Multi-Media Exposure to Polycyclic Aromatic Hydrocarbons at Lake Chaohu, the Fifth Largest Fresh Water Lake in China: Residual Levels, Sources and Carcinogenic Risk
Abstract
:1. Introduction
2. Materials and Methods
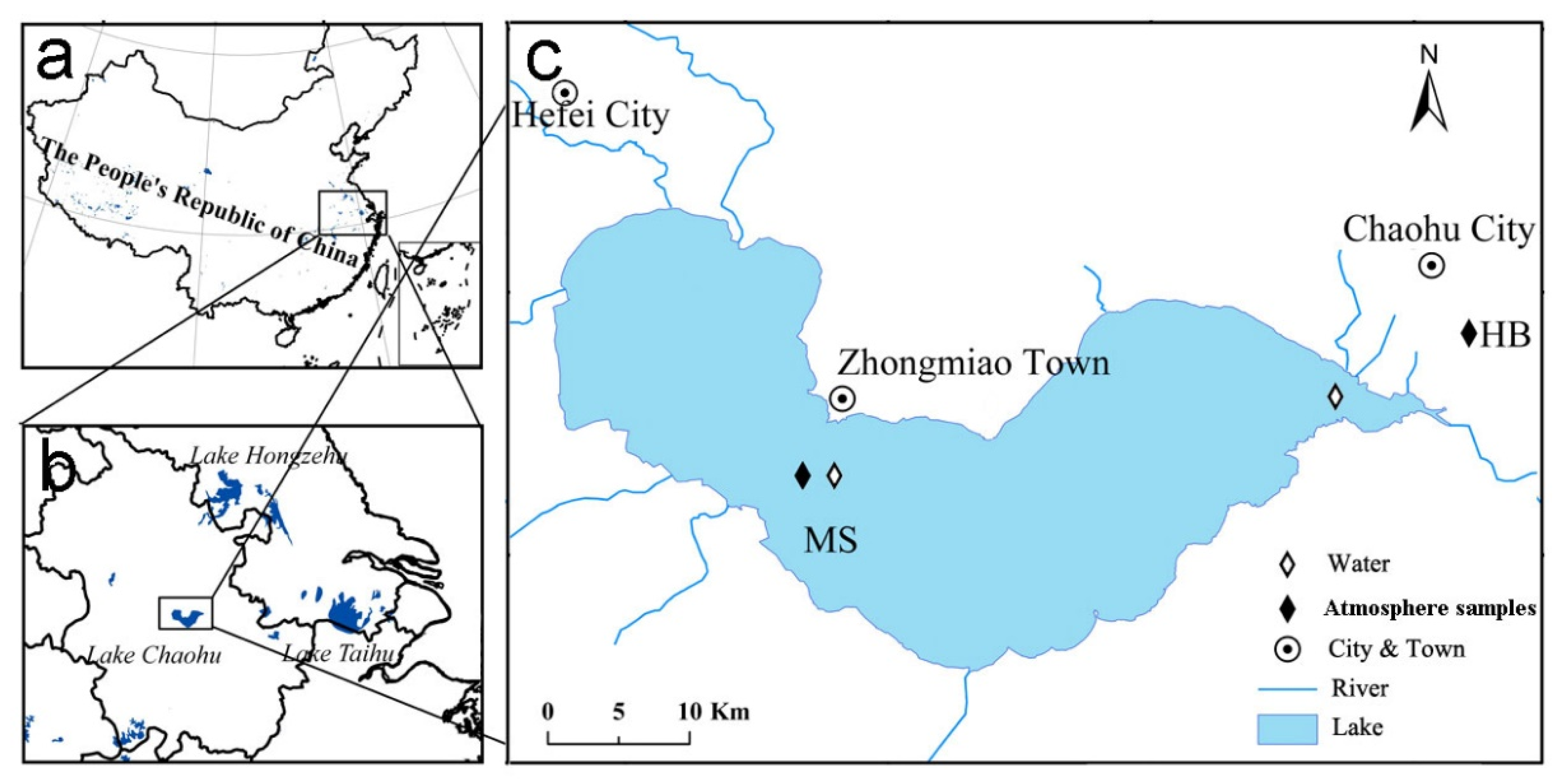
2.1. Sample Collection and Pretreatment
2.2. Instrument Analysis
2.3. Positive Matrix Factorization (PMF)
2.4. Multi-Pathway Exposure and Risk Assessment
3. Results and Discussions
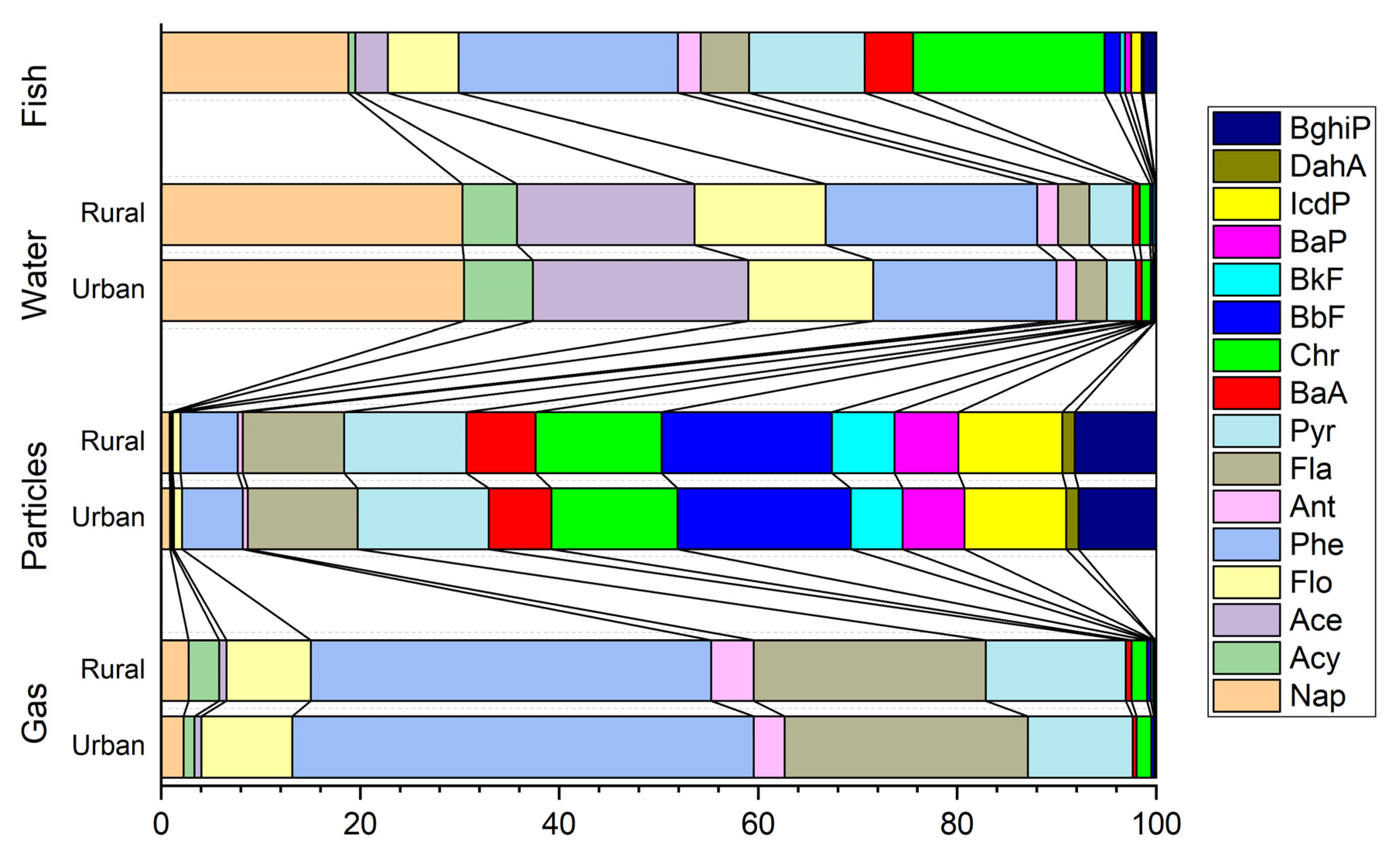
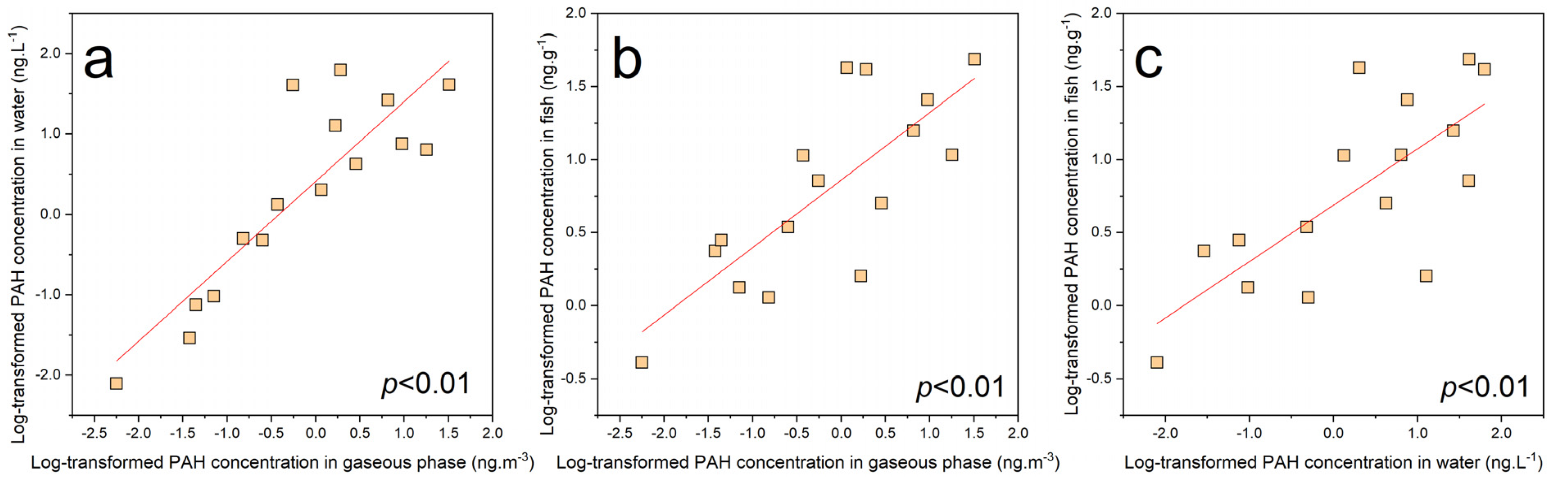
3.1. PAH Residual Levels in Environment Media
3.2. Source Apportionment
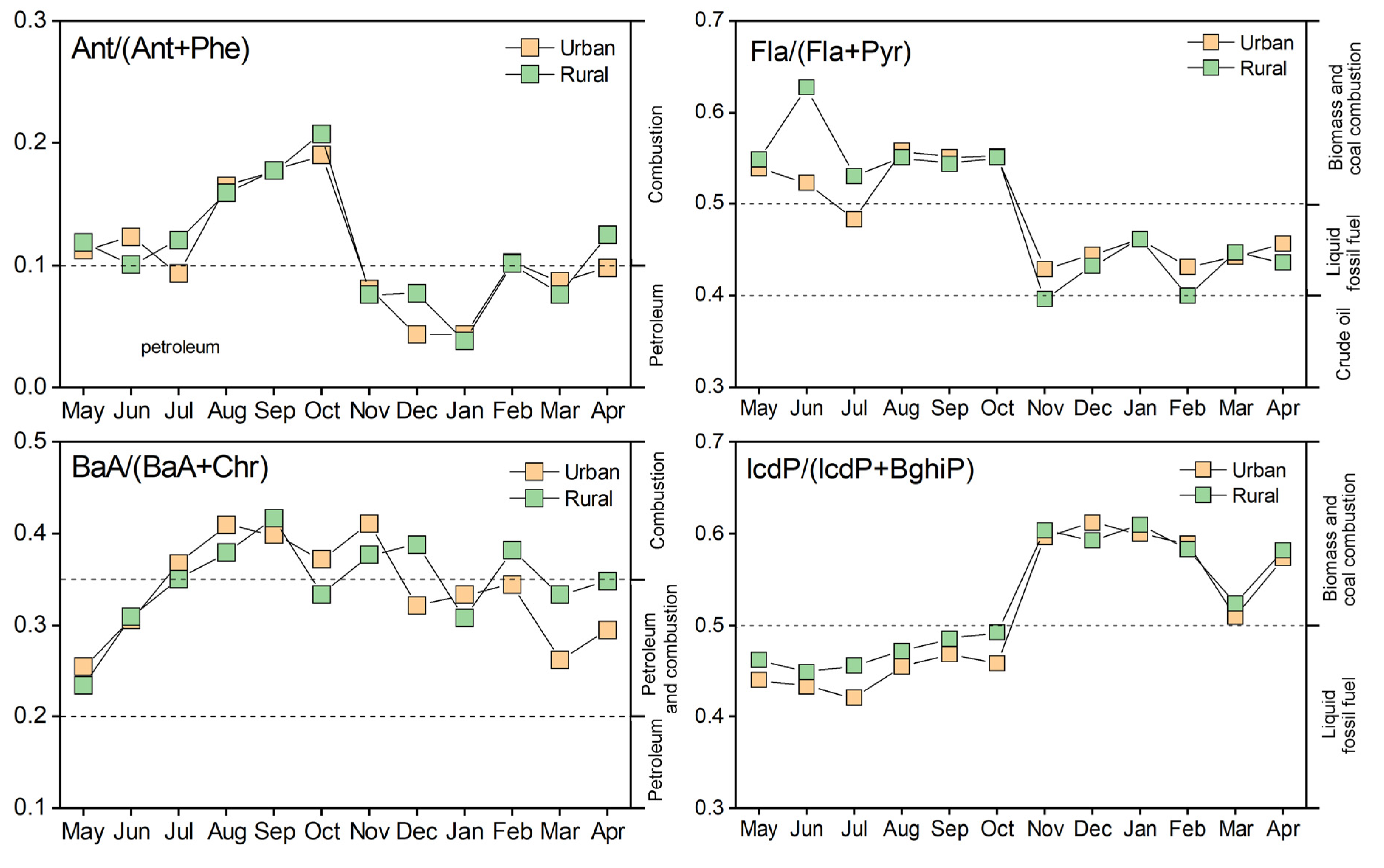
3.2.1. Molecular Diagnostic Ratios
3.2.2. PMF Results
3.3. Exposure through Different Pathways
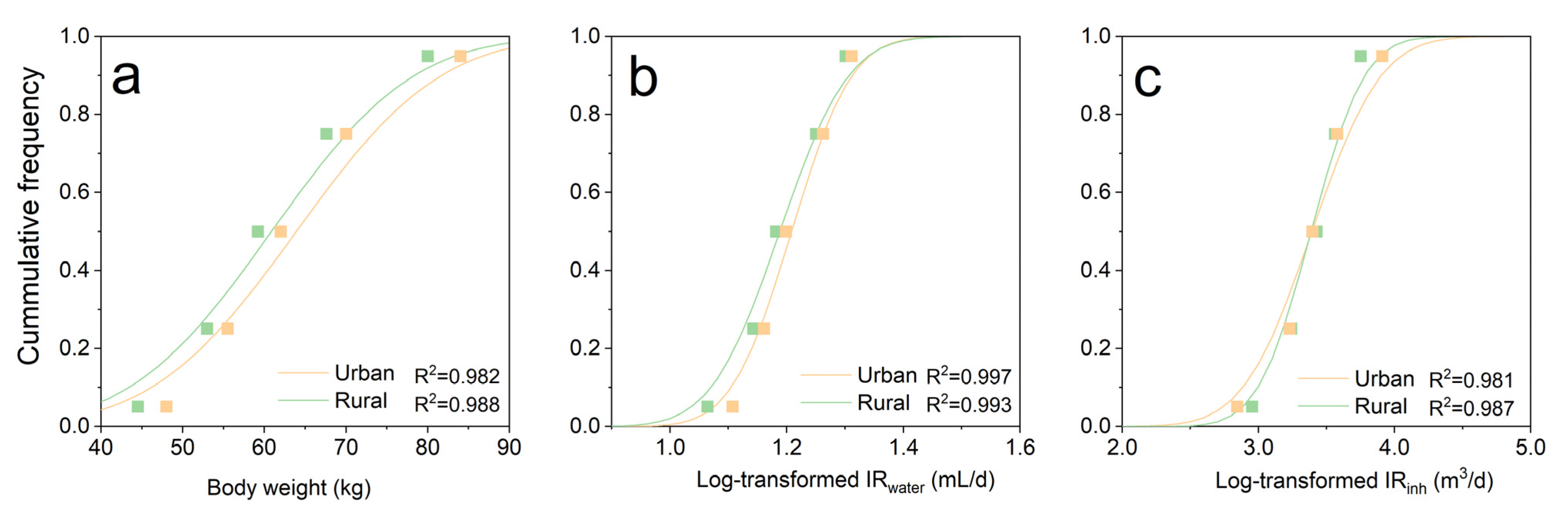
3.3.1. Derivation of Exposure Parameters
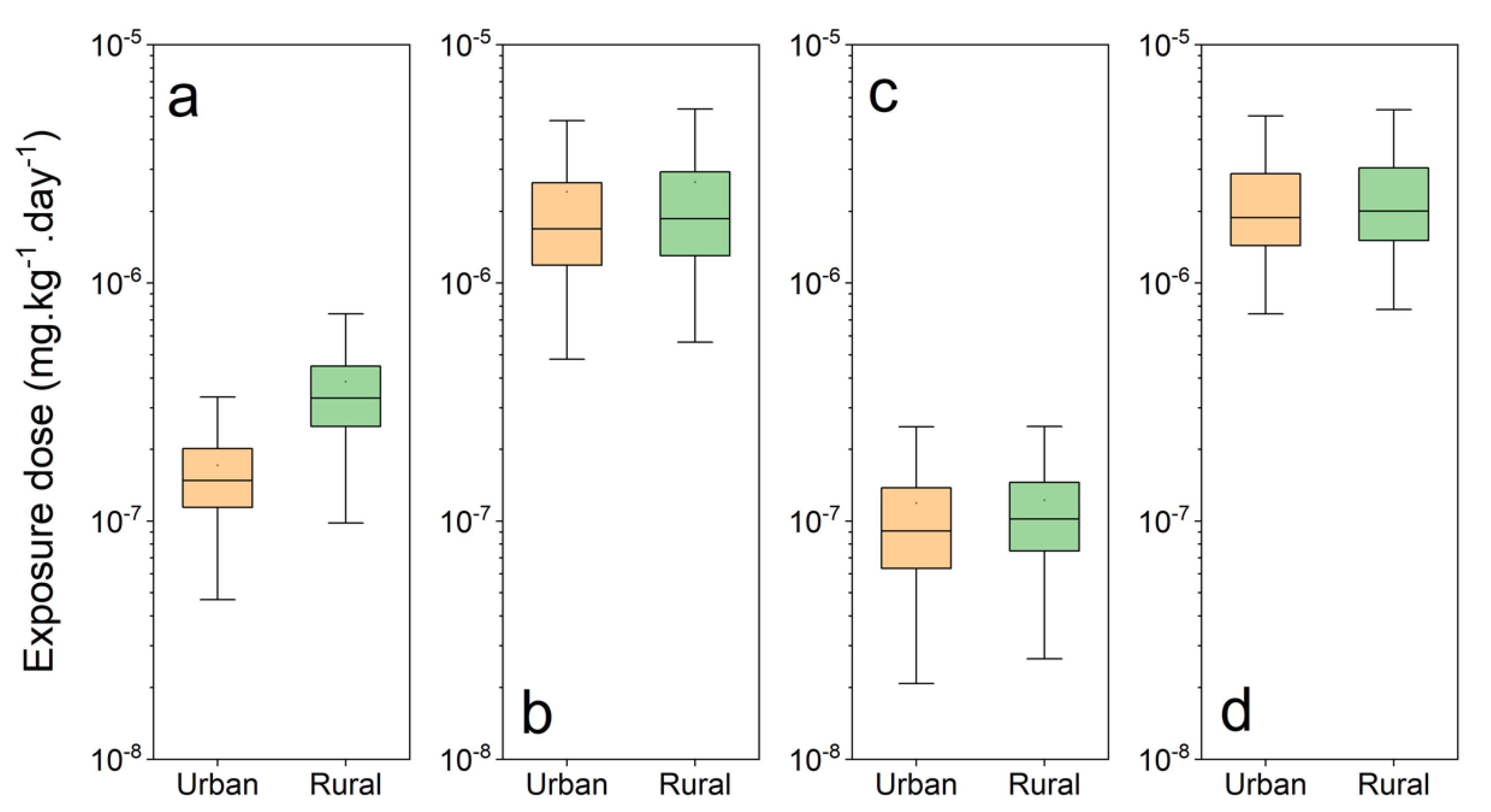
3.3.2. Estimation of Exposure Doses
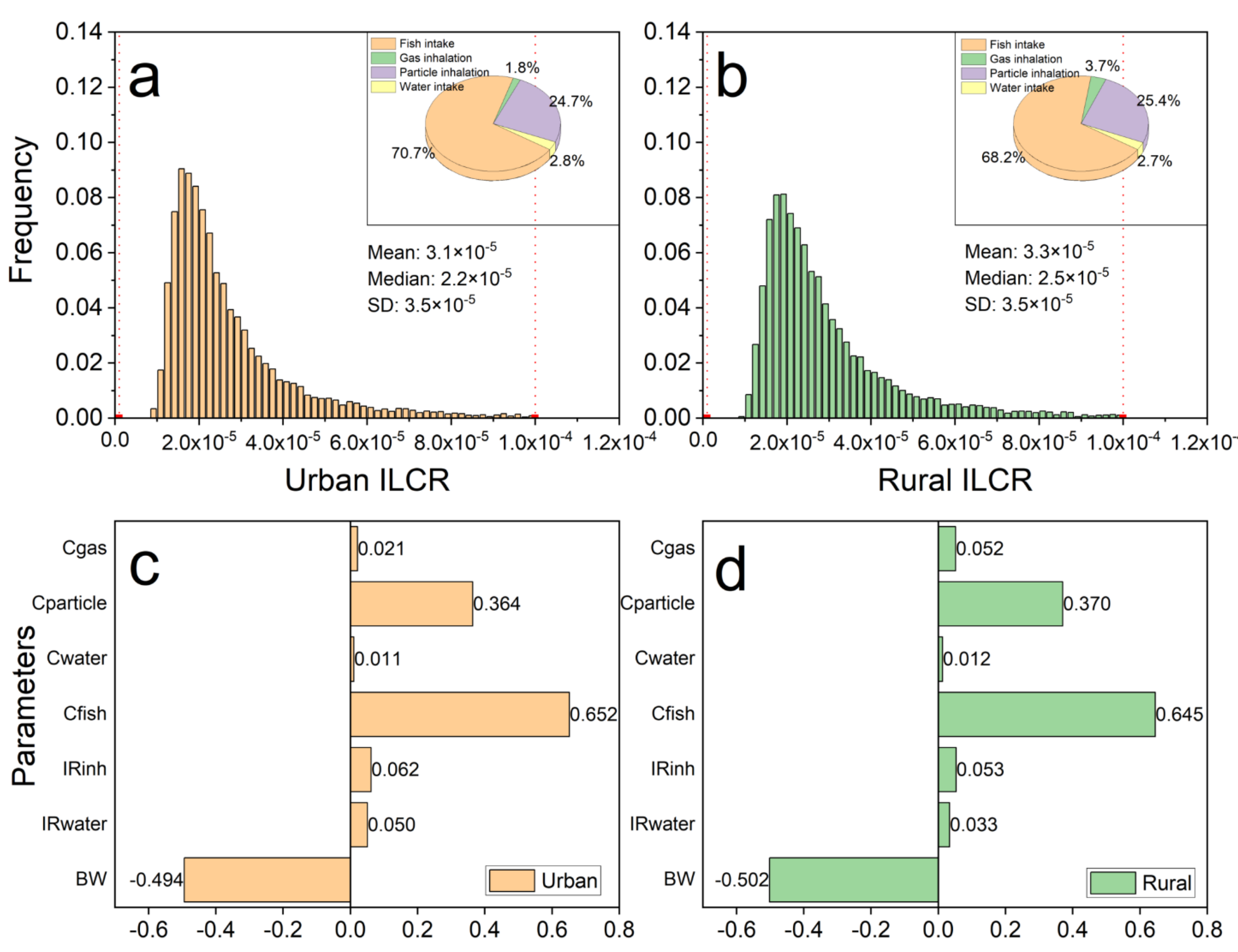
3.4. Carcinogenic Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Xu, F.-L.; Qin, N.; Zhu, Y.; He, W.; Kong, X.-Z.; Barbour, M.T.; He, Q.-S.; Wang, Y.; Ou-Yang, H.-L.; Tao, S. Multimedia fate modeling of polycyclic aromatic hydrocarbons (PAHs) in Lake Small Baiyangdian, Northern China. Ecol. Model. 2013, 252, 246–257. [Google Scholar] [CrossRef]
- Larsen, R.K.; Baker, J. Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: A comparison of three methods. Environ. Sci. Technol. 2003, 37, 1873–1881. [Google Scholar] [CrossRef]
- Fernandes, M.; Sicre, M.-A.; Boireau, A.; Tronczynski, J. Polyaromatic hydrocarbon (PAH) distributions in the Seine River and its estuary. Mar. Pollut. Bull. 1997, 34, 857–867. [Google Scholar] [CrossRef]
- Man, Y.B.; Mo, W.Y.; Zhang, F.; Wong, M.H. Health risk assessments based on polycyclic aromatic hydrocarbons in freshwater fish cultured using food waste-based diets. Environ. Pollut. 2020, 256, 113380. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.G.; Doust, E.; Cherrie, J.W.; Hurley, J.F. Lung cancer mortality and exposure to polycyclic aromatic hydrocarbons in British coke oven workers. BMC Public Health 2013, 13, 962. [Google Scholar] [CrossRef] [Green Version]
- Kuang, D.; Zhang, W.; Deng, Q.; Zhang, X.; Huang, K.; Guan, L.; Hu, D.; Wu, T.; Guo, H. Dose-response relationships of polycyclic aromatic hydrocarbons exposure and oxidative damage to DNA and lipid in coke oven workers. Environ. Sci. Technol. 2013, 47, 7446–7456. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Duan, X.; Qin, N.; Li, J.; Tian, J.; Zhong, Y.; Chen, L.; Fan, R.; Yu, Y.; Wu, G.; et al. Internal biomarkers and external estimation of exposure to polycyclic aromatic hydrocarbons and their relationships with cancer mortality in a high cancer incidence area. Sci. Total Environ. 2019, 688, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Rogge, W.F.; Hildemann, L.M.; Mazurek, M.A.; Cass, G.R.; Simoneit, B.R. Sources of fine organic aerosol.3. Road dust, tire debris, and organonetallic brake lining dust-roads as sources and sinks. Environ. Sci. Technol. 1993, 27, 1892–1904. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.; Ma, Y.; Qiu, X.; Ren, A. Association of polycyclic aromatic hydrocarbons in housewives’ hair with hyper-tension. Chemosphere 2016, 153, 315–321. [Google Scholar] [CrossRef]
- Qin, N.; He, W.; Kong, X.Z.; Liu, W.X.; He, Q.S.; Yang, B.; Ouyang, H.L.; Wang, Q.M.; Xu, F.L. Ecological risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the water from a large Chinese lake based on multiple indicators. Ecol. Indic. 2013, 24, 599–608. [Google Scholar] [CrossRef]
- Andra, S.S.; Austin, C.; Wright, R.O.; Arora, M. Reconstructing pre-natal and early childhood exposure to multi-class organic chemicals using teeth: Towards a retrospective temporal exposome. Environ. Int. 2015, 83, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Li, Q.; Wang, H.; Wang, B.; Wang, X.; Ren, A.; Tao, S. Risk of human exposure to polycyclic aromatic hydrocarbons: A case study in Beijing, China. Environ. Pollut. 2015, 205, 70–77. [Google Scholar] [CrossRef]
- Lohmann, R.; Jurado, E.; Pilson, M.E.; Dachs, J. Oceanic deep water formation as a sink of persistent organic pollutants. Geophys. Res. Lett. 2006, 33, 735. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Li, J.; Cheng, H.; Li, X.; Xu, W.; Jones, K.C. Distribution of organochlorine pesticides in the Northern South China Sea: Implications for land outflow and air−sea exchange. Environ. Sci. Technol. 2007, 41, 3884–3890. [Google Scholar] [CrossRef]
- Simonich, S.L.; Hites, R.A. Organic Pollutant Accumulation in Vegetation. Environ. Sci. Technol. 1995, 29, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Simcik, M.F.; Eisenreich, S.J.; Lioy, P.J. Source apportionment and source/sink relationships of PAHs in the coastal atmosphere of Chicago and Lake Michigan. Atmos. Environ. 1999, 33, 5071–5079. [Google Scholar] [CrossRef]
- Franz, T.P.; Eisenreich, S.J.; Holsen, T.M. Dry Deposition of Particulate Polychlorinated Biphenyls and Polycyclic Aromatic Hydrocarbons to Lake Michigan. Environ. Sci. Technol. 1998, 32, 3681–3688. [Google Scholar] [CrossRef]
- Qin, N.; He, W.; Liu, W.; Kong, X.; Xu, F.; Giesy, J.P. Tissue distribution, bioaccumulation, and carcinogenic risk of polycyclic aromatic hydrocarbons in aquatic organisms from Lake Chaohu, China. Sci. Total Environ. 2020, 749, 141577. [Google Scholar] [CrossRef]
- Xu, F.-L.; Wu, W.-J.; Wang, J.-J.; Qin, N.; Wang, Y.; He, Q.-S.; He, W.; Tao, S. Residual levels and health risk of polycyclic aromatic hydrocarbons in freshwater fishes from Lake Small Bai-Yang-Dian, Northern China. Ecol. Model. 2011, 222, 275–286. [Google Scholar] [CrossRef]
- He, Y.; Qin, N.; He, W.; Xu, F. The impacts of algae biological pump effect on the occurrence, source apportionment and toxicity of SPM-bound PAHs in lake environment. Sci. Total Environ. 2021, 753, 141980. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huo, S.; Yu, Z.; Xi, B.; Zeng, X.; Wu, F. Spatial distribution, potential risk assessment, and source apportionment of polycyclic aromatic hydrocarbons (PAHs) in sediments of Lake Chaohu, China. Environ. Sci. Pollut. Res. 2014, 21, 12028–12039. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Kong, X.-Z.; He, W.; He, Q.-S.; Liu, W.-X.; Xu, F.-L. Dustfall-bound polycyclic aromatic hydrocarbons (PAHs) over the fifth largest Chinese lake: Residual levels, source apportionment, and correlations with suspended particulate matter (SPM)-bound PAHs in water. Environ. Sci. Pollut. Res. 2021, 1–13. [Google Scholar] [CrossRef]
- Wang, R.; Huang, Q.; Cai, J.; Wang, J. Seasonal variations of atmospheric polycyclic aromatic hydrocarbons (PAHs) sur-rounding Chaohu Lake, China: Source, partitioning behavior, and lung cancer risk. Atmos. Pollut. Res. 2021, 12. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, Y.-S.; Wang, J.-Z.; Peng, S.-C.; Chen, T.-H.; Yin, D.-Q. Identification and determination of the contribution of iron–steel manufacturing industry to sediment-associated polycyclic aromatic hydrocarbons (PAHs) in a large shallow lake of eastern China. Environ. Sci. Pollut. Res. 2016, 23, 22037–22046. [Google Scholar] [CrossRef]
- Qin, N.; He, W.; Kong, X.-Z.; Liu, W.; He, Q.-S.; Yang, B.; Ouyang, H.-L.; Wang, Q.-M.; Xu, F.-L. Atmospheric partitioning and the air–water exchange of polycyclic aromatic hydrocarbons in a large shallow Chinese lake (Lake Chaohu). Chemosphere 2013, 93, 1685–1693. [Google Scholar] [CrossRef]
- Paatero, P. Least squares formulation of robust non-negative factor analysis. Chemom. Intell. Lab. Syst. 1997, 37, 23–35. [Google Scholar] [CrossRef]
- Liu, C.; Tian, F.; Chen, J.; Li, X.; Qiao, X. A comparative study on source apportionment of polycyclic aromatic hydrocarbons in sediments of the Daliao River, China: Positive matrix factorization and factor analysis with non-negative constraints. Chin. Sci. Bull. 2010, 55, 915–920. [Google Scholar] [CrossRef]
- Nisbet, I.C.T.; Lagoy, P.K. Toxic equaivalency factors (TEFs) for Polycyclic Aromatic-Hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Taghvaee, S.; Sowlat, M.H.; Hassanvand, M.S.; Yunesian, M.; Naddafi, K.; Sioutas, C. Source-specific lung cancer risk assessment of ambient PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) in central Tehran. Environ. Int. 2018, 120, 321–332. [Google Scholar] [CrossRef]
- Exposure Factors Handbook; EPA/600/P-95/002; US EPA: Washington, DC, USA, 1997.
- Li, J.; Zhang, G.; Li, X.; Qi, S.H.; Liu, G.Q.; Peng, X.Z. Source seasonality of polycyclic aromatic hydrocarbons (PAHs) in a subtropical city, Guangzhou, South China. Sci. Total Environ. 2006, 355, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tao, S.; Liu, W.; Dou, H.; Liu, Y.; Zhao, J.; Little, M.G.; Tian, Z.; Wang, J.; Wang, L.; et al. Seasonal and spatial occurrence and distribution of atmospheric polycyclic aromatic hydrocarbons (PAHs) in rural and urban areas of the North Chinese plain. Environ. Pollut. 2008, 156, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Yin, Y.; Chen, K.; Zhen, X.; Zhang, X.; Jiang, H.; Wang, H.; Kuang, X.; Cui, Y.; Dai, M.; et al. Concentration and atmospheric transport of PM2.5-bound polycyclic aromatic hydrocarbons at Mount Tai, China. Sci. Total Environ. 2021, 786, 147513. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.E.; Dickhut, R.M. Gaseous exchange of polycyclic aromatic hydrocarbons across the air−water interface of Southern Chesapeake Bay. Environ. Sci. Technol. 1997, 31, 1623–1629. [Google Scholar] [CrossRef]
- Mandalakis, M.; Tsapakis, M.; Tsoga, A.; Stephanou, E.G. Gas-particle concentrations and distribution of aliphatic hydro-carbons, PAHs, PCBs and PCDD/Fs in the atmosphere of Athens (Greece). Atmos. Environ. 2002, 36, 4023–4035. [Google Scholar] [CrossRef]
- Matos, J.; Silveira, C.; Cerqueira, M. Particle-bound polycyclic aromatic hydrocarbons in a rural background atmosphere of southwestern Europe. Sci. Total Environ. 2021, 87, 147666. [Google Scholar] [CrossRef]
- Xia, Z.; Duan, X.; Qiu, W.; Liu, D.; Wang, B.; Tao, S.; Jiang, Q.; Lu, B.; Song, Y.; Hu, X. Health risk assessment on dietary exposure to polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Sci. Total Environ. 2010, 408, 5331–5337. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Zhang, Q.; Zhao, H.; Yang, Y.; Zhang, Y.; Xie, Q.; Chen, J. Seasonal variation, air-water exchange, and multivariate source apportionment of polycyclic aromatic hydrocarbons in the coastal area of Dalian, China. Environ. Pollut. 2019, 244, 405–413. [Google Scholar] [CrossRef]
- Křůmal, K.; Mikuška, P.; Horák, J.; Hopan, F.; Kuboňová, L. Influence of boiler output and type on gaseous and particulate emissions from the combustion of coal for residential heating. Chemosphere 2021, 278, 130402. [Google Scholar] [CrossRef]
- Teixeira, E.C.; Agudelo-Castañeda, D.M.; Mattiuzi, C.D.P. Contribution of polycyclic aromatic hydrocarbon (PAH) sources to the urban environment: A comparison of receptor models. Sci. Total Environ. 2015, 538, 212–219. [Google Scholar] [CrossRef]
- Feng, J.; Song, N.; Yu, Y.; Li, Y. Differential analysis of FA-NNC, PCA-MLR, and PMF methods applied in source apportionment of PAHs in street dust. Environ. Monit. Assess. 2020, 192, 727. [Google Scholar] [CrossRef]
- Dickhut, R.M.; Canuel, E.A.; Gustafson, K.E.; Liu, K.; Arzayus, K.M.; Walker, S.E.; Edgecombe, G.; Gaylor, M.O.; Macdonald, E.H. Automotive sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay Region. Environ. Sci. Technol. 2000, 34, 4635–4640. [Google Scholar] [CrossRef]
- Davis, E.; Walker, T.R.; Adams, M.; Willis, R.; Norris, G.A.; Henry, R.C. Source apportionment of polycyclic aromatic hy-drocarbons (PAHs) in small craft harbor (SCH) surficial sediments in Nova Scotia, Canada. Sci. Total Environ. 2019, 691, 528–537. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser river basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Galarneau, E. Source specificity and atmospheric processing of airborne PAHs: Implications for source apportionment. Atmospheric Environ. 2008, 42, 8139–8149. [Google Scholar] [CrossRef]
- Katsoyiannis, A.; Sweetman, A.; Jones, K.C. PAH molecular diagnostic ratios applied to atmospheric sources: A critical evaluation using two decades of source inventory and air concentration data from the UK. Environ. Sci. Technol. 2011, 45, 8897–8906. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.M.; Smith, A.D.J.T.; Luhana, L. Source Apportionment of Atmospheric Polycyclic Aromatic Hydrocarbons Collected from an Urban Location in Birmingham, UK. Environ. Sci. Technol. 1996, 30, 825–832. [Google Scholar] [CrossRef]
- Chen, Y.; Sheng, G.; Bi, X.; Feng, Y.; Mai, B.; Fu, J. Emission Factors for Carbonaceous Particles and Polycyclic Aromatic Hydrocarbons from Residential Coal Combustion in China. Environ. Sci. Technol. 2005, 39, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-Q.; Liu, J.-H. Estimating coal production peak and trends of coal imports in China. Energy Policy 2010, 38, 512–519. [Google Scholar] [CrossRef]
- Xiuge, Z.; Xiao-li, D. Exposure Factors Handbook of Chinese Population; Ministry of Environmental Protection of the People′s Republic of China: Beijing, China, 2014.
- Chen, S.-C.; Liao, C.-M. Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pol-lution sources. Sci. Total Environ. 2006, 366, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-M.; Chio, C.-P.; Chen, W.-Y.; Ju, Y.-R.; Li, W.-H.; Cheng, Y.-H.; Liao, V.; Chen, S.-C.; Ling, M.-P. Lung cancer risk in relation to traffic-related nano/ultrafine particle-bound PAHs exposure: A preliminary probabilistic assessment. J. Hazard. Mater. 2011, 190, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; He, Y. Monitoring Report on Nutrition and Health Status of Chinese Residents (2010–2013) Volume I: Dietary and Nutrient Intake; People’s Medical Publishing House Co., LTD: Beijing, China, 2018. [Google Scholar]
- Falcó, G.; Domingo, J.L.; Llobet, J.M.; Teixidó, A.; Casas, C.; Müller, L. Polycyclic Aromatic Hydrocarbons in Foods: Human Exposure through the Diet in Catalonia, Spain. J. Food Prot. 2003, 66, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Martorell, I.; Nieto, A.; Nadal, M.; Perello, G.; Marce, R.M.; Domingo, J.L. Human exposure to polycyclic aromatic hydro-carbons (PAHs) using data from a duplicate diet study in Catalonia, Spain. Food Chem. Toxicol. 2012, 50, 4103–4108. [Google Scholar] [CrossRef] [PubMed]

| Abbreviation | PAHs | Gaseous (%) | Particulate (%) | Water (%) | Aquatic Organisms (%) | TEF |
|---|---|---|---|---|---|---|
| Nap | Naphthalene | 46 | 47 | 81 | 115 | 0.001 |
| Acy | Acenaphthene; | 51 | 48 | 87 | 101 | 0.001 |
| Ace | Acenaphthylene | 67 | 50 | 81 | 117 | 0.001 |
| Flo | Fluorene | 75 | 57 | 103 | 105 | 0.001 |
| Phe | Phenanthrene | 83 | 69 | 108 | 107 | 0.001 |
| Ant | Anthracene | 77 | 71 | 93 | 101 | 0.01 |
| Fla | Fluoranthene | 98 | 87 | 89 | 113 | 0.001 |
| Pyr | Pyrene | 124 | 88 | 89 | 122 | 0.001 |
| BaA | Benzo[a]anthracene | 99 | 97 | 63 | 102 | 0.1 |
| Chr | Chrysene | 92 | 102 | 62 | 119 | 0.01 |
| BbF | Benzo[b]fluoranthene | 121 | 103 | 43 | 105 | 0.1 |
| BkF | Benzo[k]fluorant hene | 90 | 111 | 44 | 102 | 0.1 |
| BaP | Benzo[a]pyrene; | 108 | 103 | 60 | 87 | 1 |
| IcdP | Dibenz[a,h]anthracene | 102 | 119 | 31 | 89 | 0.1 |
| DahA | Indeno [1,2,3-cd]pyrene | 127 | 118 | 24 | 93 | 1 |
| BghiP | Benzo[ghi]perylene | 65 | 115 | 24 | 110 | 0.01 |
| Categories | Media | Unit | Urban | Rural | Kruskal-Wallis Test | ||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | GM | Min | Max | GM ± SD | ||||
| PAH16 | Gas | ng·m−3 | 22.1 | 186 | 49.5 ± 46.0 | 10.9 | 183 | 72.3 ± 54.3 | p = 0.157 |
| Particle | ng·m−3 | 3.41 | 82.5 | 13.8 ± 25.6 | 2.74 | 69.3 | 14.6 ± 22.4 | p = 0.773 | |
| Water | ng·L−1 | 57 | 409 | 171 ± 119 | 59.6 | 779 | 169 ± 188 | p = 0.544 | |
| Fish | ng·g−1 | 18.5 | 1029 | 114 ± 315 | 18.5 | 1029 | 114 ± 315 | ||
| BaPeq | Gas | ng·m−3 | 0.04 | 0.38 | 0.14 ± 0.10 | 0.06 | 1.05 | 0.31 ± 0.26 | p = 0.010 |
| Particle | ng·m−3 | 0.38 | 10.1 | 1.57 ± 2.99 | 0.31 | 9.00 | 1.71 ± 2.74 | p = 0.840 | |
| Water | ng·L−1 | 0.17 | 1.57 | 0.46 ± 0.42 | 0.26 | 1.36 | 0.54 ± 0.36 | p = 0.862 | |
| Fish | ng·g−1 | 0.29 | 20.2 | 1.75 ± 6.24 | 0.29 | 20.2 | 1.75 ± 6.24 | ||
| Media | Unit | Distribution Mode | Urban | Rural | References |
|---|---|---|---|---|---|
| Water | ng·L | Log-Normal | LN(−0.77, 0.68) | LN(−0.62, 0.56) | Measured |
| Gas | ng·m−3 | Log-Normal | LN(−1.96, 0.67) | LN(−1.18, 0.71) | Measured |
| Particle | ng·m−3 | Log-Normal | LN(0.45, 1.10) | LN(0.54, 1.11) | Measured |
| Fish | ng·g−3 | Log-Normal | LN(0.56, 1.48) | LN(0.56, 1.48) | Measured |
| BW | Kg | Normal | N(63.90, 13.86) | LN(60.86, 13.64) | [53] |
| IR(water) | mL·d−3 | Log-Normal | LN(7.81, 0.91) | LN(7.80, 0.70) | [53] |
| IR(inh) | m3·d−1 | Log-Normal | LN(2.78,0.19) | LN(2.74, 0.21) | [53] |
| IR(fish) | g·d−3 | Constant | 19.0 | 11.1 | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, N.; He, W.; He, Q.; Kong, X.; Liu, W.; Wang, Q.; Xu, F. Multi-Media Exposure to Polycyclic Aromatic Hydrocarbons at Lake Chaohu, the Fifth Largest Fresh Water Lake in China: Residual Levels, Sources and Carcinogenic Risk. Atmosphere 2021, 12, 1241. https://doi.org/10.3390/atmos12101241
Qin N, He W, He Q, Kong X, Liu W, Wang Q, Xu F. Multi-Media Exposure to Polycyclic Aromatic Hydrocarbons at Lake Chaohu, the Fifth Largest Fresh Water Lake in China: Residual Levels, Sources and Carcinogenic Risk. Atmosphere. 2021; 12(10):1241. https://doi.org/10.3390/atmos12101241
Chicago/Turabian StyleQin, Ning, Wei He, Qishuang He, Xiangzhen Kong, Wenxiu Liu, Qin Wang, and Fuliu Xu. 2021. "Multi-Media Exposure to Polycyclic Aromatic Hydrocarbons at Lake Chaohu, the Fifth Largest Fresh Water Lake in China: Residual Levels, Sources and Carcinogenic Risk" Atmosphere 12, no. 10: 1241. https://doi.org/10.3390/atmos12101241
APA StyleQin, N., He, W., He, Q., Kong, X., Liu, W., Wang, Q., & Xu, F. (2021). Multi-Media Exposure to Polycyclic Aromatic Hydrocarbons at Lake Chaohu, the Fifth Largest Fresh Water Lake in China: Residual Levels, Sources and Carcinogenic Risk. Atmosphere, 12(10), 1241. https://doi.org/10.3390/atmos12101241






