Relative Risk Functions for Estimating Excess Mortality Attributable to Outdoor PM2.5 Air Pollution: Evolution and State-of-the-Art
Abstract
1. Introduction
2. Estimation of Attributable Deaths Due to PM2.5 Exposure
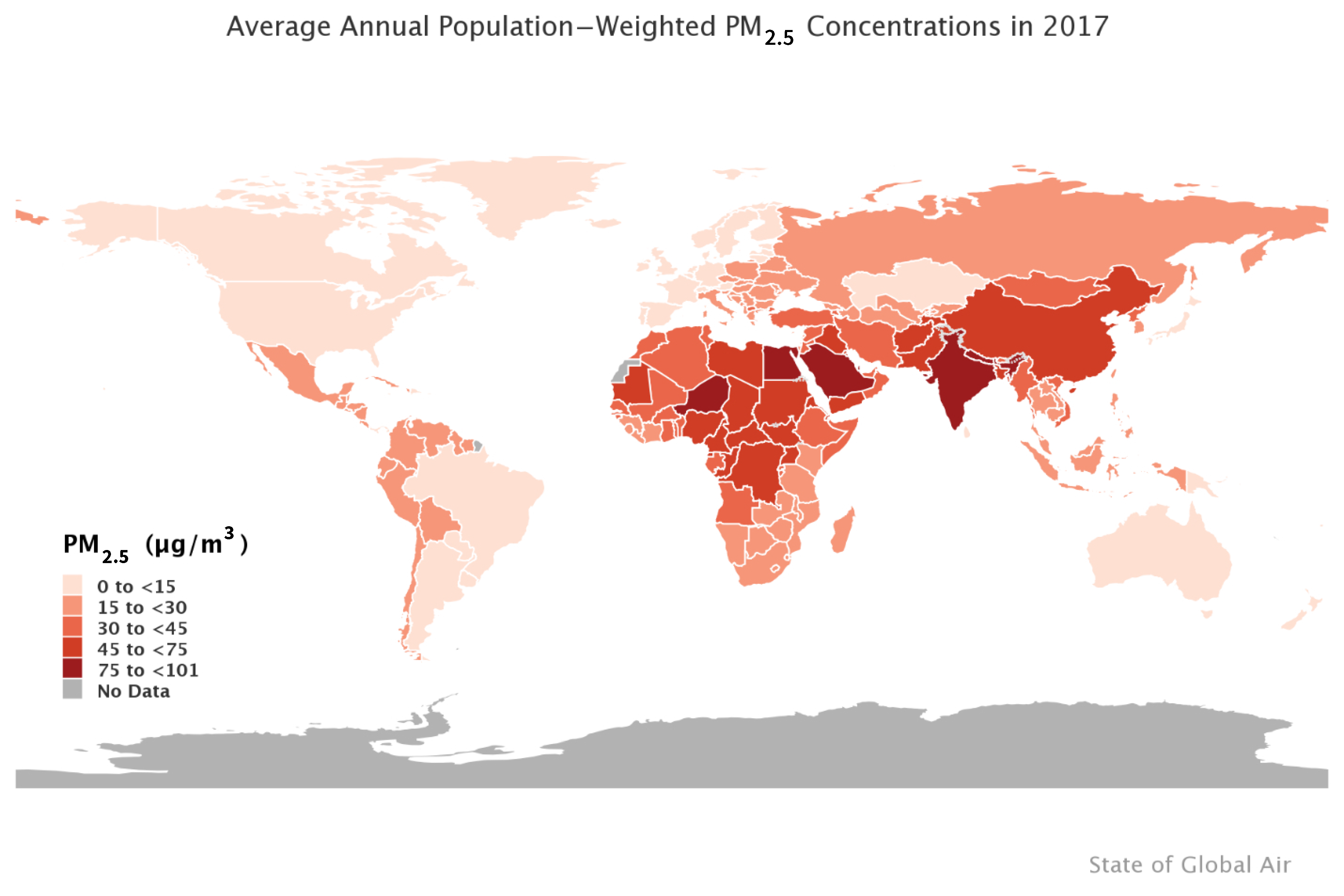
3. Estimators of the PM2.5 Mortality Relative Risk over the Global Concentration Range
3.1. The Integrated Exposure-Response (IER) Relative Risk Model
3.2. Relative Risk Models Using Only Ambient Air Pollution Cohort Studies
3.2.1. Shape Constrained Health Impact Function (SCHIF)
3.2.2. Global Exposure Mortality Model (GEMM)
4. Recommendations for Conducting Burden and Benefits Assessments
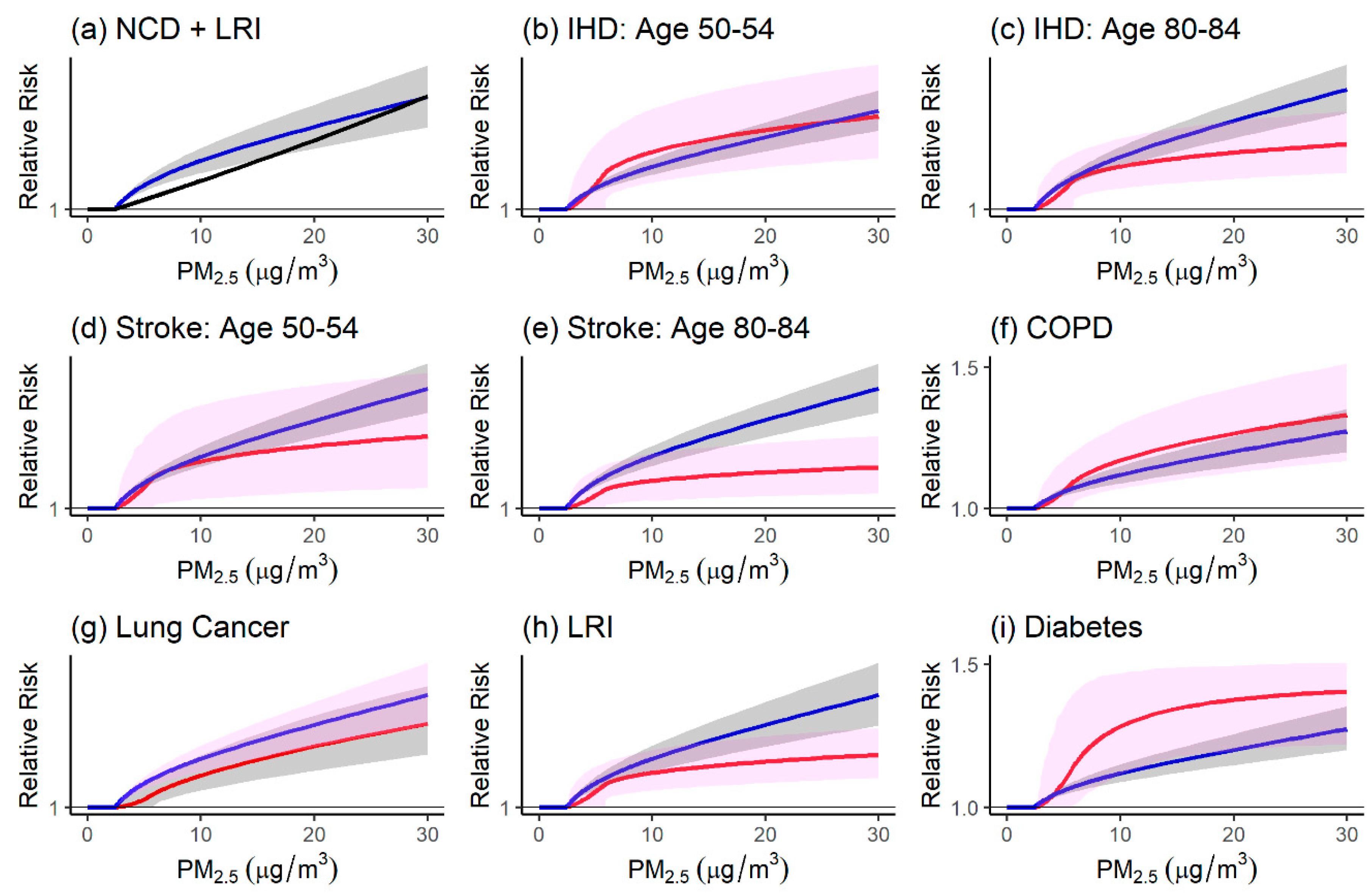
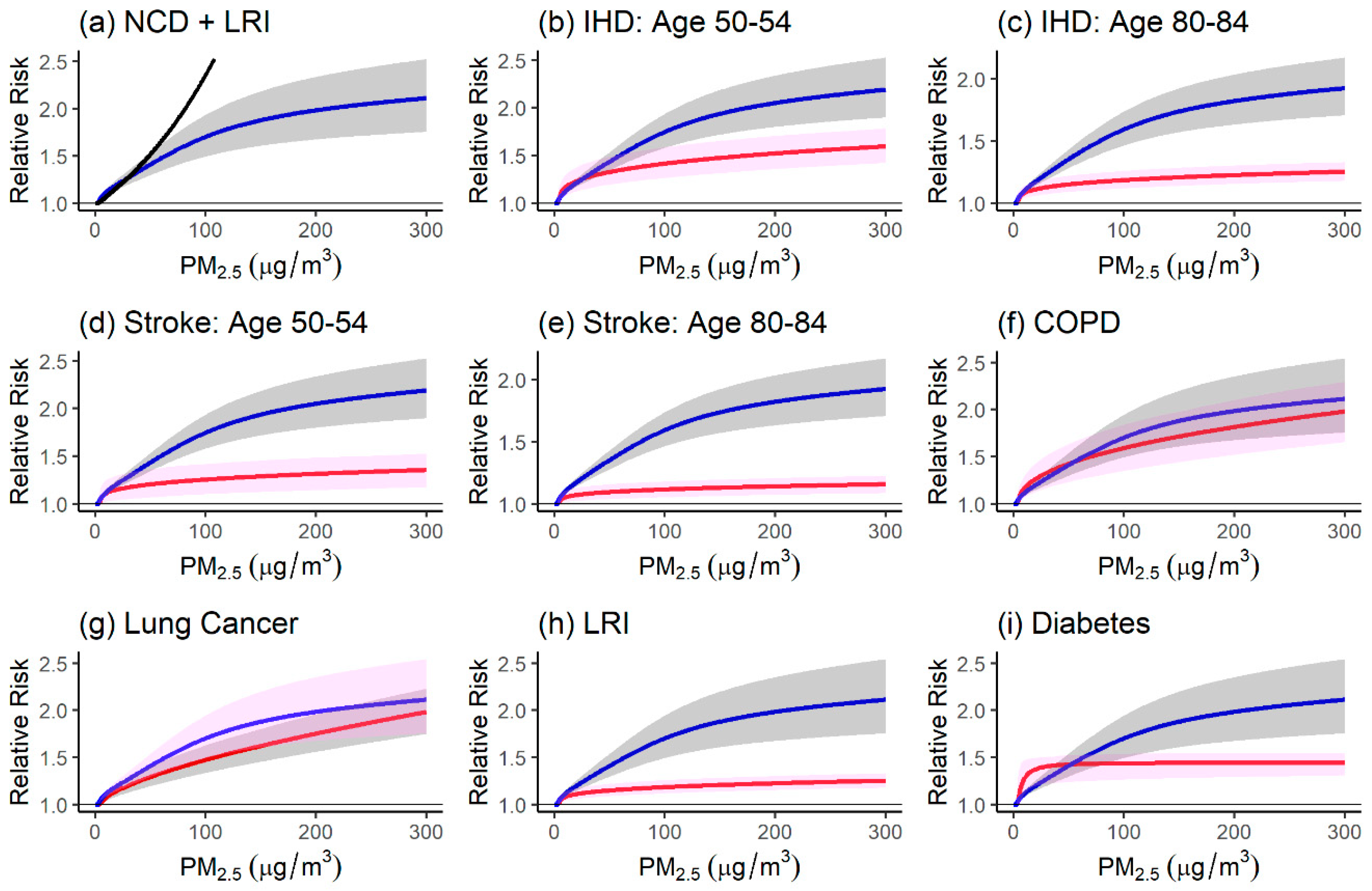
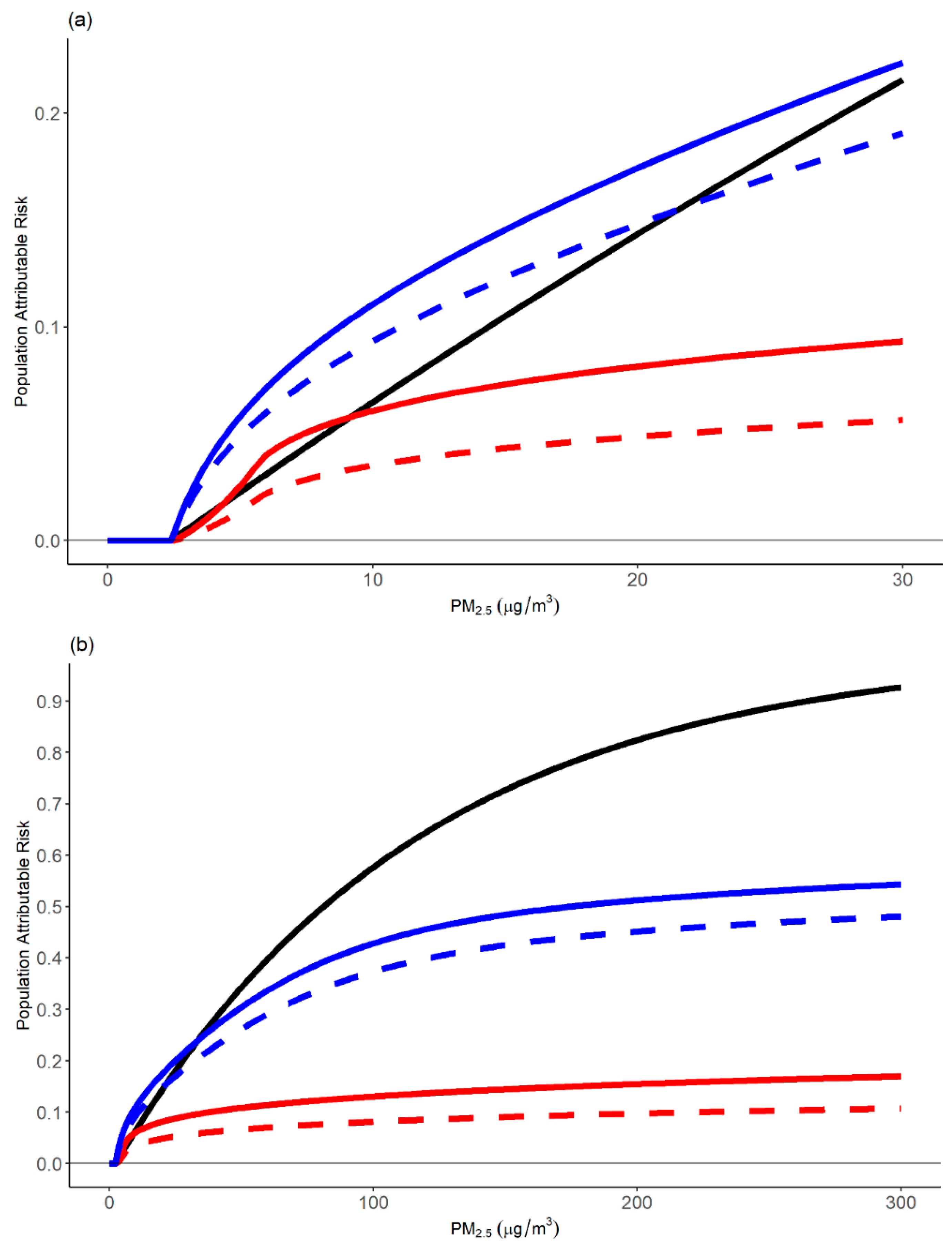
4.1. Comparison of the Relative Risk Modeling Approaches
4.1.1. Comparison of Relative Risk Estimates and Population Attributable Fractions
4.1.2. Strengths and Limitations of Modeling Approaches
4.2. Should Estimates Be Based on All-Natural Cause or Cause-Specific Mortality?
5. Recommendations and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Health Effects Institute (HEI). State of Global Air 2019; Special Report; Health Effects Institute: Boston, MA, USA, 2019. [Google Scholar]
- Shaddick, G.; Thomas, M.L.; Amini, H.; Broday, D.; Cohen, A.; Frostad, J.; Green, A.; Gumy, S.; Liu, Y.; Martin, R.V.; et al. Data integration for the assessment of population exposure to ambient air pollution for global burden of disease assessment. Environ. Sci. Technol. 2018, 52, 9069–9078. Available online: https://arxiv.org/abs/1609.00141 (accessed on 31 March 2020). [CrossRef]
- United Nations. About the Sustainable Development Goals. 2019. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 31 March 2020).
- Pappin, A.J.; Christidis, T.; Pinault, L.L.; Crouse, D.L.; Tjepkema, M.; Erickson, A.C.; Brauer, M.; Weichenthal, S.; van Donkelaar, A.; Martin, R.V.; et al. Examining the shape of the association between low levels of fine particulate matter and mortality across three cycles of the Canadian Census Health and Environment Cohort. Environ. Health Perspect. 2019, 127, 107008. [Google Scholar] [CrossRef] [PubMed]
- Di, Q.; Wang, Y.; Zanobetti, A.; Wang, Y.; Koutrakis, P.; Choirat, C.; Dominici, F.; Schwartz, J.D. Air pollution and mortality in the Medicare population. N. Engl. J. Med. 2017, 376, 2513–2522. [Google Scholar] [CrossRef]
- U.S. EPA (United States Environmental Protection Agency). Final Report: Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2009); EPA/600/R-08/139F; Environmental Protection Agency: Washington, DC, USA, 2009. Available online: http://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=216546 (accessed on 31 March 2020).
- Thurston, G.D.; Kipen, H.; Annesi-Maesano, I.; Balmes, J.; Brook, R.D.; Cromar, K.; De Matteis, S.; Forastiere, F.; Forsberg, B.; Frampton, M.W.; et al. A joint ERS/ATS policy statement: What constitutes an adverse health effect of air pollution? An analytical framework. Eur. Respir. J. 2017, 49, 1600419. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.T.; Dewanji, A.; Dominici, F.; Goldberg, M.S.; Cohen, A.; Krewski, D. On the relationship between time-series studies, dynamic population studies, and estimating loss of life due to short-term exposure to environmental risks. Environ. Health Perspect. 2003, 111, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Apte, J.S.; Brauer, M.; Cohen, A.J.; Ezzati, M.; Pope, C.A.I.I.I. Ambient PM2.5 reduces global and regional life expectancy. Environ. Sci. Technol. Lett. 2018, 5, 546–551. [Google Scholar] [CrossRef]
- Hammitt, J.K.; Morfeld, P.; Tuomisto, J.T.; Erren, T.C. Premature Deaths, Statistical Lives, and Years of Life Lost: Identification, Quantification, and Valuation of Mortality Risks. Risk Anal. 2020, 40, 674–695. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ezzati, M.; Lopez, A.D.; Rodgers, A.; Vander Hoorn, S. Comparative quantification of health risks: Conceptual framework and methodological issues. Popul. Health Metr. 2003, 1, 1–20. [Google Scholar] [CrossRef]
- GBD MAPS Working Group. Burden of Disease Attributable to Coal-Burning and Other Major Sources of Air Pollution in China; Special Report 20; Health Effects Institute: Boston, MA, USA, 2016. [Google Scholar]
- GBD MAPS Working Group. Burden of Disease Attributable to Major Air Pollution Sources in India; Special Report 21; Health Effects Institute: Boston, MA, USA, 2018. [Google Scholar]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. Available online: http://dx.doi.org/10.1016/S0140-673630505-6 (accessed on 31 March 2020). [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Burnett, R.T.; Pope, C.A.I.I.I.; Ezzati, M.; Olives, C.; Lim, S.S.; Mehta, S.; Shin, H.H.; Singh, G.; Hubbell, B.; Brauer, M.; et al. An Integrated Risk Function for Estimating the Global Burden of Disease Attributable to Ambient Fine Particulate Matter Exposure. Environ. Health Perspect. 2014, 122, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.; Spadaro, J. Global Mortality and Long-Term Ambient Exposure to Fine Particulate Matter: A New Relative Risk Estimator. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef] [PubMed]
- World Bank; Institute for Health Metrics and Evaluation. The Cost of Air Pollution: Strengthening the Economic Case for Action; World Bank: Washington, DC, USA, 2016; Available online: https://openknowledge.worldbank.org/handle/10986/25013 (accessed on 31 March 2020).
- Lelieveld, J.; Pozzer, A.; Pöschl, U.; Fnais, M.; Haines, A.; Münzel, T. Loss of life expectancy from air pollution compared to other risk factors: A worldwide perspective. Cardiovasc. Res. 2020. Online publication. [Google Scholar] [CrossRef]
- U.S. EPA. Regulatory Impact Analysis for the Final Revisions to the National Ambient Air Quality Standards for Particulate Matter; Office of Air Quality Planning and Standards, Health and Environmental Impacts Division: Research Triangle Park, NC, USA, 2012. [Google Scholar]
- Pope, C.A.I.I.I.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J. Am. Med Assoc. 2002, 278, 1132–1141. [Google Scholar] [CrossRef]
- Cohen, A.J.; Anderson, H.R.; Ostro, B.; Pandey, K.D.; Krzyzanowski, M.; Kuenzli, N.; Gutschmidt, K.; Pope, C.A.; Romieu, I.; Samet, J.M.; et al. Mortality impacts of urban air pollution. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Due to Selected Major Risk Factors; Ezzati, M., Lopez, A.D., Rodgers, A., Murray, C.J.L., Eds.; World Health Organization: Geneva, Switzerland, 2004; Volume 2. [Google Scholar]
- Pope, C.A.I.I.I.; Burnett, R.T.; Krewski, D.; Jerrett, M.; Shi, Y.; Calle, E.; Thun, M.J. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: Shape of the exposure-response relationship. Circulation 2009, 120, 941–948. [Google Scholar] [CrossRef]
- Pope, C.A.I.I.I.; Burnett, R.T.; Turner, M.C.; Cohen, A.; Krewski, D.; Jerrett, M.; Gapstur, S.; Thun, M.J. 2.0.1.1. Lung Cancer and Cardiovascular Disease Mortality Associated with Particulate Matter Exposure from Ambient Air Pollution and Cigarette Smoke: Shape of the Exposure-Response Relationships. Environ. Health Perspect. 2011, 119, 1616–1621. [Google Scholar] [CrossRef]
- Turner, M.C.; Jerrett, M.; Pope, I.I.I.C.A.; Krewski, D.; Gapstur, S.M.; Diver, W.R.; Beckerman, B.S.; Marshall, J.D.; Su, J.; Crouse, D.L.; et al. Long-Term Ozone Exposure and Mortality in a Large Prospective Study. Am. J. Respir. Crit. Care Med. 2016, 193, 1134–1142. [Google Scholar] [CrossRef]
- Pope, C.A.I.I.I.; Cohen, A.J.; Burnett, R.T. Cardiovascular Disease and Fine Particulate Matter: Lessons and Limitations of an Integrated Exposure Response Approach. Circ. Res. 2018, 122, 1645–1647. [Google Scholar] [CrossRef]
- Yin, P.; Brauer, M.; Cohen, A.; Burnett, R.T.; Liu, J.; Liu, Y.; Liang, R.; Wang, W.; Qi, J.; Wang, L.; et al. Long-term fine particulate matter exposure and nonaccidental and cause-specific mortality in a large national cohort of Chinese men. Environ. Health Perspect. 2017, 125, 117002. [Google Scholar] [CrossRef]
- Nasari, M.N.; Szyszkowicz, M.; Chen, H.; Crouse, D.L.; Turner, M.C.; Jerrett, M.; Pope, C.A.I.I.I.; Hubbell, B.; Fann, N.; Cohen, A.; et al. A Class of Non-Linear Exposure-Response Models Suitable for Health Impact Assessment Applicable to Large Cohort Studies of Ambient Air Pollution. Air Qual. Atmos. Health 2016, 9, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Fantke, P.; McKone, T.E.; Tainio, M.; Jolliet, O.; Apte, J.S.; Stylianou, K.S.; Illner, N.; Marshall, J.D.; Choma, E.F.; Evans, J.S. Global Effect Factors for Exposure to Fine Particulate Matter. Environ. Sci. Technol. 2019, 53, 6855–6868. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Hystad, P.; Yusuf, S.; Brauer, M. Pollution Health Impacts—The Knowns and Unknowns for Reliable Global Burden Calculations. Cardiovasc. Res. 2020, cvaa092. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burnett, R.; Cohen, A. Relative Risk Functions for Estimating Excess Mortality Attributable to Outdoor PM2.5 Air Pollution: Evolution and State-of-the-Art. Atmosphere 2020, 11, 589. https://doi.org/10.3390/atmos11060589
Burnett R, Cohen A. Relative Risk Functions for Estimating Excess Mortality Attributable to Outdoor PM2.5 Air Pollution: Evolution and State-of-the-Art. Atmosphere. 2020; 11(6):589. https://doi.org/10.3390/atmos11060589
Chicago/Turabian StyleBurnett, Richard, and Aaron Cohen. 2020. "Relative Risk Functions for Estimating Excess Mortality Attributable to Outdoor PM2.5 Air Pollution: Evolution and State-of-the-Art" Atmosphere 11, no. 6: 589. https://doi.org/10.3390/atmos11060589
APA StyleBurnett, R., & Cohen, A. (2020). Relative Risk Functions for Estimating Excess Mortality Attributable to Outdoor PM2.5 Air Pollution: Evolution and State-of-the-Art. Atmosphere, 11(6), 589. https://doi.org/10.3390/atmos11060589



