Influence of Ammonium Sulfate Seed Particle on Optics and Compositions of Toluene Derived Organic Aerosol in Photochemistry
Abstract
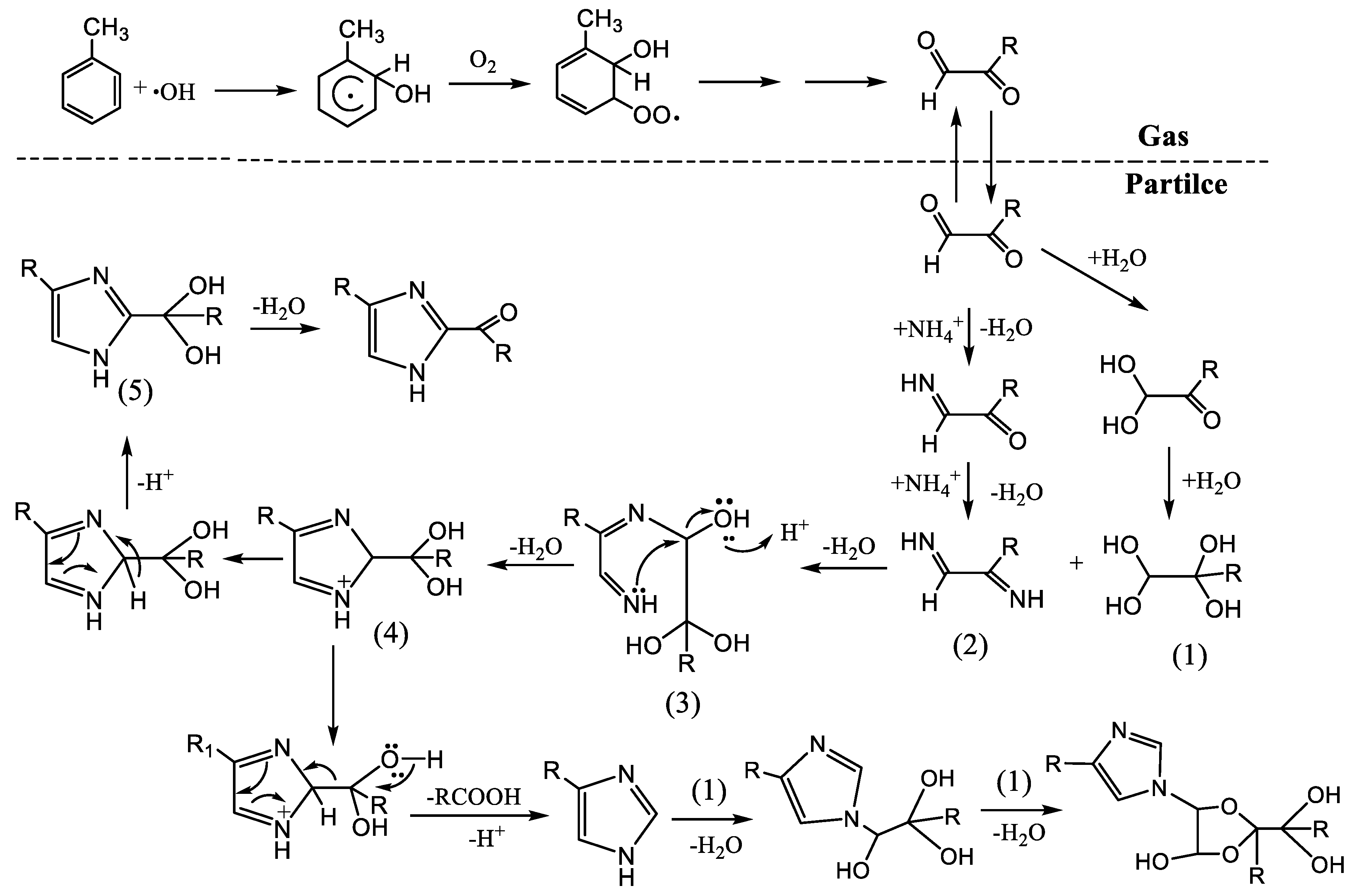
1. Introduction
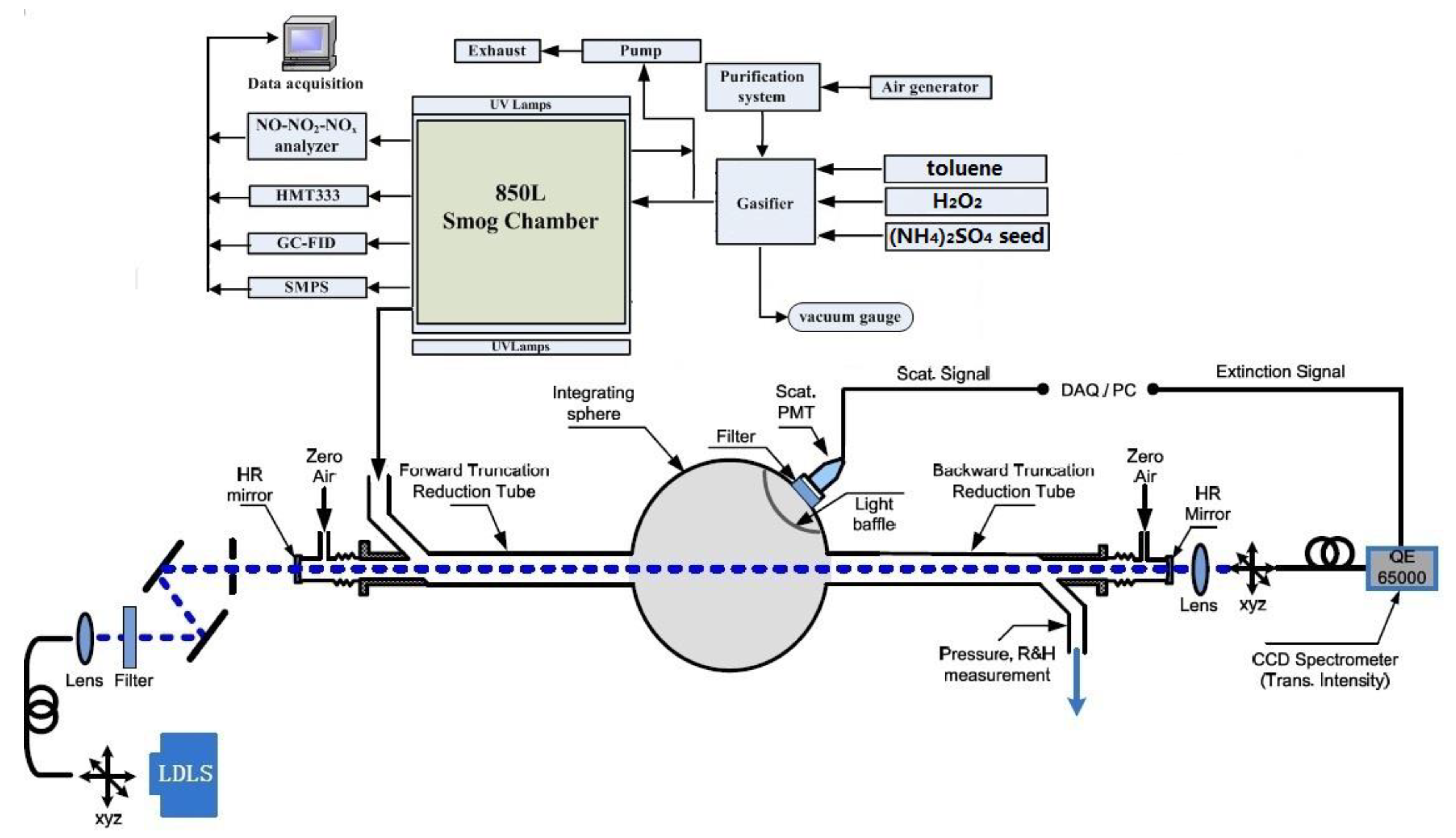
2. Experiments
2.1. Material
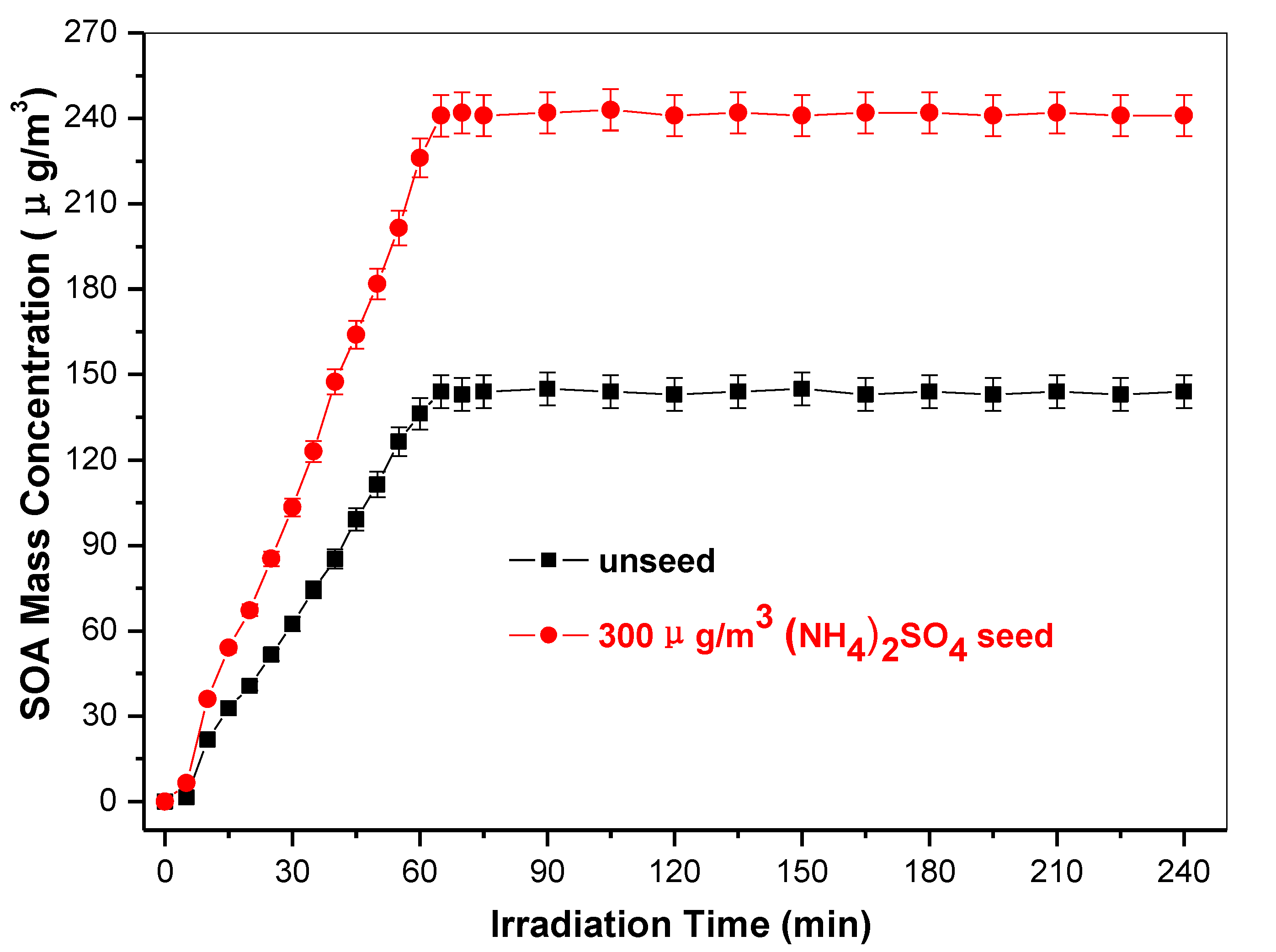
2.2. Toluene SOA Particles Formation without and in Presence of (NH4)2SO4 Seed
2.3. Aerosol Albedometer
2.4. Characterization Compositions of Toluene SOA
3. Results
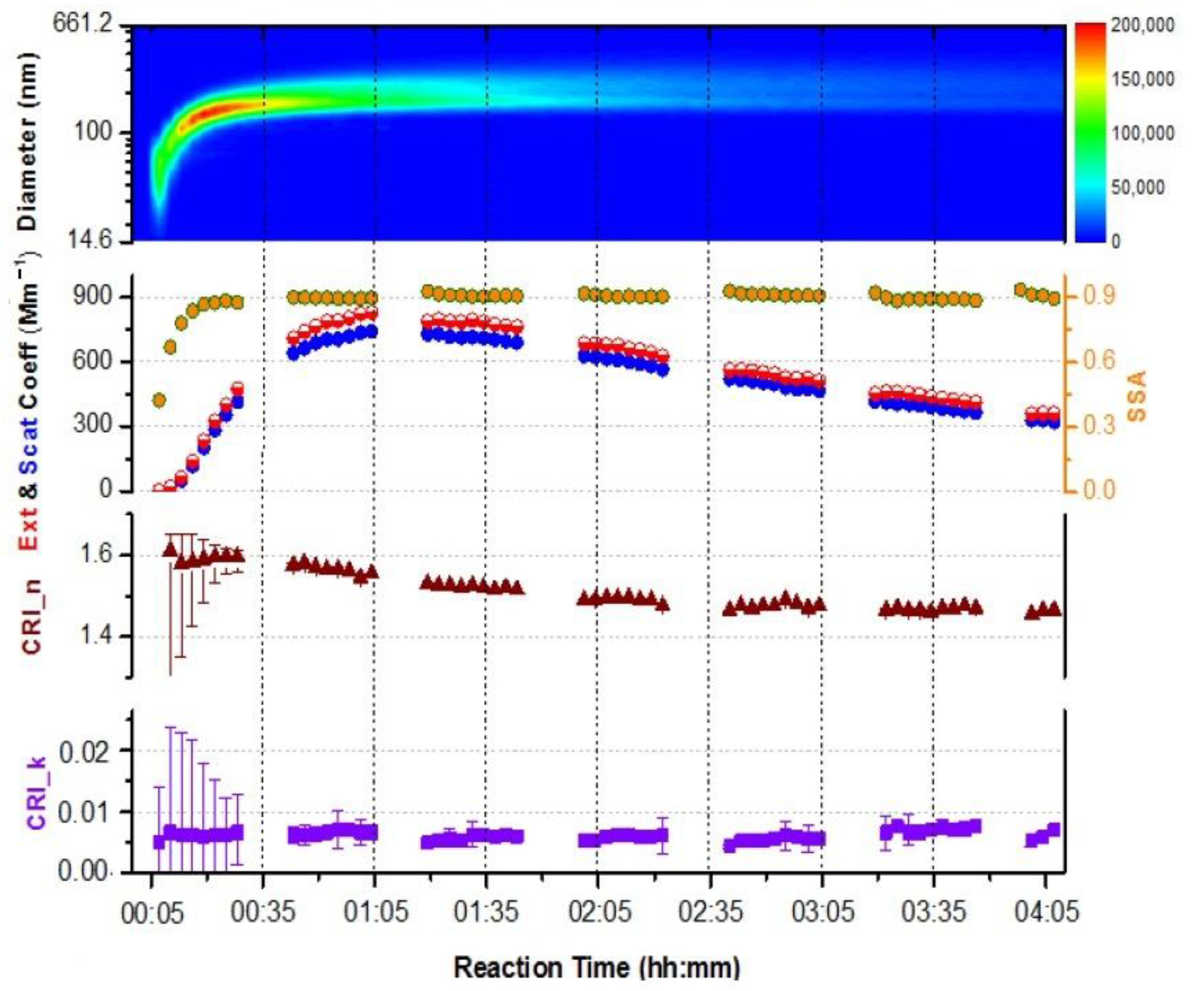
3.1. Validation of the Retrieved CRI from the Albedometer
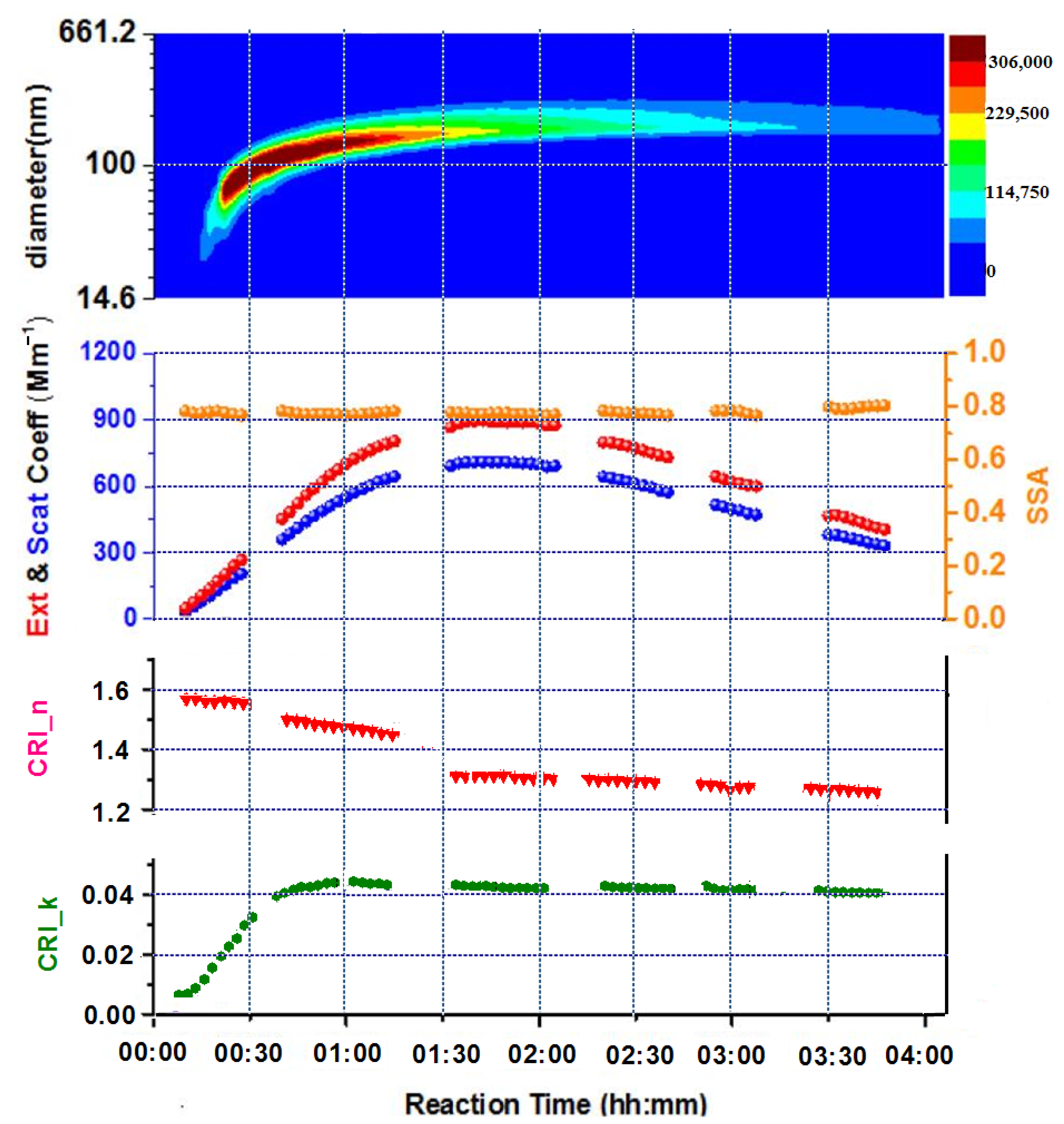
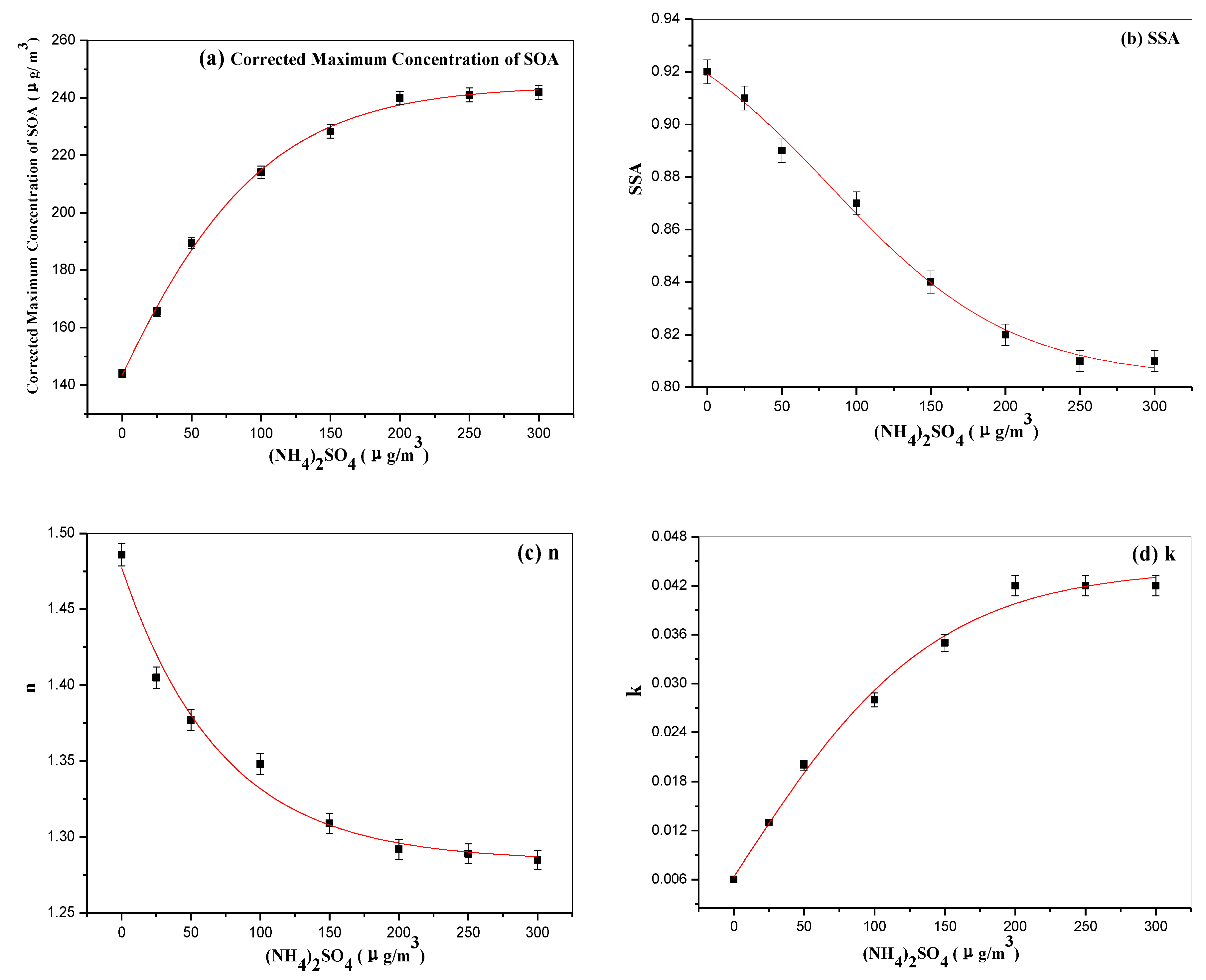
3.2. Optics of SOA without and in Presence of (NH4)2SO4 Seed
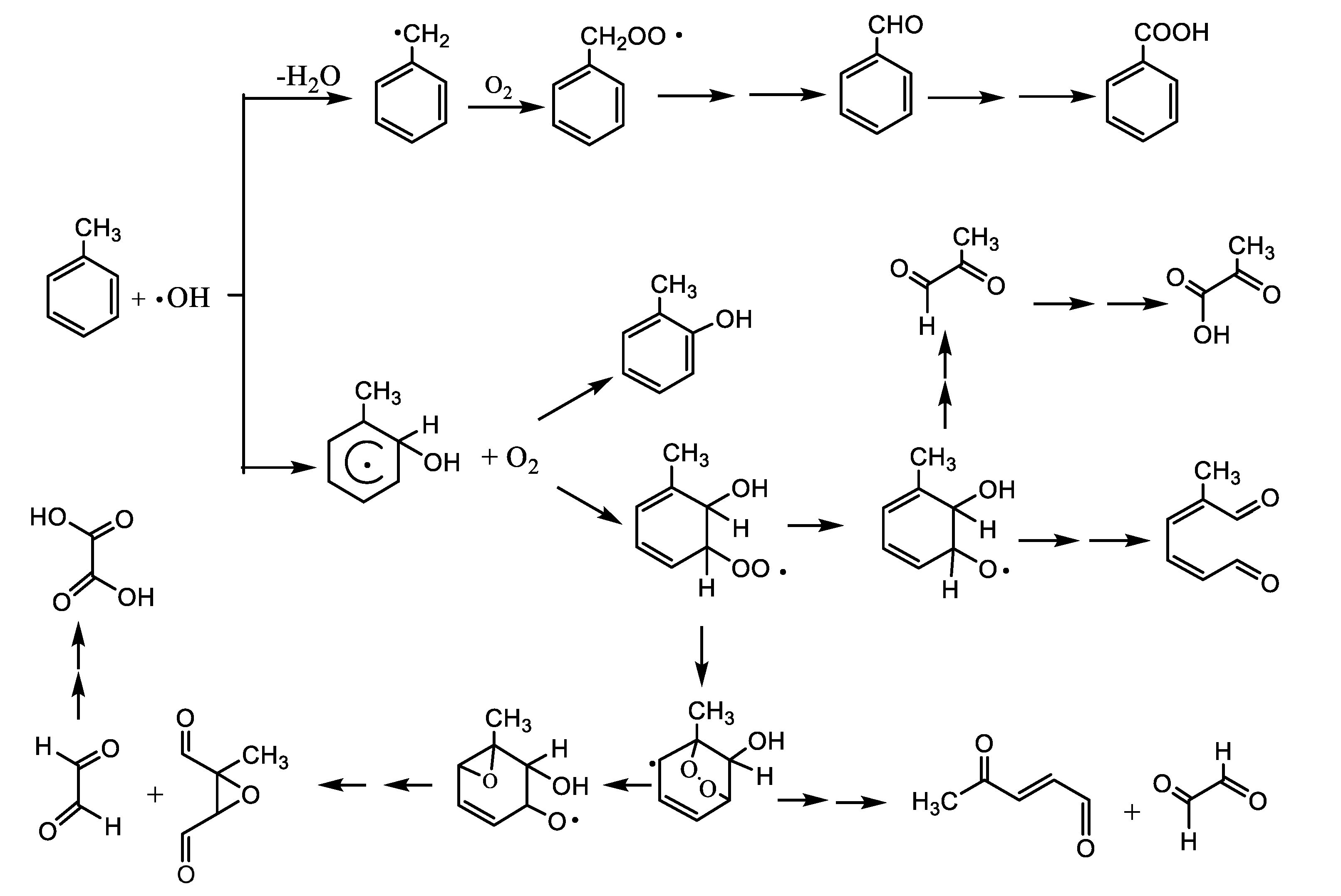
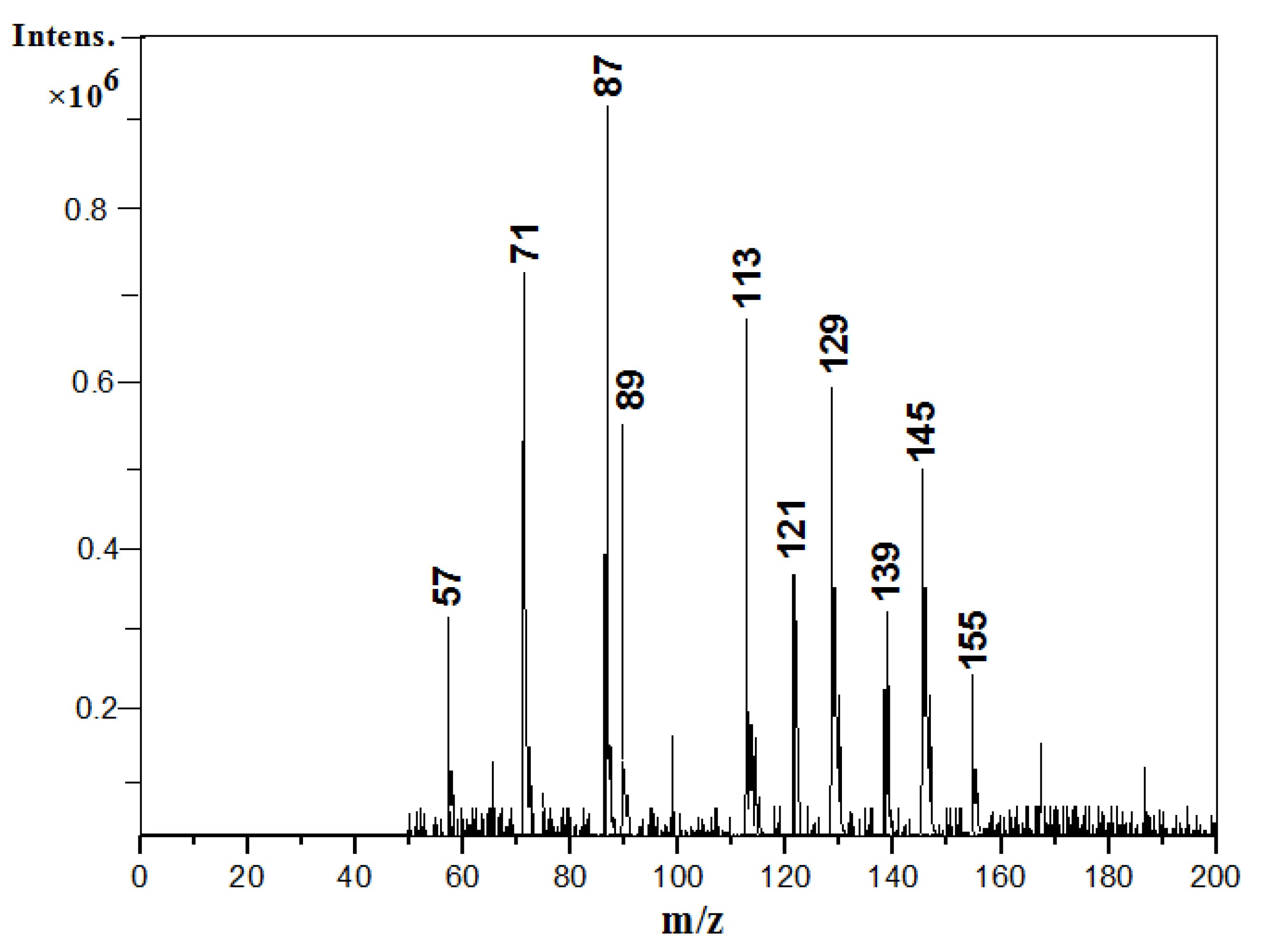
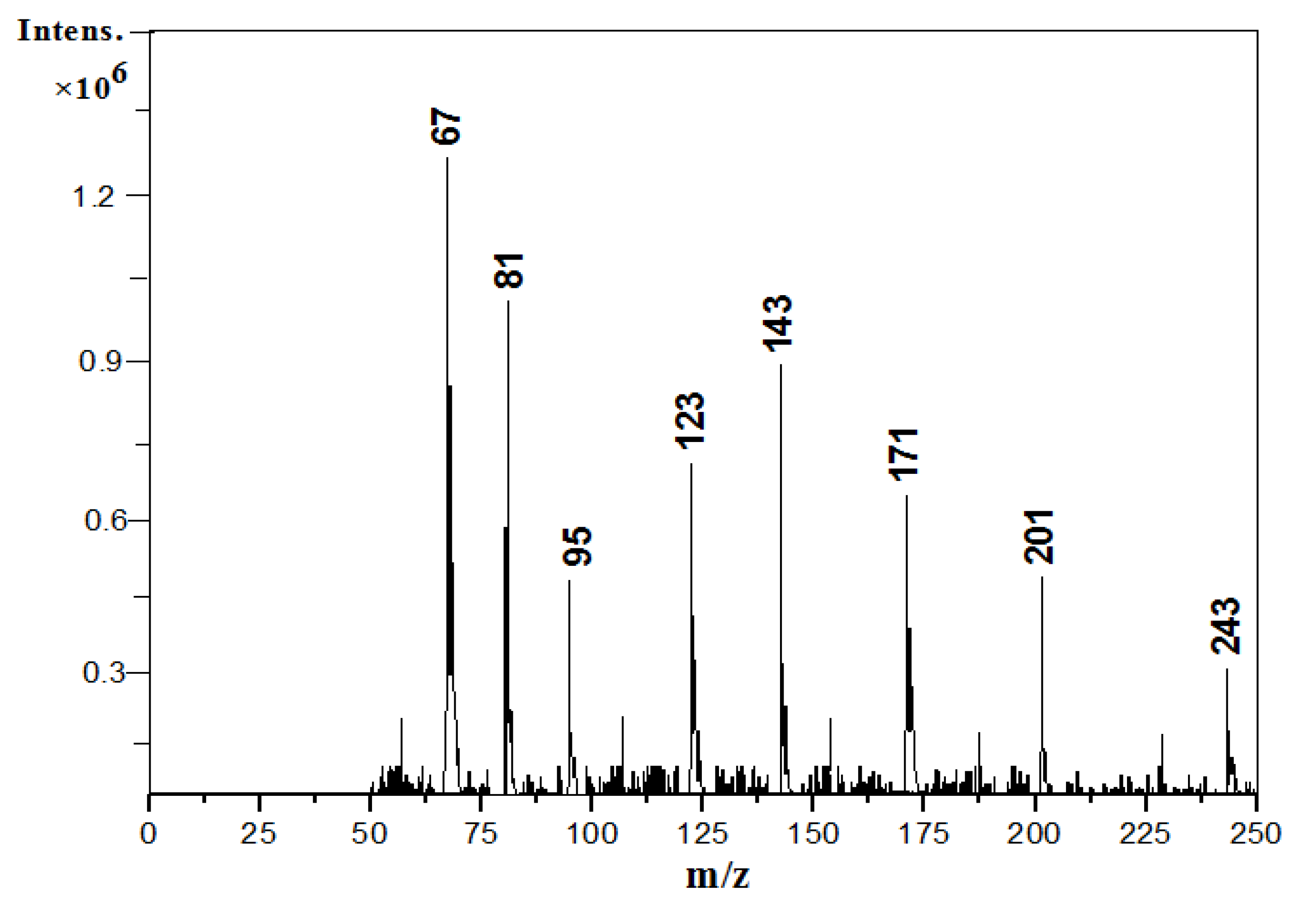
3.3. Components of Toluene SOA without and in Presence of (NH4)2SO4 Seed
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Drozd, G.T.; Zhao, Y.; Saliba, G.; Frodin, B.; Maddox, C.; Chang, M.-C.O.; Maldonado, H.; Sarder, S.; Weber, R.J.; Robinson, A.L.; et al. Detailed Speciation of Intermediate Volatility and Semivolatile Organic Compound Emissions from Gasoline Vehicles: Effects of Cold-Starts and Implications for Secondary Organic Aerosol Formation. Environ. Sci. Technol. 2018, 53, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Derwent, R.G. Representing Organic Compound Oxidation in Chemical Mechanisms for Policy-Relevant Air Quality Models under Background Troposphere Conditions. Atmosphere 2020, 11, 171. [Google Scholar] [CrossRef]
- Baltensperger, U. Aerosols in Clearer Focus. Science 2010, 329, 1474–1475. [Google Scholar] [CrossRef] [PubMed]
- Donateo, A.; Feudo, T.L.; Marinoni, A.; Dinoi, A.; Avolio, E.; Merico, E.; Calidonna, C.R.; Contini, D.; Bonasoni, P. Characterization of In Situ Aerosol Optical Properties at Three Observatories in the Central Mediterranean. Atmosphere 2018, 9, 369. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, Y.; Lyu, R.; Wu, R.; Dai, L.; Zhao, Y.; Chen, F.; Zhang, J.; Yu, H.; Guan, M. Seasonal and spatial variations of optical properties of light absorbing carbon and its influencing factors in a typical polluted city in Yangtze River Delta, China. Atmos. Environ. 2019, 199, 45–54. [Google Scholar] [CrossRef]
- Moise, T.; Flores, J.M.; Rudich, Y. Optical Properties of Secondary Organic Aerosols and Their Changes by Chemical Processes. Chem. Rev. 2015, 115, 4400–4439. [Google Scholar] [CrossRef]
- Nakayama, T.; Matsumi, Y.; Sato, K.; Imamura, T.; Yamazaki, A.; Uchiyama, A. Laboratory studies on optical properties of secondary organic aerosols generated during the photooxidation of toluene and the ozonolysis of α-pinene. J. Geophys. Res. Space Phys. 2010, 115. [Google Scholar] [CrossRef]
- Nakayama, T.; Sato, K.; Matsumi, Y.; Imamura, T.; Yamazaki, A.; Uchiyama, A. Wavelength and NOx dependent complex refractive index of SOAs generated from the photooxidation of toluene. Atmos. Chem. Phys. 2013, 13, 531–545. [Google Scholar] [CrossRef]
- Li, K.; Wang, W.; Ge, M.F.; Li, J.; Wang, D. Optical properties of secondary organic aerosols generated by photooxidation of aromatic hydrocarbons. Sci. Rep. 2014, 4, 4922. [Google Scholar] [CrossRef]
- Li, K.; Li, J.; Wang, W.; Li, J.; Peng, C.; Wang, D.; Ge, M. Effects of Gas-Particle Partitioning on Refractive Index and Chemical Composition of m-Xylene Secondary Organic Aerosol. J. Phys. Chem. A 2018, 122, 3250–3260. [Google Scholar] [CrossRef]
- Langridge, J.M.; Richardson, M.S.; Lack, D.A.; Brock, C.A.; Murphy, D.M. Limitations of the Photoacoustic Technique for Aerosol Absorption Measurement at High Relative Humidity. Aerosol Sci. Technol. 2013, 47, 1163–1173. [Google Scholar] [CrossRef]
- Varma, R.M.; Ball, S.M.; Brauers, T.; Dorn, H.-P.; Heitmann, U.; Jones, R.L.; Platt, U.; Pöhler, D.; Ruth, A.A.; Shillings, A.J.L.; et al. Light extinction by secondary organic aerosol: An intercomparison of three broadband cavity spectrometers. Atmos. Meas. Tech. 2013, 6, 6685–6727. [Google Scholar] [CrossRef]
- Zhao, W.X.; Dong, M.L.; Chen, W.D.; Gu, X.J.; Hu, C.J.; Gao, X.M.; Huang, W.; Zhang, W.J. Wavelength resolved optical extinction measurements of aerosols using broad-band cavity-enhanced absorption spectroscopy over the spectral range of 445–480 nm. Anal. Chem. 2013, 85, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xu, X.; Dong, M.; Chen, W.; Gu, X.; Hu, C.; Huang, Y.; Gao, X.; Huang, W.; Zhang, W. Development of a cavity-enhanced aerosol albedometer. Atmos. Meas. Tech. 2014, 7, 2551–2566. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, W.; Zhang, Q.; Wang, S.; Fang, B.; Chen, W.; Venables, D.S.; Wang, X.; Pu, W.; Wang, X.; et al. Optical properties of atmospheric fine particles near Beijing during the HOPE-J3A campaign. Atmos. Chem. Phys. 2016, 16, 6421–6439. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, W.; Xu, X.; Fang, B.; Zhang, Q.; Qian, X.; Zhang, W.; Chen, W.; Pu, W.; Wang, X. Dependence of columnar aerosol size distribution, optical properties, and chemical components on regional transport in Beijing. Atmos. Environ. 2017, 169, 128–139. [Google Scholar] [CrossRef]
- Ge, S.; Xu, Y.; Jia, L. Effects of inorganic seeds on secondary organic aerosol formation from photochemical oxidation of acetone in a chamber. Atmos. Environ. 2017, 170, 205–215. [Google Scholar] [CrossRef]
- Tajuelo, M.; Rodríguez, A.M.; Baeza-Romero, M.T.T.; Aranda, A.; Díaz-De-Mera, Y.; Rodríguez, D. Secondary organic aerosol formation from α-methylstyrene atmospheric degradation: Role of NO level, relative humidity and inorganic seed aerosol. Atmos. Res. 2019, 230, 104631. [Google Scholar] [CrossRef]
- Shao, P.; Tian, H.; Sun, Y.; Liu, H.; Wu, B.; Liu, S.; Liu, X.; Wu, Y.; Liang, W.; Wang, Y.; et al. Characterizing remarkable changes of severe haze events and chemical compositions in multi-size airborne particles (PM1, PM2.5 and PM10) from January 2013 to 2016–2017 winter in Beijing, China. Atmos. Environ. 2018, 189, 133–144. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Liu, Z.-J.; Wu, J.; Zhang, Y.-F.; Han, S.-Q.; Zheng, X.-J.; Zhou, L.-D.; Feng, Y.-C.; Zhu, T. Characterization of chemical compositions in size-segregated atmospheric particles during severe haze episodes in three mega-cities of China. Atmos. Res. 2017, 187, 138–146. [Google Scholar] [CrossRef]
- Sun, Z.; Duan, F.; He, K.; Du, J.; Zhu, L. Sulfate–nitrate–ammonium as double salts in PM2.5: Direct observations and implications for haze events. Sci. Total. Environ. 2019, 647, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Hao, L.; Cai, S.; Gu, X.; Zhang, W.; Hu, C.; Wang, Z.; Fang, L.; Zhang, W. Effects of inorganic seed aerosols on the particulate products of aged 1,3,5-trimethylbenzene secondary organic aerosol. Atmos. Environ. 2017, 152, 490–502. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, W.; Cai, S.; Liao, Y.; Zhao, W.; Hu, C.; Gu, X.; Fang, L.; Zhang, W. Mass spectrometric study of aged benzene secondary organic aerosol in the presence of dry ammonium sulfate. J. Atmos. Chem. 2016, 73, 329–344. [Google Scholar] [CrossRef]
- Assaf, E.; Fittschen, C. Cross section of OH radical overtone transition near 7028 cm–1 and measurement of the rate constant of the reaction of OH with HO2 radicals. J. Phys. Chem. A 2016, 120, 7051–7059. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Huang, M.; Cai, S.; Xu, X.; Yang, Z.; Zhao, W.; Hu, C.; Gu, X.; Zhang, W. Characterization of single scattering albedo and chemical components of aged toluene secondary organic aerosol. Atmos. Pollut. Res. 2019, 10, 1736–1744. [Google Scholar] [CrossRef]
- Bones, D.L.; Henricksen, D.K.; Mang, S.A.; Gonsior, M.; Bateman, A.P.; Nguyen, T.B.; Cooper, W.J.; Nizkorodov, S.A. Appearance of strong absorbers and fluorophores in limonene-O3 secondary organic aerosol due to NH4+-mediated chemical aging over long time scales. J. Geophys. Res. Space Phys. 2010, 115. [Google Scholar] [CrossRef]
- Bueno, P.A.; Havey, D.K.; Mulholland, G.W.; Hodges, J.T.; Gillis, K.A.; Dickerson, R.R.; Zachariah, M.R. Photoacoustic Measurements of Amplification of the Absorption Cross Section for Coated Soot Aerosols. Aerosol Sci. Technol. 2011, 45, 1217–1230. [Google Scholar] [CrossRef]
- Washenfelder, R.A.; Flores, J.M.; Brock, C.A.; Brown, S.S.; Rudich, Y. Broadband measurements of aerosol extinction in the ultraviolet spectral region. Atmos. Meas. Tech. 2013, 6, 861–877. [Google Scholar] [CrossRef]
- Thompson, J.E.; Barta, N.; Policarpio, D.; Duvall, R. A fixed frequency aerosol albedometer. Opt. Express 2008, 16, 2191–2205. [Google Scholar] [CrossRef]
- Miles, R.E.H.; Rudić, S.; Orr-Ewing, A.J.; Reid, J.P. Influence of Uncertainties in the Diameter and Refractive Index of Calibration Polystyrene Beads on the Retrieval of Aerosol Optical Properties Using Cavity Ring Down Spectroscopy. J. Phys. Chem. A 2010, 114, 7077–7084. [Google Scholar] [CrossRef]
- Huang, M.; Hao, L.; Gu, X.; Hu, C.; Zhao, W.; Wang, Z.; Fang, L.; Zhang, W. Effects of inorganic seed aerosols on the growth and chemical composition of secondary organic aerosol formed from OH-initiated oxidation of toluene. J. Atmos. Chem. 2013, 70, 151–164. [Google Scholar] [CrossRef]
- Jathar, S.H.; Mahmud, A.; Barsanti, K.C.; Asher, W.E.; Pankow, J.F.; Kleeman, M.J. Water uptake by organic aerosol and its influence on gas/particle partitioning of secondary organic aerosol in the United States. Atmos. Environ. 2016, 129, 142–154. [Google Scholar] [CrossRef]
- Reid, J.S.; Eck, T.F.; Christopher, S.A.; Koppmann, R.; Dubovik, O.; Eleuterio, D.P.; Holben, B.N.; Reid, E.A.; Zhang, J. A review of biomass burning emissions part III: Intensive optical properties of biomass burning particles. Atmos. Chem. Phys. 2005, 5, 827–849. [Google Scholar] [CrossRef]
- Lambe, A.T.; Chhabra, P.S.; Onasch, T.B.; Brune, W.H.; Hunter, J.F.; Kroll, J.H.; Cummings, M.J.; Brogan, J.F.; Parmar, Y.; Worsnop, D.R.; et al. Effect of oxidant concentration, exposure time, and seed particles on secondary organic aerosol chemical composition and yield. Atmos. Chem. Phys. 2015, 15, 3063–3075. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric Degradation of Volatile Organic Compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef]
- Hinks, M.L.; Montoya-Aguilera, J.; Ellison, L.; Lin, P.; Laskin, A.; Laskin, J.; Shiraiwa, M.; Dabdub, D.; Nizkorodov, S.A. Effect of relative humidity on the composition of secondary organic aerosol from the oxidation of toluene. Atmos. Chem. Phys. Online 2018, 18, 1643–1652. [Google Scholar] [CrossRef]
- Sato, K.; Takami, A.; Kato, Y.; Seta, T.; Fujitani, Y.; Hikida, T.; Shimono, A.; Imamura, T. AMS and LC/MS analyses of SOA from the photooxidation of benzene and 1,3,5-trimethyl benzene in the presence of NOx: Effects of chemical structure on SOA aging. Atmos. Chem. Phys. 2012, 12, 4667–4682. [Google Scholar] [CrossRef]
- Suh, I.; Zhang, R.; Molina, L.T.; Molina, M.J. Oxidation Mechanism of Aromatic Peroxy and Bicyclic Radicals from OH-Toluene Reactions. J. Am. Chem. Soc. 2003, 125, 12655–12665. [Google Scholar] [CrossRef]
- Carlton, A.G.; Turpin, B.; Altieri, K.E.; Seitzinger, S.; Reff, A.; Lim, H.-J.; Ervens, B. Atmospheric oxalic acid and SOA production from glyoxal: Results of aqueous photooxidation experiments. Atmos. Environ. 2007, 41, 7588–7602. [Google Scholar] [CrossRef]
- Laskin, J.; Laskin, J.; Nizkorodov, S.A. Chemistry of Atmospheric Brown Carbon. Chem. Rev. 2015, 115, 4335–4382. [Google Scholar] [CrossRef]
- Updyke, K.M.; Nguyen, T.B.; Nizkorodov, S.A. Formation of brown carbon via reactions of ammonia with secondary organic aerosols from biogenic and anthropogenic precursors. Atmos. Environ. 2012, 63, 22–31. [Google Scholar] [CrossRef]
- Kampf, C.J.; Jakob, R.; Hoffmann, T. Identification and characterization of aging products in the glyoxal/ammonium sulfate system-implications for light-absorbing material in atmospheric aerosols. Atmos. Chem. Phys. 2012, 12, 6323–6333. [Google Scholar] [CrossRef]
- Lee, A.K.Y.; Zhao, R.; Li, R.; Liggio, J.; Li, S.-M.; Abbatt, J.P.D. Formation of Light Absorbing Organo-Nitrogen Species from Evaporation of Droplets Containing Glyoxal and Ammonium Sulfate. Environ. Sci. Technol. 2013, 47, 12819–12826. [Google Scholar] [CrossRef] [PubMed]
- Maxut, A.; Mechakra, H.; Nozière, B.; Fenet, B. Formation mechanisms and yields of small imidazoles from reactions of glyoxal with NH4+in water at neutral pH. Phys. Chem. Chem. Phys. 2015, 17, 20416–20424. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Zhang, X.; Jing, S.; Peng, Y.; Qiao, L.; Zhou, M.; Huang, D.D.; Wang, Q.; Li, X.; et al. Estimating Secondary Organic Aerosol Production from Toluene Photochemistry in a Megacity of China. Environ. Sci. Technol. 2019, 53, 8664–8671. [Google Scholar] [CrossRef]
- Liu, Y.; Liggio, J.; Staebler, R.; Li, S.-M. Reactive uptake of ammonia to secondary organic aerosols: Kinetics of organonitrogen formation. Atmos. Chem. Phys. 2015, 15, 13569–13584. [Google Scholar] [CrossRef]
- Trainic, M.; Riziq, A.A.; Lavi, A.; Flores, J.M.; Rudich, Y. The optical, physical and chemical properties of the products of glyoxal uptake on ammonium sulfate seed aerosols. Atmos. Chem. Phys. 2011, 11, 9697–9707. [Google Scholar] [CrossRef]
- Pósfai, M.; Xu, H.; Anderson, J.R.; Buseck, P.R. Wet and dry sizes of atmospheric aerosol particles: An AFM-TEM Study. Geophys. Res. Lett. 1998, 25, 1907–1910. [Google Scholar] [CrossRef]
- Cruz, C.N.; Pandis, S.N. Deliquescence and Hygroscopic Growth of Mixed Inorganic-Organic Atmospheric Aerosol. Environ. Sci. Technol. 2000, 34, 4313–4319. [Google Scholar] [CrossRef]
- Meyer, N.K.; Duplissy, J.; Gysel, M.; Metzger, A.; Dommen, J.; Weingartner, E.; Alfarra, M.R.; Fletcher, C.; Good, N.; McFiggans, G.; et al. Analysis of the hygroscopic and volatile properties of ammonium sulphate seeded and un-seeded SOA particles. Atmos. Chem. Phys. 2008, 8, 8629–8659. [Google Scholar] [CrossRef]
- Hallquist, M.; Wenger, J.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef]
- Lu, Z.; Hao, J.; Takekawa, H.; Hu, L.; Li, J. Effect of high concentrations of inorganic seed aerosols on secondary organic aerosol formation in the m-xylene/NOx photooxidation system. Atmos. Environ. 2009, 43, 897–904. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, T.; Huang, M.; Zhao, W.; Hu, C.; Gu, X.; Zhang, W. Influence of Ammonium Sulfate Seed Particle on Optics and Compositions of Toluene Derived Organic Aerosol in Photochemistry. Atmosphere 2020, 11, 961. https://doi.org/10.3390/atmos11090961
Lu T, Huang M, Zhao W, Hu C, Gu X, Zhang W. Influence of Ammonium Sulfate Seed Particle on Optics and Compositions of Toluene Derived Organic Aerosol in Photochemistry. Atmosphere. 2020; 11(9):961. https://doi.org/10.3390/atmos11090961
Chicago/Turabian StyleLu, Tingting, Mingqiang Huang, Weixiong Zhao, Changjin Hu, Xuejun Gu, and Weijun Zhang. 2020. "Influence of Ammonium Sulfate Seed Particle on Optics and Compositions of Toluene Derived Organic Aerosol in Photochemistry" Atmosphere 11, no. 9: 961. https://doi.org/10.3390/atmos11090961
APA StyleLu, T., Huang, M., Zhao, W., Hu, C., Gu, X., & Zhang, W. (2020). Influence of Ammonium Sulfate Seed Particle on Optics and Compositions of Toluene Derived Organic Aerosol in Photochemistry. Atmosphere, 11(9), 961. https://doi.org/10.3390/atmos11090961




