in Lagos, averaged roadside levels were more than a factor of 3 higher than the urban background locations (Figure 2). The mean roadside site TVOC level of 543 ± 158 µgm−3 in Lagos was not significantly different (p > 0.05) from the averaged roadside site TVOC level in Nairobi, which was found to be 462 ± 247 µgm−3. The average urban background TVOC levels were very similar between Lagos and Nairobi, at 143 ± 51 µgm−3 and 142 ± 34 µgm−3, respectively. Measurements were only made on a single day for each site in Lagos, explaining the reduced intra-site variation.
Ojuelegba had the highest TVOC concentration of all sites studied, at 800 µgm−3, most likely owing to its proximity to a major intersection of a main route (A1) out of the city and is one of the key transport nodes of Lagos that connects the city’s mainland to Lagos Island. It also has a very high population density, high commerce and pockets of industrial activity. Mushin and Yaba had the next total highest VOC levels. Mushin is largely a congested residential area with high levels of commercial activities coupled with poor sanitary condition and lies at the intersection of major roads from Lagos, Shomolu and Ikeja. Yaba has one of the busiest market sites in Lagos and is a major transport hub. TVOC at Iyana Ipaja, Obalende, Ojoto and Oshodi ranged between 395 and 488 µgm−3, with these sites representing busy commercial areas with relatively high volumes of traffic, dumpsites and regular burning of refuse, particularly at the Olusosun refuse dump in Ojota. Oshodi has industrial activities and major motor parks (transportation hubs) contributing to the TVOC concentration. The four urban background sites were in quieter residential areas, away from major traffic routes, and have TVOC concentrations substantially lower, in the range of 95–191 µgm−3.
TVOC concentrations recorded in both cities were substantially higher than those normally recorded at European roadside locations, e.g., Ghent street = 54 µgm−3 [62], but in a similar range to roadside locations in cities in more developing countries, e.g., Hanoi, Vietnam = 507 µgm−3; Addis Ababa, Ethiopia = 318 µgm−3 [62]; and Delhi, India = 241–734 µgm−3 [63]. Whilst the measurement of TVOC allows an overall understanding of VOC pollution at a particular site, more detailed classification of the VOC composition is necessary to understand the potential detrimental health impacts.
3.3. Relative Contribution TVOC
Despite having much lower TVOC concentrations at the urban background sites compared to roadside, the composition by chemical class was very similar for each given location (Figure 3). The ambient VOC loading at each location monitored for both cities was dominated by hydrocarbons (57.1–7.1%) and aromatics (16.8–35.4%), demonstrating the ubiquitous significance of traffic emissions across the cities. The urban background site in Nairobi had a higher non-alcohol oxygenate contribution (13.3% compared to 3.9%) and slightly lower aromatic fraction (25.7% compared to 32.6%) than the nearby roadside locations, suggesting a higher contribution from non-traffic sources. A similar trend was seen at the Lagos urban background sites; the notable difference between the two cities is the presence of a considerable alcohol fraction observed in the Lagos air samples (6.2%), which was substantially less in the Nairobi samples (0.8%). Very few terpenes were seen in any of the Nairobi samples (all <0.3%) or in most of the Lagos samples (<0.5%). Mushin, Iyana Ipaja, Obalende and Ojota were the exception. where terpenes made up 4.1, 0.8, 1.2 and 1.5% of the total VOC pool, respectively.
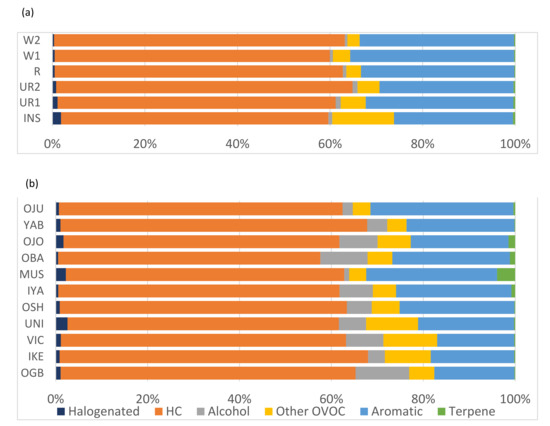
Figure 3.
Total VOCs composition by chemical class across all measurement sites in (a) Nairobi and (b) Lagos.
3.4. Quantification of VOC Pollutants in Air
Figure 3 shows that roadside sites in both cities were dominated by saturated aliphatic hydrocarbons. Speciation of the fraction (Table 2) showed that the n-alkanes pentane and hexane were the most abundant VOCs, followed by 2-methyl butane and 2-methyl pentane. It is worth noting that the selected sorbent was not capable of trapping hydrocarbons lighter than pentane, so their detection was not possible in this study by the analytics used. However, their presence in substantial quantities in roadside air, as well as in combustive emissions from industrial sites, has been shown previously [64,65]. The dominant aromatics (the second largest contributing class) observed at all locations were toluene followed by benzene with lower, but still substantial, levels of xylenes, trimethyl benzene isomers, ethylbenzene and ethyltoluene isomers. Both aliphatic and aromatic hydrocarbons are produced from diesel and petrol vehicles and are present in both exhaust and evaporative fuel emissions and are also formed during the incineration/electrification of biogas [62,64,66,67].

Table 2.
Target VOCs averaged (±standard deviation) across urban background (UB) and roadside (RS) sites in Nairobi and Lagos, coloured by maximal value per sample group.
The exact composition of VOC emissions from vehicles is dependent on a number of factors, including the age of the vehicles, the way in which they are driven and maintained and the type and quality of fuel used. In developing countries such as Kenya and Nigeria, vehicles tend to be older and have fewer modern adaptations such as catalytic converters [68,69]. Old cars are often shipped to Africa from Europe [70], leading to higher VOC pollution for a given traffic volume.
Oxygenate concentrations were more variable between the cities. Acetone (9.3 µgm−3) was the most prominent OVOC at Nairobi roadside locations (compared to 5.3 Lagos µgm−3), whereas ethanol was the highest OVOC in Lagos, at 26.2 µgm−3, but considerably lower in Nairobi, at 3.6 µgm−3. Anthropogenic sources of acetone include vehicular emissions and secondary production by the oxidation of anthropogenic hydrocarbons; however, the levels were not significantly raised in the measurements taken later in the day, as primary emissions would appear to dominate. Solvent use, biomass burning and vegetation can also contribute [71,72,73].
Ethanol, the most abundant OVOC in Lagos, has multiple sources, including vegetation, biomass combustion and release from various industrial processes, and there are several ethanol distilleries in the city [74] which are known local sources of ethanol [75]. Ethanol is also increasingly being used as a biofuel across the globe [76]. Whist there are plans to introduce a proportion of ethanol into fuel in Lagos [77], this has not yet been implemented. There also exists a scheme to replace solid fuel cookers in homes with ethanol burning, which could also be a potential source [78]. 2-butanone was also abundant in Lagos roadside air, found at concentrations of 12.2 µgm−3, compared to 2.3 in residential areas, the most likely source of which is car exhaust butane oxidation [79]; however, this large increase from urban background was not seen in Nairobi (2.8 µgm−3 roadside, 2.3 µgm−3 urban background).
Limonene was responsible for the higher terpene levels measured in Mushin; as biogenic sources are unlikely in this area, other potential sources include various industrial processes, where it is becoming increasingly used as a “green alternative” industrial solvent [80].
The VOCs measured at the urban background sites in both cities were still dominated by the vehicle-derived pentane, hexane, methyl butane and methyl pentane, as well as the aromatics, benzene and toluene. Acetone was the next highest contributor and had a larger contribution to TVOC in the urban background locations than roadside. It was higher in absolute concentration in Nairobi urban background compared to roadside (10.4 µgm−3 compared to 9.4 µgm−3), and only slightly lower at the Lagos urban background sites (5.3 µgm−3 compared to 4.0 µgm−3), suggesting that traffic is not major source. Ethanol was lower in absolute concentration in the urban background (10.8 µgm−3 vs. 26.2 µgm−3) in Lagos but on average had a higher contribution to TVOC of 7.1% (range 5.9–11.6%), compared to 5.4% (range 1.0–10.2%) at the roadside.
The ratio of toluene to benzene (T:B) can be a useful indicator of emission age, owing to the shorter tropospheric lifetime of toluene compared to benzene (ca. 225 h for benzene, 50 h for toluene [81]; as the distance from the emission source increases, the T:B decreases. This is reflected in the ratios measured across the sites in Nairobi (Figure 4); a decrease in ratio was seen as the sampling moved away from the road sites to the urban background site, as can be seen in Figure 4 (range of T:B was 2.4–2). In Lagos, the opposite trend was observed (T:B 1.31), which was potentially caused by alternative sources of toluene further from roads, such as industrial emissions. The ranges for the ratios were similar to those reported previously, with ratios between 0.36 and 3 measured in several Chinese cities [60,65,82], 3.22 in Antwerp, Belgium [83] and values of 2.92 to 3.91 in Canadian cities [84], over similar geographic scales.
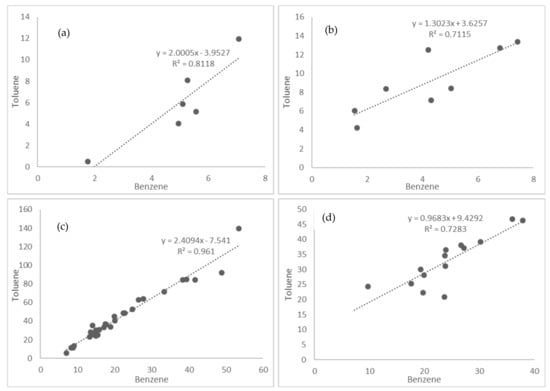
Figure 4.
Ratio of toluene to benzene measurements at (a) urban background site, Nairobi; (b) urban background/residential sites, Lagos; (c) roadside, Nairobi; and (d) roadside, Lagos.
The overall lower T:B ratio values recorded in Lagos possibly result from the greater fraction of diesel vehicles in the city compared to Nairobi and hence greater contributions from diesel combustion emissions, as the car fleet in west Africa is dominated by vehicles with diesel engines [31]. Diesel engines are known to produce lower T:B ratios than petrol equivalents, particularly for older vehicles with worn and high millage engines, such as those found in many developing cities [68,85]. Both pollutants have alternate sources; benzene has a considerable contribution from domestic biomass burning, which could explain the raised background levels in Nairobi, and industry-sourced toluene the background level in Lagos.
3.5. Classification of Sources of VOCs
By examining correlations between concentrations of different VOCs, information can be determined regarding sources of the pollutants, with strong positive correlations being indicative of common sources [86] and weak correlations being associated with different or multiple emission sources [87]. Figure 5 shows the correlation between the quantified VOCs across all sample sites in (a) Nairobi and (b) Lagos to determine common atmospheric sources. It has been previously reported that good correlations between the aromatic species can be found in areas where traffic is the dominant source [88]. Indeed, the largest cluster of high correlations for both cities is seen for the aromatics, alongside the smaller alkanes. Additionally, part of this cluster, albeit with weaker correlations, are the larger ≥C10 n-alkanes and butanone.
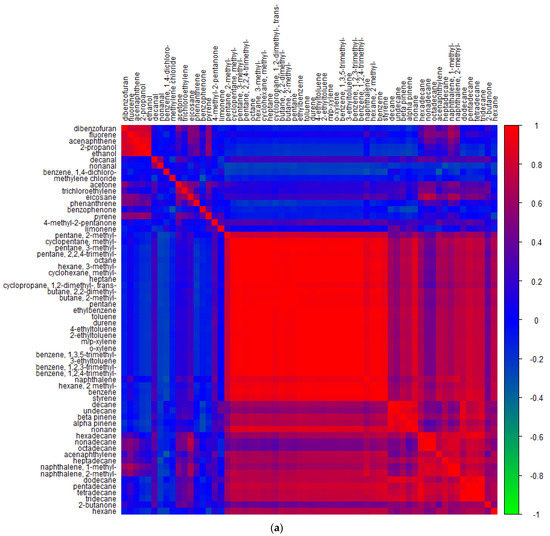
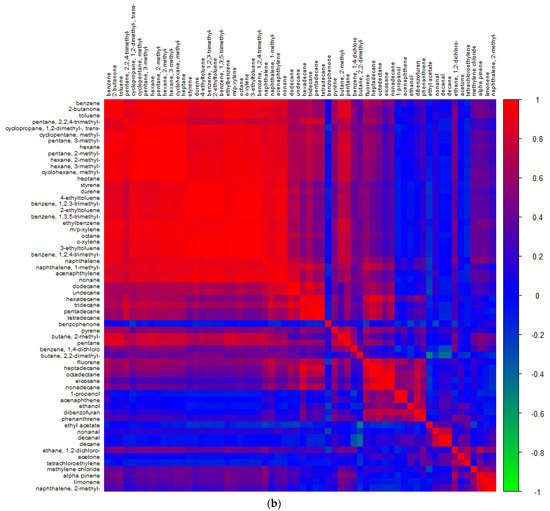
Figure 5.
Pearson correlation coefficients for target VOCs measured that were above LOD in (a) Nairobi air and (b) Lagos air, displayed with hierarchical clustering.
In Nairobi air, a smaller cluster with strong correlations was seen for pyrene and some other PAHs, which had little correlation with the vehicle exhaust VOCs, so these were likely derived from other sources, such as biomass burning and solid waste combustion. Acetone could also be seen to have some correlation within this cluster and has also been documented to be present in biomass burning emissions [89]. Furthermore, there was moderate correlation with some of the larger alkanes and methyl naphthalenes, which are also products of combustion [90].
In Lagos, ethanol was clustered with larger alkanes and had a moderate strength correlation with PAHs (>0.6) but had poor correlation with the vehicle emission cluster of aromatics and small alkanes, this suggesting an alternative combustion source than vehicle emission. Acetone in Lagos had no correlation with roadside emissions, suggesting alternate sources most likely of industrial origin.
3.6. Ozone Forming Potential
In the presence of sunlight, the reactions of VOCs and NOx emitted from vehicle exhausts can form ozone, which has the potential to have significant detrimental effects on health [91,92]. The ozone forming potential (OFP) of the targeted VOCs monitored at the roadside, commercial and urban background sites in Nairobi and Lagos (Table 2) was calculated and can be seen in Table 3. Calculations were made using the following determination:
where maximum incremental reactivity (MIR) is defined as the maximum amount of ozone formed per unit of VOC added to a system [93].
The total OFPs for the roadside locations in both Nairobi and Lagos were 1055.4 µgm−3 and 1184.1 µgm−3, respectively, and were larger than their respective background sites, by a factor of 5.3 and 4.8, respectively. Individually, aromatic compounds were the largest contributors to the total OFP at roadside locations in Nairobi, with the three largest being toluene (178.0 µgm−3), m/p-xylene (114.8 µgm−3) and 1,2,4-trimethylbenzene (108.6 µgm−3) at 16.8%, 10.8% and 10.2% of the total OFP, respectively. At the background location in Nairobi, however, the three largest contributors to the total OFP were hexane (45.1 µgm−3), 2-methylpentane (26.9 µgm−3) and toluene (23.8 µgm−3) at 45%, 26% and 23% of the total OFP, respectively; these VOCs are typical vehicle emissions, indicating that whilst the OFP of VOCs detected at the background site was lower than that calculated at the roadside, the sources of VOCs are still traffic dominated [94,95].
In Lagos, m/p-xylene (195.0 µgm−3), toluene (131.6 µgm−3) and pentane (118.4 µgm−3) were the three largest contributors to the total OFP at roadside/commercial locations, at 16.4%, 11.1% and 10.0%, respectively. As per the background location in Nairobi, the highest contributing VOCs and the respective OFP at the background location in Lagos could be attributed to traffic-based VOCs pentane (43.1 µgm−3), toluene (36.4 µgm−3) and m/p-xylene (33.2 µgm−3).
Previous studies have shown a wide range of OFP values, owing to discrepancies between the number of VOCs monitored, the types of vehicles used in the respective cities, the vehicle fuel type and ambient conditions. Olajire and Azeez (2014) [34] previously measured a smaller number of VOCs in Lagos, specifically focusing on BTEX compounds, along with trichloroethylene. For these VOCs, a total OFP of 12,760 µgm−3 was calculated across three sites, with o-xylene having the largest OFP, ranging from 2949 to 4241 µgm−3. For the same BTEX VOCs monitored in this study, we calculated a total OFP of 457.64 µgm−3 at roadside/commercial areas in Lagos. This difference in total OFP between these studies is due to considerably larger concentrations of xylenes measured by Olajire and Azeez (2014), by a factor of 35.9 and 3.1 for o-xylene and m/p-xylene, respectively, measured across all sites. Olajire and Azeez (2014) noted that high concentrations of xylene isomers in their study could be attributed to industrial sources as well as vehicle exhaust emissions, possibly explaining the discrepancy.
Other measurements of VOC concentrations in Lagos have been made by Olumayede (2014) [96]. They determined a lower OFP in Benin City, Nigeria than determined in this study for Lagos roadside sites (1184 µgm−3) (although also monitoring fewer species) at a total of 281.1 µgm−3, with the largest contributor being m,p-xylene at 25.7 µgm−3. This difference in the calculated OFPs between the two cities was due to significantly larger concentrations of VOCs measured in our study compared to that by Olumayede (2014) with concentrations ranging from 10.5 times lower for m,p-xylene to 4.3 times lower for ethylbenzene. This is likely due to the wider range of site locations used in our study, ranging from background sites to road intersections.
OFP has been calculated for a range of locations around the world; for example, Do et al., (2013) [62] previously measured VOCs in Ghent, Belgium; Hanoi, Vietnam; and Jimma and Addis Ababa, Ethiopia. Whilst only 28 VOCs were monitored in their study, the OFP calculations made for Hanoi were on a similar level to those calculated for roadside locations in Nairobi and Lagos in this study at 1308 µgm−3. The OFP calculated for BTEX VOCs measured by Do et al., (2013) in Hanoi (928.83 µgm−3) was higher than that calculated for the same VOCs at the roadside locations in Nairobi and Lagos (333.85 µgm−3 and 389.95 µgm−3,g). However, the BTEX related OFP calculated in Ghent was lower (64.42 µgm−3) than that calculated at the roadside Nairobi and Lagos sites and also lower than the Lagos urban background site (78.22 µgm−3). Finally, BTEX OFP calculated at Addis Ababa (368 µgm−3) was lower than that calculated at the Lagos roadside site, but larger than the Nairobi roadside site.
Duan et al., (2008) [97] monitored VOC concentrations and calculated the respective OFPs (937 µgm−3) during an ozone episode in Beijing, with formaldehyde, xylenes, trimethylbenzene, acetaldehyde and propene being the largest contributors to total OFP. However, it is important to note that within their measurements, Duan et al., (2008) monitored a number of short chained alkanes and alkenes as well as formaldehyde and acetaldehyde, which could not be monitored within this study, that could contribute to the total OFP and will be considered in future campaigns in order to fully constrain the OFP. The calculated OFP from VOCs measured at roadside locations in this study were also larger than that made in a previous study at roadside locations in Hong Kong by Huang et al., (2015) [65], in which the total OFP was calculated as 567.3 µgm−3 in 2003 and proceeded to decrease to 300 µgm−3 by 2011.
The concentrations of VOCs detected, and the resultant OFP, depends upon the fuel type. Chang et al., (2001) [98] studied the tailpipe emission factors of gasoline and liquefied petroleum gas (LPG) powered vehicles in Taiwan and calculated a larger OFP per kilometre for gasoline powered vehicles compared to that running on LPG (1828 mg/km compared to 965 mg/km). In addition, Wang et al., (2020) [99] monitored VOC emission factors and calculated the respective OFPs per kilometre for a number of vehicle types: light duty gasoline vehicles, light duty diesel trucks, heavy duty diesel trucks and liquefied petroleum gas electric hybrid buses. They determined that VOCs emitted from heavy duty diesel truck vehicles had the largest OFP of all vehicles tested (2489.4 mg/km), with liquefied petroleum gas electric vehicles having the lowest OFP at 124.7 mg/km.
NOx emissions and ambient NOx concentrations also determine the rate and concentration of ozone formed. When the concentrations of NOx are high, the formation of ozone is limited by peroxy radicals formed through the reactions of VOCs, whereas when NOx concentrations are low, ozone formation is NOx limited [97,100]; during this field campaign, the concentration of NOx was not measured.

Table 3.
Calculated ozone forming potential for targeted VOCs across roadside and urban background sites in Nairobi and Lagos coloured by maximal value per sample group. Maximum incremental reactivity (MIR) determined from Carter (2010) [93] unless stated otherwise. NA indicates that an MIR value for the compound could not be found, and ND indicates that the target compound was not detected in the sample.
Table 3.
Calculated ozone forming potential for targeted VOCs across roadside and urban background sites in Nairobi and Lagos coloured by maximal value per sample group. Maximum incremental reactivity (MIR) determined from Carter (2010) [93] unless stated otherwise. NA indicates that an MIR value for the compound could not be found, and ND indicates that the target compound was not detected in the sample.
| OFP (µgm−3) | ||||
|---|---|---|---|---|
| Nairobi | Lagos | |||
| Roadside | Urban Background | Roadside/ Commercial | Urban Background | |
| 1,2-dichloro propane | ND | ND | ND | ND |
| 1-butanol | ND | ND | ND | ND |
| 1-propanol a | ND | ND | 2.06 | 0.85 |
| 2-butanone b | 4.10 | 3.40 | 18.12 | 3.42 |
| 2-ethyltoluene | 15.43 | 2.07 | 15.09 | 1.34 |
| 2-propanol | ND | ND | ND | ND |
| 3-ethyltoluene | 65.55 | 6.72 | 57.42 | 8.42 |
| 4-ethyltoluene | 8.39 | 1.38 | 13.05 | 2.09 |
| 4-methyl-2-pentanone | 1.71 | 0.78 | ND | ND |
| Acenaphthene | NA | NA | NA | NA |
| Acenaphthylene | NA | NA | NA | NA |
| Acetone | 3.38 | 3.74 | 1.92 | 1.42 |
| Alpha pinene | 1.49 | 0.45 | 0.54 | 0.23 |
| Benzene | 15.55 | 3.56 | 17.45 | 3.02 |
| Benzene, 1,2,3-trimethyl- | 45.13 | 4.55 | 27.17 | 2.15 |
| Benzene, 1,2,4-trimethyl- | 108.57 | 10.82 | 81.34 | 9.14 |
| Benzene, 1,3,5-trimethyl- | 35.63 | 3.65 | 32.22 | 3.06 |
| Benzene, 1,4-dichloro- | 0.13 | 0.18 | 0.05 | 0.02 |
| Benzophenone | 1.80 | 1.58 | 1.16 | 0.95 |
| Beta pinene | 0.04 | 0.02 | ND | ND |
| Bromodichloro-methane | ND | ND | ND | ND |
| Butane, 2,2-dimethyl- | 9.51 | 0.96 | 15.07 | 14.86 |
| Butane, 2-methyl- | NA | NA | NA | NA |
| Butyl acetate | ND | ND | ND | ND |
| Carbon tetrachloride | ND | ND | ND | ND |
| Chloroform | ND | ND | ND | ND |
| Cyclohexane, methyl- | 4.68 | 0.43 | 8.87 | 1.26 |
| Cyclopentane, methyl- | 16.51 | 3.24 | 60.64 | 9.33 |
| Cyclopropane, 1,2-dimethyl-, trans- | NA | NA | NA | NA |
| Decanal | NA | NA | NA | NA |
| Decane | 1.09 | 0.69 | 1.29 | 1.13 |
| Dibenzofuran | NA | NA | NA | NA |
| Dodecane | 0.51 | 0.15 | 0.69 | 0.14 |
| Durene | 14.91 | 1.39 | 5.83 | 0.09 |
| Eicosane | 0.14 | 0.10 | 0.04 | 0.01 |
| Ethane, 1,1,1-trichloro | ND | ND | ND | ND |
| Ethane, 1,2-dichloro- | ND | ND | 0.22 | 0.11 |
| Ethanol | 5.49 | 1.81 | 40.07 | 16.37 |
| Ethyl acetate | ND | ND | 2.03 | 1.54 |
| Ethylbenzene | 25.48 | 3.34 | 46.00 | 5.59 |
| Fluorene | NA | NA | NA | NA |
| Heptadecane | 1.22 | 0.87 | 0.11 | 0.03 |
| Heptane | 9.99 | 1.13 | 13.56 | 1.86 |
| Hexadecane | 0.90 | 0.45 | 0.15 | 0.04 |
| Hexane | 86.91 | 45.14 | 71.01 | 13.39 |
| Hexane, 2 methyl- | 13.53 | 1.50 | 6.99 | 1.40 |
| Hexane, 3-methyl- | 12.99 | 1.09 | 1.48 | 0.21 |
| Limonene | 2.14 | 1.68 | 28.39 | 1.09 |
| m/p-xylene (average) | 114.82 | 12.94 | 194.95 | 33.21 |
| Methane, dibromochloro- | ND | ND | ND | ND |
| Methylene chloride | ND | ND | 0.17 | 0.04 |
| Naphthalene | 5.04 | 2.14 | 12.02 | 2.77 |
| Naphthalene, 1-methyl- | 1.47 | 1.16 | 1.50 | 0.21 |
| Naphthalene, 2-methyl- | 1.01 | 0.70 | 2.91 | 0.55 |
| Nonadecane | 0.29 | 0.20 | 0.03 | 0.01 |
| Nonanal | NA | NA | NA | NA |
| Nonane | 0.66 | 0.29 | 3.54 | 0.97 |
| Octadecane | 0.57 | 0.40 | 0.07 | 0.02 |
| Octane | 1.85 | 0.50 | 5.18 | 0.85 |
| o-Xylene | 81.90 | 8.79 | 67.69 | 11.77 |
| pentadecane | 0.70 | 0.22 | 0.27 | 0.05 |
| Pentane | 70.98 | 9.55 | 118.40 | 43.1 |
| Pentane, 2,2,4-trimethyl- | 9.51 | 0.64 | 2.26 | 0.40 |
| Pentane, 2,4-dimethyl- | ND | ND | ND | ND |
| Pentane, 2-methyl- | 50.06 | 26.85 | 15.54 | 2.6 |
| Pentane, 3-methyl- | 18.70 | 2.05 | 50.72 | 7.25 |
| Phenanthrene | NA | NA | NA | NA |
| Pyrene | NA | NA | NA | NA |
| Styrene | 3.77 | 0.93 | 5.28 | 0.62 |
| Tetrachloroethylene | ND | ND | 0.01 | 0 |
| Tetradecane | 0.64 | 0.24 | 0.28 | 0.01 |
| Toluene | 178.00 | 23.76 | 131.56 | 36.4 |
| Trichloroethylene | 1.28 | 1.05 | ND | ND |
| Tridecane | 0.53 | 0.17 | 0.62 | 0.09 |
| Undecane | 0.76 | 0.31 | 1.08 | 0.09 |
| Total | 1055.44 | 199.78 | 1184.11 | 245.58 |
MIR values from Carter (2010) [93] unless stated otherwise. a MIR value from (EPA) [101] b MIR value from Do et al., (2013) [62].
3.7. BTEX Concentrations in Air
BTEX compounds in general are an important class of compounds to consider when examining the composition of the troposphere. They are abundant in ambient air, have high ozone forming potential [102] and have well documented harmful effects on human health [50], falling within the classification of the World Health Organization’s hazardous air pollutants.
Benzene in particular is of interest owing to its effects on human health, being a known carcinogen [103], which has resulted in the production of guideline values for benzene concentrations for indoor and ambient air in various countries. According to WHO guidelines, the concentrations of airborne benzene that are associated with an excess life-time risk of leukaemia of 1/10,000, 1/100,000 and 1/1,000,000 are 17, 1.7 and 0.17 µgm−3, respectively [104]. In Europe, the ambient benzene concentration is restricted by the EU 2000/69/EC directive to 5 µgm−3 as an annual average. Aside from the acute health effects of BTEX compounds, they are also important compounds in the photochemical formation of ozone and as such can have health and environmental impacts far from the primary emission source [40,105].
Figure 6 shows the concentrations of BTEX compounds across the sites monitored. The dominance of these compounds in the ambient VOC matrix, produced primarily by motor vehicles, shows the major influence of traffic sources even at locations several hundred meters away from busy roads. In Nairobi as expected, levels were lowest at the urban background site, with levels of benzene, toluene and total BTEX recorded at 4.9 µgm−3, 5.9 µgm−3 and 14.8 µgm−3, respectively. At Waiyaki Way, the site with greatest traffic intensity in Nairobi, average benzene concentrations were 28.5 µgm−3, toluene 60.8 µgm−3 and BTEX 135.8 µgm−3. At the residential/urban background sites in Lagos, benzene ranged from 2.1 to 6.2 µgm−3, toluene 7.2 to 10.9 µgm−3 and total BTEX 16.1 to 26.7 µgm−3, and the most polluted site for BTEX (and TVOC) was Ojuelegba, which saw average concentrations of benzene of 36.5 µgm−3, toluene 46.5 µgm−3 and total BTEX 172 µgm−3. Gasoline fuels contain mainly paraffinic and aromatic fractions [106]; in an analysis of neat gasoline and diesel by Chin and Batterman (2012), concentrations of 6140 mg L−1 and 64 mg L−1 have been detected. Whilst benzene may be present within the fuel before combustion, it can also be continuously formed from other fractions in fuel through a series of reactions [106] and can also form in the catalytic converter [107].
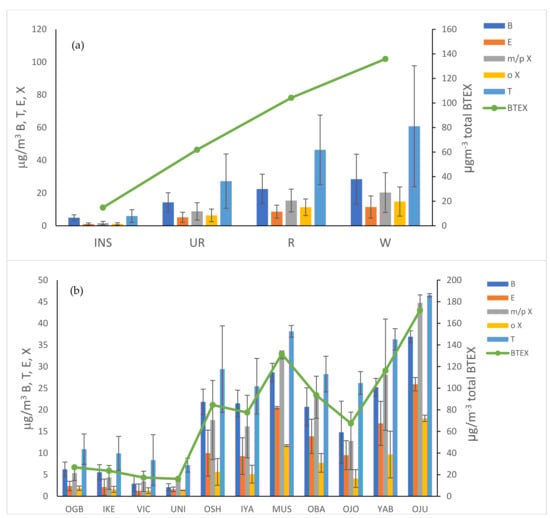
Figure 6.
Concentrations of BTEX compounds across site measured in (a) Nairobi and (b) Lagos.
All roadside/commercial sites investigated in this study exceeded the EU limit value for benzene by between 3 and 7.5 times. In the urban background, Victoria Island and the University of Lagos were the only sites below the limit, levels in Nairobi were close to the limit and all other sites in Lagos exceeded it. The results here are, however, temporally limited and restricted to a few measurements, and it should be noted that the EU regulatory limit is averaged over a one year period. Whilst levels of benzene in cities in the US and Europe are normally below the EU limit of 5 µgm−3 even at roadside locations [62,108,109], this is often not the case in large developing cities. Whilst regulations have resulted in lower benzene content in fuels [66], in many African countries, where the limits are less regulated, gasoline can contain up to 5% benzene [110]. The Do et al. study [62] measured BTEX at roadside location in Hanoi, Vietnam, and Addis Ababa and Jimma in Ethiopia, where benzene concentrations were 5.4–49.4 µgm−3, and Dehghani et al. [50] took roadside measurements in Shiraz, Iran and recorded concentrations in the range of 26.2–34.4 µgm−3 [50], which are in a similar range to those observed in this study.
Unlike much of Africa, China has been the focus of several VOC studies in recent years [51,60,86,95,97,111]. These have demonstrated BTEX levels to be high, particularly in roadside locations. For example, Wang et al., (2002) [86] demonstrated mean roadside levels of benzene between 20 and 51.5, toluene between 39.1 and 85.9, ethylbenzene between 3 and 24.1 and xylene between 14.2 and 95.65 µgm−3. These exceed the highest levels observed in Nairobi and Lagos in this study, and China, along with India [112] and SE Asia [113], has some of the highest recorded BTEX pollution in the world.
3.8. Exposure Calculations
The health effects of airborne pollutants are dependent not only on their concentration but on the length of exposure time and the inhalation rate of the exposed individual. An average inhalation rate of 20 m3 air/day is widely used to determine the inhaled dose for a given air pollutant for adults [103,114]. Table 4 shows the average BTEX absorption typical of an adult who is exposed to the air measured at the roadside in both cities for 8 h, as a typical informal market seller or hawker would be exposed to, for a typical commute time for both cities, and for working an outdoor job in the urban background for 8 h. Salau et.al., (2013) [115] found that a significant proportion of residents of peri-urban area of Ota in Ogun State, north of Lagos, commute to Lagos for work using mostly the public transport network, private cars, commercial motorcycles and tricycles, and residents reported to spend an average of 2–3 h commuting daily. In urban Nairobi, 89% of adult commuting is by walking and/or matatus (privately-operated paratransit), with typical total daily commute times of around an hour [116].

Table 4.
Potential exposure doses of workers in Nairobi and Lagos.
Benzene is carcinogenic to humans, and no level of exposure can be deemed safe [104]; thus, measurement of personal exposure to benzene is important to consider. From the measurements carried out in this pilot study, an 8-h exposure (a typical working day) to benzene was 4.4 times higher (144 µg compared to 33 µg) at the roadside in Nairobi and 5.7 times higher in Lagos (161 µg compared to 28 µg) when compared to the local urban background (Table 4). Exposure between the cities was similar within scenarios; however, the extended commute time experienced in Lagos inevitable leads to a higher (2.7 times) exposure that in Nairobi. There are a limited number of studies on personal exposure to benzene to relate these figures to. One example is the Duarte-Davidson et al., (2001) [103] study to estimate total daily exposure in the UK to benzene for various populations (using 1995 benzene air quality measurements). In this study an urban living individual could expect a daily exposure of 89–95 µg, so an individual working at the roadside in Nairobi or Lagos will exceed this dose by >50% with the roadside exposure alone. Benzene levels in the UK have also decreased from 1995 to present with annual mean concentrations of benzene now consistently below 2 µgm−3 due to the introduction of catalytic converters on car exhausts in the 1990s [117].
To further put this into perspective, the inhaled dose of benzene from a cigarette is on average 40 µg per cigarette [118], so working a roadside job in one of these cities can be equated to smoking 3–4 cigarettes a day. Whilst not investigated in this study, indoor exposure to BTEX when using solid fuel for heating and cooking is likely to far exceed the exposure from outdoors, as has been observed previously for particulate pollution in the region [119]. The total daily exposure of a citizen of Nairobi or Lagos is therefore likely to greatly exceed that of a citizen in a city in a developed country such as the UK, and this high exposure will be disproportionately affecting the most deprived residents of the cities, who live and work in the busiest most polluted areas.
3.9. Key Messages and Recommendations
- This study revealed very high levels of traffic-related VOCs in both Nairobi and Lagos and highlights that exposure to VOCs should be investigated alongside exposure to the more commonly measured air pollutants (NOx, O3, PM and SO2).
- Key similarities between the two cities were the high levels of aliphatic and aromatic hydrocarbons, including the carcinogen benzene, from vehicle exhausts and fuel evaporation, showing the dominance of traffic as a pollution source in such environments.
- Other emissions differed between the cities, particularly the OVOCs. These had a higher contribution to TVOC in Lagos, mostly due to the high abundance of ethanol, likely from the ethanol refining industry.
- While studies from China show an even more severe problem, exposures in these two major African cities are greater than those typical in European and North American cities.
- In terms of burden of disease estimates, VOCs should not be prioritized over PM2.5, but for air quality policy in the two cities, targeted VOC sampling such as that in this study can reveal a wealth of information regarding air pollution sources and determining the photo-chemical age of air masses in complex urban environments and providing evidence to motivate targeted interventions to improve air quality.
4. Conclusions
VOCs were measured at selected sites in Nairobi and Lagos to examine the extent of VOC pollution in the cities. Whilst there are a handful of other VOC studies in Lagos, these are the first measurements we are aware of examining VOCs in Nairobi. The preliminary results demonstrated in this study show that compared to European and US cities, VOC pollution concentrations and the ozone forming potential of the VOCs in the two SSA cities is high, but in a similar range to other developing cities both within the continent and beyond. Consequently, exposure levels of outdoor workers in these cities to harmful VOCs, such as benzene, and their secondary reaction products, are substantially higher. This pilot study represents a useful snapshot into VOC pollution in SSA, a region with huge challenges to overcome in air quality and shows that studies like this one are crucial to raise awareness in the scientific community to the exposure to air pollution of millions of vulnerable people. It also highlights how more VOC measurements are needed, alongside measurements of other pollutants, including radicals, to determine which species limit ozone production and therefore where future mitigation strategies can be best focused.
Author Contributions
Conceptualization—R.L.C., R.P., E.B., M.G., J.D.V.H., Methodology—M.J.W., Formal Analysis—R.L.C., R.P., Investigation—R.L.C., R.P., E.B., E.W., M.N., Resources—M.G., J.N., M.O., A.A.A., R.A., Data curation—R.L.C., Writing—original draft preparation—R.L.C., R.P., Writing—review and editing—M.G., M.J.W., K.P.W., P.S.M., J.D.V.H., Visualization—R.L.C., K.P.W., Supervision—M.G., A.A.A., R.A., P.S.M., J.D.V.H., Project administration—M.G., J.D.V.H., Funding acquisition—M.G., J.N., M.O., J.D.V.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Leicester’s QR Global Challenges Research Fund (Research England), the British Council Newton Utafiti Award 275904011, and the NERC Knowledge Exchange Fellowship of J. D. Vande Hey NE/N005406/1. Additionally, J. D. Vande Hey acknowledges funding from the NIHR HPRU in Environmental Exposures and Health at the University of Leicester.
Data Availability Statement
Raw data is available at https://osf.io/5wq4p/.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aghedo, A.M.; Schultz, M.G.; Rast, S. The influence of African air pollution on regional and global tropospheric ozone. Atmos. Chem. Phys. Discuss. 2007, 7, 1193–1212. [Google Scholar] [CrossRef] [Green Version]
- Schultz, M.G.; Heil, A.; Hoelzemann, J.; Spessa, A.; Thonicke, K.; Goldammer, J.G.; Held, A.C.; Pereira, J.M.C.; Bolscher, M.V.H. Global wildland fire emissions from 1960 to 2000. Glob. Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef]
- Lamancusa, C.; Wagstrom, K. Global transport of dust emitted from different regions of the Sahara. Atmos. Environ. 2019, 214, 116734. [Google Scholar] [CrossRef]
- Monks, P.; Granier, C.; Fuzzi, S.; Stohl, A.; Williams, M.; Akimoto, H.; Amann, M.; Baklanov, A.; Baltensperger, U.; Bey, I.; et al. Atmospheric composition change—Global and regional air quality. Atmos. Environ. 2009, 43, 5268–5350. [Google Scholar] [CrossRef] [Green Version]
- Anenberg, S.C.; Miller, J.; Henze, D.K.; Minjares, R.; Achakulwisut, P. The global burden of transportation tailpipe emissions on air pollution-related mortality in 2010 and 2015. Environ. Res. Lett. 2019, 14, 094012. [Google Scholar] [CrossRef]
- Gaita, S.M.; Boman, J.; Gatari, M.J.; Wagner, A.; Jonsson, S.K. Characterization of Size-Fractionated Particulate Matter and Deposition Fractions in Human Respiratory System in a Typical African City: Nairobi, Kenya. Aerosol Air Qual. Res. 2016, 16, 2378–2385. [Google Scholar] [CrossRef] [Green Version]
- Gatari, M.J.; Boman, J.; Wagner, A. Characterization of aerosol particles at an industrial background site in Nairobi, Kenya. X-Ray Spectrom. 2009, 38, 37–44. [Google Scholar] [CrossRef]
- Ngo, N.S.; Gatari, M.; Yan, B.; Chillrud, S.N.; Bouhamam, K.; Kinney, P.L. Occupational exposure to roadway emissions and inside informal settlements in sub-Saharan Africa: A pilot study in Nairobi, Kenya. Atmos. Environ. 2015, 111, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, F.D.; Gatari, M.; Ng’Ang’A, D.; Poynter, A.; Blake, R. Airborne particulate matter monitoring in Kenya using calibrated low-cost sensors. Atmos. Chem. Phys. Discuss. 2018, 18, 15403–15418. [Google Scholar] [CrossRef] [Green Version]
- Amegah, A.K.; Agyei-Mensah, S. Urban air pollution in Sub-Saharan Africa: Time for action. Environ. Pollut. 2017, 220, 738–743. [Google Scholar] [CrossRef]
- United Nations. Revision of World Urbanization Prospects; United Nations: New York, NY, USA, 2018. [Google Scholar]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.; Balde, A.B.; Bertollini, R.; Bose O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef] [Green Version]
- UNICEF. Silent Suffocation in Africa; UNICEF: New York, NY, USA, 2019. [Google Scholar]
- WHO. Burden of Disease from Ambient Air Pollution for 2012; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Assamoi, E.-M.; Liousse, C. A new inventory for two-wheel vehicle emissions in West Africa for 2002. Atmos. Environ. 2010, 44, 3985–3996. [Google Scholar] [CrossRef]
- Efe, S.I.; Efe, A.T. Spatial distribution of particulate matter (PM10) in Warri metropolis, Nigeria. Environment 2008, 28, 385–394. [Google Scholar] [CrossRef]
- Fayiga, A.O.; Ipinmoroti, M.O.; Chirenje, T. Environmental pollution in Africa. Environ. Dev. Sustain. 2018, 20, 41–73. [Google Scholar] [CrossRef]
- Kinney, P.L.; Gichuru, M.G.; Volavka-Close, N.; Ngo, N.; Ndiba, P.K.; Law, A.; Gachanja, A.; Gaita, S.M.; Chillrud, S.N.; Sclar, E. Traffic impacts on PM2.5 air quality in Nairobi, Kenya. Environ. Sci. Policy 2011, 14, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Naidja, L.; Ali-Khodja, H.; Khardi, S. Sources and levels of particulate matter in North African and Sub-Saharan cities: A literature review. Environ. Sci. Pollut. Res. 2018, 25, 12303–12328. [Google Scholar] [CrossRef] [PubMed]
- Odhiambo, G.O.; Kinyua, A.M.; Gatebe, C.K.; Awange, J. Motor vehicles air pollution in Nairobi, Kenya. Res. J. Environ. Earth Sci. 2010, 2, 178–187. [Google Scholar]
- UNEP. Towards a Green Economy: Pathways to Sustainable Development and Poverty Eradication; UNEP: Nairobi, Kenya, 2011. [Google Scholar]
- Van Vliet, E.D.S.; Kinney, P.L. Impacts of roadway emissions on urban particulate matter concentrations in sub-Saharan Africa: New evidence from Nairobi, Kenya. Environ. Res. Lett. 2007, 2, 045028. [Google Scholar] [CrossRef]
- Croitoru, L.; Chang, J.C.; Kelly, A. The Cost of Air Pollution in Lagos (English); World Bank Group: Washington, DC, USA, 2019. [Google Scholar]
- Hopkins, J.R.; Evans, M.J.; Lee, J.D.; Lewis, A.C.; Marsham, J.H.; McQuaid, J.B.; Parker, D.J.; Stewart, D.J.; Reeves, C.E.; Purvis, R.M. Direct estimates of emissions from the megacity of Lagos. Atmos. Chem. Phys. Discuss. 2009, 9, 8471–8477. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, C.M.; Hourcade, R.; Lefebvre, B.; Pilot, E. A Scoping Review on Air Quality Monitoring, Policy and Health in West African Cities. Int. J. Environ. Res. Public Health 2020, 17, 9151. [Google Scholar] [CrossRef] [PubMed]
- Obanya, H.E.; Amaeze, N.H.; Togunde, O.; Otitoloju, A. Air Pollution Monitoring Around Residential and Transportation Sector Locations in Lagos Mainland. J. Health Pollut. 2018, 8, 180903. [Google Scholar] [CrossRef]
- Clark, S.N.; Alli, A.S.; Brauer, M.; Ezzati, M.; Baumgartner, J.; Toledano, M.B.; Hughes, A.F.; Nimo, J.; Moses, J.B.; Terkpertey, S.; et al. High-resolution spatiotemporal measurement of air and environmental noise pollution in Sub-Saharan African cities: Pathways to Equitable Health Cities Study protocol for Accra, Ghana. BMJ Open 2020, 10, e035798. [Google Scholar] [CrossRef]
- Nkosi, V.; Wichmann, J.; Voyi, K. Indoor and outdoor PM10 levels at schools located near mine dumps in Gauteng and North West Provinces, South Africa. BMC Public Health 2017, 17, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obioh, I.; Ezeh, G.; Abiye, O.; Alpha, A.; Ojo, E.; Ganiyu, A. Atmospheric particulate matter in Nigerian megacities. Toxicol. Environ. Chem. 2013, 95, 379–385. [Google Scholar] [CrossRef]
- Doumbia, M.; Toure, N.E.; Silue, S.; Yoboue, V.; Diedhiou, A.; Hauhouot, C. Emissions from the Road Traffic of West African Cities: Assessment of Vehicle Fleet and Fuel Consumption. Energies 2018, 11, 2300. [Google Scholar] [CrossRef] [Green Version]
- Gatari, M.J.; Boman, J. Black carbon and total carbon measurements at urban and rural sites in Kenya, East Africa. Atmos. Environ. 2003, 37, 1149–1154. [Google Scholar] [CrossRef]
- Baumbach, G.; Vogt, U.; Hein, K.; Oluwole, A.; Ogunsola, O.; Olaniyi, H.; Akeredolu, F. Air pollution in a large tropical city with a high traffic density—Results of measurements in Lagos, Nigeria. Sci. Total Environ. 1995, 169, 25–31. [Google Scholar] [CrossRef]
- Olajire, A.; Azeez, L. Source apportionment and ozone formation potential of volatile organic compounds in Lagos (Nigeria). Chem. Ecol. 2013, 30, 156–168. [Google Scholar] [CrossRef]
- Bailey, J.; Schmidt, B.; Williams, M. Speciated hydrocarbon emissions from vehicles operated over the normal speed range on the road. Atmos. Environ. Part A Gen. Top. 1990, 24, 43–52. [Google Scholar] [CrossRef]
- Inomata, S.; Tanimoto, H.; Fujitani, Y.; Sekimoto, K.; Sato, K.; Fushimi, A.; Yamada, H.; Hori, S.; Kumazawa, Y.; Shimoto, A.; et al. On-line measurements of gaseous nitro-organic compounds in diesel vehicle exhaust by pro-ton-transfer-reaction mass spectrometry. Atmos. Environ. 2013, 73, 195–203. [Google Scholar] [CrossRef]
- Lloyd, A.C.; Cackette, T.A. Diesel Engines: Environmental Impact and Control. J. Air Waste Manag. Assoc. 2001, 51, 809–847. [Google Scholar] [CrossRef] [Green Version]
- Yamada, H.; Misawa, K.; Suzuki, D.; Tanaka, K.; Matsumoto, J.; Fujii, M.; Tanaka, K. Detailed analysis of diesel vehicle exhaust emissions: Nitrogen oxides, hydrocarbons and particulate size distributions. Proc. Combust. Inst. 2011, 33, 2895–2902. [Google Scholar] [CrossRef]
- Huang, H.; Hu, H.; Zhang, J.; Liu, X. Characteristics of volatile organic compounds from vehicle emissions through on–road test in Wuhan, China. Environ. Res. 2020, 188, 109802. [Google Scholar] [CrossRef]
- Krzyżanowski, M.; Kuna-Dibbert, B.; Schneider, J. Health Effects of Transport-Related Air Pollution; World Health Organization Europe: Geneva, Switzerland, 2005. [Google Scholar]
- Keyte, I.J.; Albinet, A.; Harrison, R.M. On-road traffic emissions of polycyclic aromatic hydrocarbons and their oxy- and nitro- derivative compounds measured in road tunnel environments. Sci. Total Environ. 2016, 566, 1131–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, M.G.; Carbone, C.; Faedo, D.; Ferrero, L.; Maggioni, A.; Sangiorgi, G.; Bolzacchini, E. Exhaust emissions of polycyclic aromatic hydrocarbons, n-alkanes and phenols from vehicles coming within different European classes. Atmos. Environ. 2014, 82, 391–400. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, Q.; Li, X.; Tian, F.; Qiao, X.; Chen, J.; Ding, W. Profile and source apportionment of volatile organic compounds from a complex industrial park. Environ. Sci. Process. Impacts 2019, 21, 9–18. [Google Scholar] [CrossRef]
- Wang, H.; Lou, S.; Huang, C.; Qiao, L.; Tang, X.; Chen, C.; Zeng, L.; Wang, Q.; Zhou, M.; Lu, S.; et al. Source Profiles of Volatile Organic Compounds from Biomass Burning in Yangtze River Delta, China. Aerosol Air Qual. Res. 2014, 14, 818–828. [Google Scholar] [CrossRef] [Green Version]
- Monks, P.S.; Archibald, A.T.; Colette, A.; Cooper, O.; Coyle, M.; Derwent, R.; Fowler, D.; Granier, C.; Law, K.S.; Mills, G.E.; et al. Tropospheric ozone and its precursors from the urban to the global scale from air quality to short-lived climate forcer. Atmos. Chem. Phys. Discuss. 2015, 15, 8889–8973. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Norris, C.; Johnson, K.; Cui, X.; Sun, J.; Teng, Y.; Tian, E.; Xu, W.; Li, Z.; Mo, J.; et al. Toxic volatile organic compounds in 20 homes in Shanghai: Concentrations, inhalation health risks, and the impacts of household air cleaning. Build. Environ. 2019, 157, 309–318. [Google Scholar] [CrossRef]
- Public Heath England. Indoor Air Quality Guidelines for selected Volatile Organic Compounds (VOCs) in the UK; Public Heath England: London, UK, 2019. [Google Scholar]
- Davidson, C.J.; Hannigan, J.H.; Bowen, S.E. Effects of inhaled combined Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX): Toward an environmental exposure model. Environ. Toxicol. Pharmacol. 2021, 81, 103518. [Google Scholar] [CrossRef]
- Rumchev, K.; Spickett, J.; Bulsara, M.; Phillips, M.; Stick, S. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax 2004, 59, 746–751. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, M.; Fazlzadeh, M.; Sorooshian, A.; Tabatabaee, H.R.; Miri, M.; Baghani, A.N.; Delikhoon, M.; Mahvi, A.H.; Rashidi, M. Characteristics and health effects of BTEX in a hot spot for urban pollution. Ecotoxicol. Environ. Saf. 2018, 155, 133–143. [Google Scholar] [CrossRef]
- Zhou, J.; You, Y.; Bai, Z.; Hu, Y.; Zhang, J.; Zhang, N. Health risk assessment of personal inhalation exposure to volatile organic compounds in Tianjin, China. Sci. Total Environ. 2011, 409, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, M.; Dioni, L.; Bonzini, M.; Pesatori, A.C.; Fustinoni, S.; Cavallo, D.; Carugno, M.; Albetti, B.; Marinelli, B.; Schwartz, J.; et al. Association between leukocyte telomere shortening and exposure to traffic pollution: A cross-sectional study on traffic officers and indoor office workers. Environ. Health 2009, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, M.; Autrup, H.; Møller, P.; Hertel, O.; Jensen, S.S.; Vinzents, P.; Knudsen, L.E.; Loft, S. Linking exposure to environmental pollutants with biological effects. Mutat. Res. Mutat. Res. 2003, 544, 255–271. [Google Scholar] [CrossRef]
- Blumberg, L. Comprehensive two-dimensional gas chromatography: Metrics, potentials, limits. J. Chromatogr. A 2003, 985, 29–38. [Google Scholar] [CrossRef]
- Lewis, A.; Carslaw, N.; Marriott, P.; Kinghorn, R.M.; Morrison, P.; Lee, A.L.; Bartle, K.D.; Pilling, M.J. A larger pool of ozone-forming carbon compounds in urban atmospheres. Nat. Cell Biol. 2000, 405, 778–781. [Google Scholar] [CrossRef]
- Kenya National Bureau of Statistics. Kenya Population and Housing Census Volume I: Population by County and Sub-County; Kenya National Bureau of Statistics: Nairobi, Kenya, 2019. [Google Scholar]
- Zlotnik, H. World Urbanization: Trends and Prospects. In New Forms of Urbanization; Routledge: London, UK, 2017; pp. 43–64. [Google Scholar]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, G.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metab. Off. J. Metab. Soc. 2007, 3, 211–221. [Google Scholar]
- Wilde, M.J.; Cordell, R.; Salman, D.; Zhao, B.; Ibrahim, W.; Bryant, L.; Ruszkiewicz, D.; Singapuri, A.; Free, R.C.; Gaillard, E.A.; et al. Breath analysis by two-dimensional gas chromatography with dual flame ionisation and mass spectrometric detection—Method optimisation and integration within a large-scale clinical study. J. Chromatogr. A 2019, 1594, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ho, S.S.H.; Xue, Y.; Huang, Y.; Wang, L.; Cheng, Y.; Dai, W.; Zhong, H.; Cao, J.; Lee, S.-C. Characterizations of volatile organic compounds (VOCs) from vehicular emissions at roadside environment: The first comprehensive study in Northwestern China. Atmos. Environ. 2017, 161, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tsai, J.H.; Lu, Y.T.; Chung, I.I.; Chiang, H.L. Traffic-Related Airborne VOC Profiles Variation on Road Sites and Residential Area within a Microscale in Urban Area in Southern Taiwan. Atmosphere 2020, 11, 1015. [Google Scholar] [CrossRef]
- Do, D.H.; Van Langenhove, H.; Walgraeve, C.; Hayleeyesus, S.F.; De Wispelaere, P.; Dewulf, J.; Demeestere, K. Volatile organic compounds in an urban environment: A comparison among Belgium, Vietnam and Ethiopia. Int. J. Environ. Anal. Chem. 2013, 93, 298–314. [Google Scholar] [CrossRef]
- Srivastava, A.; Joseph, A.; Patil, S.; More, A.; Dixit, R. Air toxics in ambient air of Delhi. Atmos. Environ. 2005, 39, 59–71. [Google Scholar] [CrossRef]
- Dobslaw, D.; Engesser, K.-H.; Störk, H.; Gerl, T. Low-cost process for emission abatement of biogas internal combustion engines. J. Clean. Prod. 2019, 227, 1079–1092. [Google Scholar] [CrossRef]
- Huang, Y.; Ling, Z.H.; Lee, S.C.; Ho, S.S.H.; Cao, J.J.; Blake, D.R.; Cheng, Y.; Lai, S.; Ho, K.F.; Gao, Y.; et al. Characterization of volatile organic compounds at a roadside environment in Hong Kong: An investigation of influences after air pollution control strategies. Atmos. Environ. 2015, 122, 809–818. [Google Scholar] [CrossRef] [Green Version]
- Chin, J.-Y.; Batterman, S.A. VOC composition of current motor vehicle fuels and vapors, and collinearity analyses for receptor modeling. Chemosphere 2012, 86, 951–958. [Google Scholar] [CrossRef] [Green Version]
- Doumbia, E.H.T.; Liousse, C.; Galy-Lacaux, C.; Ndiaye, S.A.; Diop, B.; Ouafo, M.; Assamoi, E.M.; Gardrat, E.; Castera, P.; Rosset, R.; et al. Real time black carbon measurements in West and Central Africa urban sites. Atmos. Environ. 2012, 54, 529–537. [Google Scholar] [CrossRef]
- Mbandi, A.M.; Böhnke, J.R.; Schwela, D.; Vallack, H.; Ashmore, M.R.; Emberson, L. Estimating On-Road Vehicle Fuel Economy in Africa: A Case Study Based on an Urban Transport Survey in Nairobi, Kenya. Energies 2019, 12, 1177. [Google Scholar] [CrossRef] [Green Version]
- Watson, J.G.; Chow, J.C.; Fujita, E.M. Review of volatile organic compound source apportionment by chemical mass balance. Atmos. Environ. 2001, 35, 1567–1584. [Google Scholar] [CrossRef]
- UNEP. Used Vehicles and the Environment Global Overview of Used Light Vehicles—Flow, Scale and Regulation; UNEP: Blackwell, UK, 2020. [Google Scholar]
- Guo, H.; Ling, Z.; Cheung, K.; Wang, D.; Simpson, I.; Blake, D. Acetone in the atmosphere of Hong Kong: Abundance, sources and photochemical precursors. Atmos. Environ. 2013, 65, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Jacob, D.J.; Field, B.D.; Jin, E.M.; Bey, I.; Li, Q.; Logan, J.A.; Yantosca, R.M.; Singh, H.B. Atmospheric budget of acetone. J. Geophys. Res. Space Phys. 2002, 107. [Google Scholar] [CrossRef]
- Singh, H.B.; O’Hara, D.; Herlth, D.; Sachse, W.; Blake, D.R.; Bradshaw, J.D.; Kanakidou, M.; Crutzen, P.J. Acetone in the atmosphere: Distribution, sources, and sinks. J. Geophys. Res. Space Phys. 1994, 99, 1805–1819. [Google Scholar] [CrossRef]
- Ohimain, E.I. Emerging bio-ethanol projects in Nigeria: Their opportunities and challenges. Energy Policy 2010, 38, 7161–7168. [Google Scholar] [CrossRef]
- Felix, J.D.; Willey, J.D.; Thomas, R.K.; Mullaugh, K.M.; Avery, G.B.; Kieber, R.J.; Mead, R.N.; Helms, J.; Giubbina, F.F.; Campos, M.L.A.M.; et al. Removal of Atmospheric Ethanol by Wet Deposition: A Global Flux Estimate. Glob. Biogeochem. Cycles 2017, 31, 348–356. [Google Scholar] [CrossRef]
- Millet, D.B.; Apel, E.; Henze, D.K.; Hill, J.; Marshall, J.D.; Singh, H.B.; Tessum, C.W. Natural and Anthropogenic Ethanol Sources in North America and Potential Atmospheric Impacts of Ethanol Fuel Use. Environ. Sci. Technol. 2012, 46, 8484–8492. [Google Scholar] [CrossRef] [PubMed]
- Ohimain, E.I. A review of the Nigerian biofuel policy and incentives (2007). Renew. Sustain. Energy Rev. 2013, 22, 246–256. [Google Scholar] [CrossRef]
- Ozier, A.; Charron, D.; Chung, S.; Sarma, V.; Dutta, A.; Jagoe, K.; Obueh, J.; Stokes, H.; Munangagwa, C.L.; Johnson, M.; et al. Building a consumer market for ethanol-methanol cooking fuel in Lagos, Nigeria. Energy Sustain. Dev. 2018, 46, 65–70. [Google Scholar] [CrossRef]
- Yáñez-Serrano, A.M.; Nölscher, A.C.; Bourtsoukidis, E.; Derstroff, B.; Zannoni, N.; Gros, V.; Lanza, M.; Brito, J.; Noe, S.M.; House, E.; et al. Atmospheric mixing ratios of methyl ethyl ketone (2-butanone) in tropical, boreal, temperate and marine environments. Atmos. Chem. Phys. Discuss. 2016, 16, 10965–10984. [Google Scholar] [CrossRef] [Green Version]
- Boutekedjiret, C.; Vian, M.A.; Chemat, F. Terpenes as Green Solvents for Natural Products Extraction; Chemat, F., Vian, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 205–219. [Google Scholar]
- Alghamdi, M.A.; Khoder, M.; Abdelmaksoud, A.S.; Harrison, R.M.; Hussein, T.; Lihavainen, H.; Al-Jeelani, H.; Goknil, M.H.; Shabbaj, I.I.; Almehmadi, F.M.; et al. Seasonal and diurnal variations of BTEX and their potential for ozone formation in the urban back-ground atmosphere of the coastal city Jeddah, Saudi Arabia. Air Qual. Atmos. Health 2014, 7, 467–480. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Z.; Du, Y.; Guo, H. Analyses of volatile organic compounds concentrations and variation trends in the air of Changchun, the northeast of China. Atmos. Environ. 2000, 34, 4459–4466. [Google Scholar] [CrossRef]
- Buczynska, A.J.; Krata, A.; Stranger, M.; Godoi, A.F.L.; Kontozova-Deutsch, V.; Bencs, L.; Naveau, I.; Roekens, E.; Van Grieken, R. Atmospheric BTEX-concentrations in an area with intensive street traffic. Atmos. Environ. 2009, 43, 311–318. [Google Scholar] [CrossRef]
- Miller, L.; Xu, X.; Wheeler, A.; Atari, D.O.; Grgicak-Mannion, A.; Luginaah, I. Spatial Variability and Application of Ratios between BTEX in Two Canadian Cities. Sci. World J. 2011, 11, 2536–2549. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shao, M.; Fu, L.; Lu, S.; Zeng, L.; Tang, D. Source profiles of volatile organic compounds (VOCs) measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar] [CrossRef]
- Wang, X.; Sheng, G.-Y.; Fu, J.-M.; Chan, C.-Y.; Lee, S.-C.; Chan, L.Y.; Wang, Z.-S. Urban roadside aromatic hydrocarbons in three cities of the Pearl River Delta, People’s Republic of China. Atmos. Environ. 2002, 36, 5141–5148. [Google Scholar] [CrossRef]
- Barletta, B.; Meinardi, S.; Simpson, I.J.; Zou, S.; Rowland, F.S.; Blake, D.R. Ambient mixing ratios of nonmethane hydrocarbons (NMHCs) in two major urban centers of the Pearl River Delta (PRD) region: Guangzhou and Dongguan. Atmos. Environ. 2008, 42, 4393–4408. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, V.; Hanai, Y.; Masunaga, S. Ambient levels of volatile organic compounds in the vicinity of petrochemical industrial area of Yokohama, Japan. Air Qual. Atmos. Health 2010, 3, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Jost, C.; Sprung, D.; Andreae, M.O.; McQuaid, J.B.; Barjat, H.; Trentmann, J. Trace gas chemistry in a young biomass burning plume over Namibia: Observations and model simulations. J. Geophys. Res. Space Phys. 2003, 108. [Google Scholar] [CrossRef]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R.T. Measurement of Emissions from Air Pollution Sources. 3. C1−C29 Organic Compounds from Fireplace Combustion of Wood. Environ. Sci. Technol. 2001, 35, 1716–1728. [Google Scholar] [CrossRef]
- Jenkin, M.E.; Hayman, G.D. Photochemical ozone creation potentials for oxygenated volatile organic compounds: Sensi-tivity to variations in kinetic and mechanistic parameters. Atmos. Environ. 1999, 33, 1275–1293. [Google Scholar] [CrossRef]
- Monks, P.S. Gas-phase radical chemistry in the troposphere. Chem. Soc. Rev. 2005, 34, 376–395. [Google Scholar] [CrossRef] [Green Version]
- Carter, W.P. Updated maximum incremental reactivity scale and hydrocarbon bin reactivities for regulatory applications. Calif. Air Resour. Board Contract 2009, 2009, 339. [Google Scholar]
- Hwa, M.-Y.; Hsieh, C.-C.; Wu, T.-C.; Chang, L.-F.W. Real-world vehicle emissions and VOCs profile in the Taipei tunnel located at Taiwan Taipei area. Atmos. Environ. 2002, 36, 1993–2002. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, Y.; Liu, J.; Mellouki, A. Levels, sources and health risks of carbonyls and BTEX in the ambient air of Beijing, China. J. Environ. Sci. 2012, 24, 124–130. [Google Scholar] [CrossRef]
- Olumayede, E.G. Atmospheric Volatile Organic Compounds and Ozone Creation Potential in an Urban Center of Southern Nigeria. Int. J. Atmos. Sci. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Duan, J.; Tan, J.; Yang, L.; Wu, S.; Hao, J. Concentration, sources and ozone formation potential of volatile organic compounds (VOCs) during ozone episode in Beijing. Atmos. Res. 2008, 88, 25–35. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lo, J.-G.; Wang, J.-L. Assessment of reducing ozone forming potential for vehicles using liquefied petroleum gas as an alternative fuel. Atmos. Environ. 2001, 35, 6201–6211. [Google Scholar] [CrossRef]
- Wang, M.; Li, S.; Zhu, R.; Zhang, R.; Zu, L.; Wang, Y.; Bao, X. On-road tailpipe emission characteristics and ozone formation potentials of VOCs from gasoline, diesel and liquefied petroleum gas fueled vehicles. Atmos. Environ. 2020, 223, 117294. [Google Scholar] [CrossRef]
- Harrison, R.M. Principles of Environmental Chemistry; RSC Publishing: Cambridge, UK, 2007. [Google Scholar]
- EPA. Tables of Maximum Incremental Reactivity (MIR) Values; EPA: Washington, DC, USA, 2001. [Google Scholar]
- Derwent, R.G.; Jenkin, M.E.; Saunders, S.M. Photochemical ozone creation potentials for a large number of reactive hydrocarbons under European conditions. Atmos. Environ. 1996, 30, 181–199. [Google Scholar] [CrossRef]
- Duarte-Davidson, R.; Courage, C.; Rushton, L.; Levy, L. Benzene in the environment: An assessment of the potential risks to the health of the population. Occup. Environ. Med. 2001, 58, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Benzene. In Air Quality Guidelines for Europe, 2nd ed.; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- Yassaa, N.; Brancaleoni, E.; Frattoni, M.; Ciccioli, P. Isomeric analysis of BTEXs in the atmosphere using β-cyclodextrin capillary chromatography coupled with thermal desorption and mass spectrometry. Chemosphere 2006, 63, 502–508. [Google Scholar] [CrossRef]
- Zhang, H.R.; Eddings, E.G.; Sarofim, A.F. Pollutant Emissions from Gasoline Combustion. 1. Dependence on Fuel Structural Functionalities. Environ. Sci. Technol. 2008, 42, 5615–5621. [Google Scholar] [CrossRef] [PubMed]
- Harley, R.A.; Hooper, D.S.; Kean, A.J.; Kirchstetter, T.W.; Hesson, J.M.; Balberan, N.T.; Stevenson, E.D.; Kendall, G.R. Effects of Reformulated Gasoline and Motor Vehicle Fleet Turnover on Emissions and Ambient Concen-trations of Benzene. Environ. Sci. Technol. 2006, 40, 5084–5088. [Google Scholar] [CrossRef]
- Von Schneidemesser, E.; Monks, P.S.; Plass-Duelmer, C. Global comparison of VOC and CO observations in urban areas. Atmos. Environ. 2010, 44, 5053–5064. [Google Scholar] [CrossRef]
- Warneke, C.; De Gouw, J.A.; Holloway, J.S.; Peischl, J.; Ryerson, T.B.; Atlas, E.; Blake, D.; Trainer, M.; Parrish, D.D. Multiyear trends in volatile organic compounds in Los Angeles, California: Five decades of decreasing emissions. J. Geophys. Res. Atmos. 2012, 117. [Google Scholar] [CrossRef]
- Agbo, K.E.; Walgraeve, C.; Eze, J.I.; Ugwoke, P.E.; Ukoha, P.O.; Van Langenhove, H. A review on ambient and indoor air pollution status in Africa. Atmos. Pollut. Res. 2021, 12, 243–260. [Google Scholar] [CrossRef]
- Liu, J.; Mu, Y.; Zhang, Y.; Zhang, Z.; Wang, X.; Liu, Y.; Sun, Z. Atmospheric levels of BTEX compounds during the 2008 Olympic Games in the urban area of Beijing. Sci. Total Environ. 2009, 408, 109–116. [Google Scholar] [CrossRef]
- Dutta, C.; Som, D.; Chatterjee, A.; Mukherjee, A.K.; Jana, T.K.; Sen, S. Mixing ratios of carbonyls and BTEX in ambient air of Kolkata, India and their associated health risk. Environ. Monit. Assess. 2008, 148, 97–107. [Google Scholar] [CrossRef]
- Nguyen, V.T.L.; Dung, N.T.; Yoneda, M.; Vinh, T.H. Preliminary assessment of BTEX concentrations indoor and outdoor air in residential homes in Hanoi, Vietnam. Vietnam J. Sic. Technol. 2017, 55, 78–84. [Google Scholar]
- Rowbotham, A.L.; Levy, L.S.; Shuker, L.K. Chromium in the environment: An evaluation of exposure of the UK general population and possible adverse health effects. J. Toxicol. Environ. Health Part B Crit. Rev. 2000, 3, 145–178. [Google Scholar]
- Salau, A.; Lawanson, T.; Odumbaku, O. Amoebic Urbanization in Nigerian Cities (The Case of Lagos and Ota). Int. J. Archit. Urban Dev. 2013, 3, 19–26. [Google Scholar]
- Salon, D.; Gulyani, S. Commuting in Urban Kenya: Unpacking Travel Demand in Large and Small Kenyan Cities. Sustainability 2019, 11, 3823. [Google Scholar] [CrossRef] [Green Version]
- DEFRA. Air Pollution in the UK 2019; DEFRA: London, UK, 2020. [Google Scholar]
- IEH. IEH Report on Benzene in the Environment: An Evaluation of Exposure of the UK General Population and Possible Adverse Health Effects; IEH Report R12; Institute for Environment and Health: Leicester, UK, 1999. [Google Scholar]
- Ezzati, M.; Kammen, D.M. Indoor air pollution from biomass combustion and acute respiratory infections in Kenya: An exposure-response study. Lancet 2001, 358, 619–624. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).