Abstract
Atmospheric particles with an aerodynamic diameter less than or equal to 2.5 micrometers (PM2.5) were collected at two sites located in the urban area of the city of Cuernavaca (Morelos) during a season when a large number of forest fires occurred. Three dicarboxylic acids (malonic, glutaric and succinic) and levoglucosan were analyzed by liquid chromatography coupled with mass spectrometry (ESI-Q-TOF) and soluble potassium (K+) was analyzed by ion chromatography. The concentration of PM2.5 increased on the days when the highest number of forest fires occurred. A strong correlation was observed between levoglucosan and K+, confirming the hypothesis that both are tracers of biomass burning (r = 0.57, p < 0.05). Levoglucosan (average 367.6 ng m−3, Site 2) was the most abundant compound, followed by succinic acid (average 101.7 ng m−3, Site 2), glutaric acid (average 63.2 ng m−3, Site 2), and malonic acid (average 46.9 ng m−3, Site 2), respectively. The ratio of C3/C4 concentrations ranged from 0.5 to 1.2, with an average of 0.8, which suggests great photochemical activity in the Cuernavaca atmosphere. The ratio of K+/levoglucosan concentrations (0.44) indicates that open fires are the main source of these tracers. The positive correlations between PM2.5 and levoglucosan and succinic and malonic acids suggest that such compounds are contributing to secondary organic aerosol particle formation.
1. Introduction
Biomass burning is a major source of particulate matter in ambient air, with the particles consisting of hundreds of organic and inorganic compounds, causing significant impacts on regional to global air quality [1], human health [2] and climate [3]. The composition of the atmospheric particles depends on diverse factors, such as the original source, the amount of emission, the meteorological conditions and the topography of the area in which generation occurs [4,5]. Due to the complex chemical composition of the emissions caused by the burning of biomass, it is not easy to find exact tracers.
Water-soluble organic compounds (WSOC) constitute an important fraction of atmospheric organic matter [6], representing between 10% and 90% of organic carbon content in atmosphere aerosols, depending on location and season. Primary organic aerosols (POA) and secondary organic aerosols (SOA) are ubiquitous in the atmosphere, and their proportion will largely depend on emission factors and prevailing weather conditions [7]. In the pyrolysis of plant products rich in cellulose and hemicellulose, a large number of low molecular weight (LMW) polar compounds have been identified, such as anhydrosugars [8], sugar alcohols [9], primary sugars and oxalic acid (C2) [10], as well as several sugar derivatives [11]. These compounds are potentially important molecular markers when trying to determine the source of both anthropogenic and natural aerosols and elucidating the processes and pathways of organics in atmospheric aerosols [12]. The most commonly used organic tracer is levoglucosan, a sugar anhydride produced via pyrolysis of cellulose and hemicelluloses; it is a highly specific molecular marker for biomass burning [13,14].
Dicarboxylic acids are another important class of WSOC in the atmosphere. These are produced by primary sources, such as biomass burning and fossil fuel combustion [15,16], but the major portion results from photooxidation of organic precursors, so dicarboxylic acids are thus considered, in general, as secondary products [17]. The diacids significantly change the hygroscopicity of atmospheric aerosols, contributing directly to the earth’s radiation balance by scattering incoming solar radiation, as well as to the increase of cloud condensation nuclei [18,19]. For its part, water-soluble potassium (K+) has been used widely as an inorganic tracer for biomass burning [20,21].
In México, climatic factors such as drought, hurricanes, and the El Niño and La Niña phenomena impact directly and indirectly the forested areas of the country, generating conditions that favor the development of forest fires of different magnitude and severity. In recent years, these forest fires have manifested extreme behavior and high resistance to control. In the state of Morelos, the number of fires and the area affected are directly related to human activities and the behavior of hydrometeorological phenomena. Specifically, the behavior of drought, or the lack of relative humidity in the environment, together with the accumulation of dead vegetative material, contributes significantly to the generation of fires [22].
Despite the relevance of these types of studies, which allow for the evaluation of the impact that these compounds have on the behavior of climate change at the local and global level, in the Mexican Republic, there are few records of the concentration levels of some of these biomarkers [23,24]. Specifically, for the state of Morelos, there is no scientific study that has reported the concentration levels of these species.
The aim of the work presented here is to characterize the concentrations of combustion tracers in ambient air, such as levoglucosan, dicarboxylic acids and water-soluble potassium in the urban environment in Morelos, in 2016, between the months of February and August, the period in which the largest number of forest fires occur.
2. Materials and Methods
2.1. Sampling Sites
The city of Cuernavaca is located in the center of the Mexican Republic, 85 km from Mexico City. According to the last census, it has a population of 365,168 inhabitants [25] (Figure 1). Two sites were selected for sampling. The first one, downtown in the city (Centro, Site 1), is an area characterized by high vehicular and commercial activity. The second site is located north of the city, within the Autonomous University of the State of Morelos (Center of Chemical Research, CIQ for its acronym in Spanish, Site 2), and is surrounded by a variety of vegetation. The sampling sites were selected according to 40 CFR Part 58 Appendix A of the USEPA Code of Federal Regulations (CFR), taking into account the geographical characteristics, relevant anthropogenic activities and behavior of the meteorological variables [26].
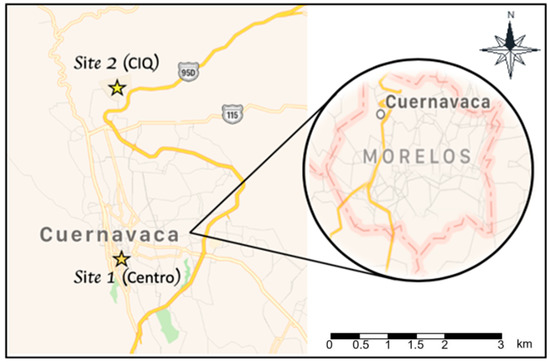
Figure 1.
Sampling sites at the city of Cuernavaca, Morelos.
2.2. Sampling of Airborne Particles
Sampling was done between February and August 2016. The particulate material was collected on fiberglass filters of 47 mm diameter and 2.0 µm pore size (Whatman, Darmstadt, Germany), which were conditioned in an oven at 550 °C for 30 min, and then transferred to a desiccator for 24 h. A low-volume (5.0 L min−1) sampler (MiniVol™ TAS, Eugene, OR, USA) was used, equipped with a 2.5 µm impactor. Sampling was carried out for periods of 24 h (12:00 a.m.–12:00 p.m.). Gravimetric analysis was carried out under controlled room temperature and humidity (22 ± 3 °C, 40% ± 5%). Before weighing, the filters were stabilized for 24 h. Three blank filters per month were used as laboratory blanks. Each filter was weighed at least three times and the three readings had to agree within 5 micrograms to be accepted. The detection limit was calculated as 3 × SD of the mass change in the blank filters divided by the volume of the corresponding exposure time (24 h). The detection limit for the measurements was 0.92 mgm−3. The mass determination of PM2.5 was performed by weighing the filters before and after the collection period with a 0.1 µg sensitivity microbalance (Citizen CX-220™, Parwanoo, India). The concentration of PM2.5 in the atmosphere in mgm−3 was obtained by dividing the mass by the volume of filtered air, adjusted to conditions of standard temperature and pressure (25 °C and 760 mm Hg).
2.3. Meteorological Parameters and Criteria Pollutants
Simultaneously, meteorological parameters were monitored at both sites (temperature, relative humidity, solar radiation, wind speed and wind direction). Additionally, only at Site 1, criteria pollutant data were obtained (O3, SO2, NOx and CO) (Table 1).

Table 1.
Meteorological conditions and criteria pollutants during the sampling period.
2.4. Extraction of Water-Soluble Organic Compounds
The sampled filters were placed in polypropylene tubes, and then 8.0 mL of Milli-Q water was added to each tube and extracted in an ultrasound bath (Branson 3210) for 1.0 h. The Erlenmeyer flasks in which the filters were introduced were fitted with cooled condensers with water at 10 °C; then the extracts were filtered through nylon membranes with a pore size 0.45 µm. Once the extracts were filtered, they were transferred to injection vials and stored at approximately 4 °C, until chromatographic analysis.
2.5. Chromatographic Analysis
The analysis of the WSOC was carried out by using Ultra-High-Performance Liquid Chromatography (UHPLC) in an Agilent 1290 Infinity LC System, coupled to a quadrupole-time-of-flight (Agilent Q-TOF 6545 mass spectrometer), equipped with an electrospray ionization source (ESI). The ESI-MS conditions were optimized by injection of single standard at a concentration of 100 ng mL−1 of four compounds (levoglucosan and glutaric, malonic and succinic acids), in both positive and negative ionization mode. No signal was recorded in positive mode. Highly abundant analytes signals were detected in negative mode. Table 2 shows optimal instrumental operating conditions.

Table 2.
Summary of source parameters for negative ion mode method.
For the separation of compounds, a Zorbax Rapid Resolution High Definition SB-C18 column with a 2.1 mm internal diameter × 50 mm and a particle pore size of 1.8 µm was used. The mobile phase was a solution of NH4OH 13 mM in methanol:water (85:15), which was used in isocratic mode. The identification of the compounds was carried out by the retention time and the molecular ion (Table 3).

Table 3.
Selected parameters for the identification of dicarboxylic acids and levoglucosan by UHPLC.
Meanwhile the analysis of soluble potassium (K+) was determined by ion chromatography (CI, Metrohm model 861 Advanced Compact with conductivity detector), without chemical suppression in a Metrosep C2_150 (Metrohm) column; the mobile phase was a solution of tartaric–dipicolinic acid (4.0:0.75 mM) at a flow rate of 1 mL min−1. The injection volume was 50 μL.
Calibrations for all studied compounds were based on serial dilutions from a stock solution made by dissolving individual compounds in solid form. Each calibration graph was made with five concentration points. The concentration range for levoglucosan and carboxylic acids was between 50 and 5000 ng mL−1, while for K+, this was between 50 and 1000 ng mL−1.
2.6. Quality Control of the Analytical Methods
To exclude the presence of the compounds of interest in the materials and reagents used, laboratory targets were done once a week. The extraction efficiency was determined by enriching filters with a mixture of the compounds studied at a concentration of 100 ppb for levoglucosan, malonic acid, succinic acid and glutaric acid and 500 ppb for K+. The enriched filters were extracted in the same way as the samples; the recovery percentages ranged from 80% to 85%. Furthermore, from the linear regression data, the detection limit of the method (LD) and quantification (LC) were determined, as well as the correlation coefficient (R) according to Miller and Miller (2002) [27] (Table 4).

Table 4.
Limits of detection and quantification of the method, and correlation coefficient for each of the detected compounds.
2.7. Wind Trajectories
To determine the behavior of winds in the study area, HYSPLIT4 model trajectories, obtained from the NOAA (National Oceanic and Atmospheric Administration), were used [28]. During the study period, two patterns were observed with respect to the direction of the winds. The first occurred between February and April; in these months, the winds came mainly from the west–southwest. During the second period, from May to July, they came mainly from the east–southeast (Figure 2).
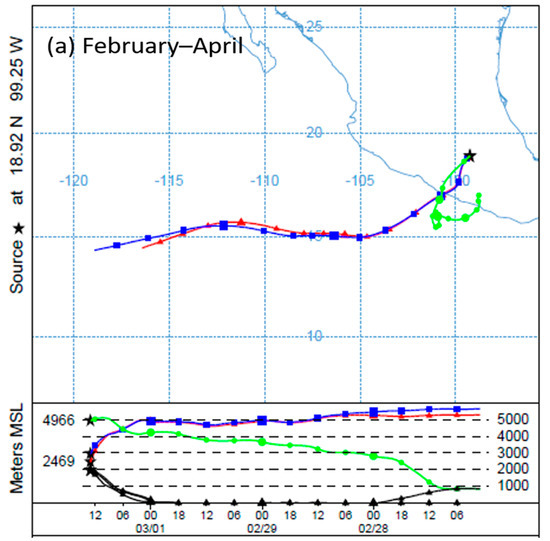
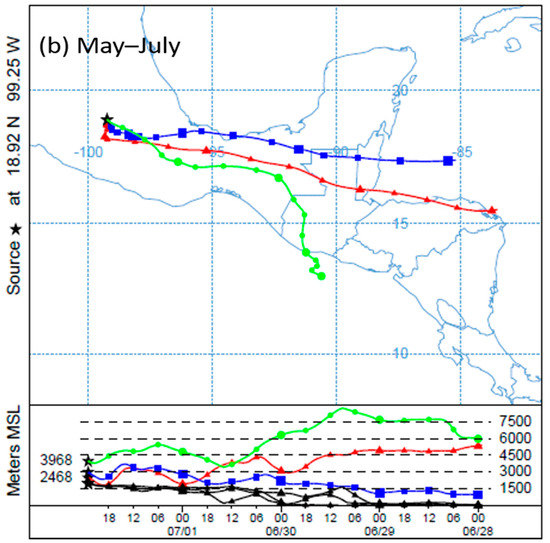
Figure 2.
Behavior of wind trajectories in Cuernavaca during February and July 2016: (a) the trend observed between February and April (west–southwest); (b) the trend observed between May and July (east–southeast). Source: 18°55′ N, 99°13′ W, heights: 500, 1000 and 2000 m above mean sea level (a.m.s.l.).
3. Results and Discussion
3.1. Concentrations of PM2.5
In total, 35 samples were taken at each site, and the results revealed that the average concentration of PM2.5 was 22 μg m−3 for Site 1 (CENTRO) and 16 μg m−3 for Site 2 (CIQ). The behavior of the PM2.5 concentration was evaluated by time series (Figure 3). The results indicate some similarity, which is probably associated with common sources influencing both sites. The highest concentration of PM2.5 was observed in February and between April and May, which is consistent with the forest fires that occurred in this period (Figure 4). According to official data, 52 fires occurred in February, 50 in March, 59 in April and 21 in May. This last month (May) is when the rains start, which allowed for the natural abatement of the fires and therefore the decrease in the concentration of PM2.5 [22].
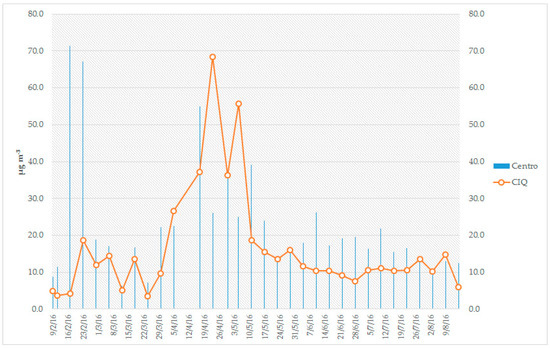
Figure 3.
Time series for the concentration of PM2.5 observed at both sites during the period studied.
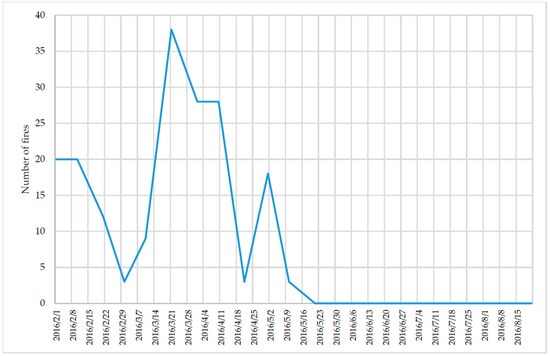
Figure 4.
Number of forest fires observed in Morelos State in 2016 [22].
3.2. Molecular Tracers Associated with Airborne Particles
Table 5 summarizes the concentrations of levoglucosan and succinic, glutaric and malonic acids associated with PM2.5 at both sampled sites in Cuernavaca and their average values with standard deviations, as well as maximum and minimum values.

Table 5.
Mean concentration and range of the selected molecular markers during study period (ng m−3).
3.2.1. Levoglucosan
Levoglucosan was the compound with the highest concentration at both sites. At the CIQ site, the average concentration was slightly higher (367.6 ng m−3, ±57.6), suggesting similar sources during the study period. Levoglucosan is the most abundant anhydrosugar reported in urban areas, due mainly to the burning of biomass [29]. Meanwhile, low concentrations found in summer could be attributed to the absence of residential heating in the warm season and better atmospheric conditions that favor atmospheric dispersion of pollutants. For its part, other minor sources such as emissions from fires for land clearance and/or accidental fires could be possible sources of levoglucosan in summer [30]. In this sense, it is important to highlight that, in the state of Morelos, about 182 forest fires occurred during the study period, which probably justifies the high concentrations of levoglucosan. Moreover, in some way, the presence of levoglucosan at this site probably indicates the transport of air masses from other regions of the state, specifically east and east–southeast, considering the behavior of wind trajectories, during the study period [28].
3.2.2. Dicarboxylic Acids
The concentration of some organic acids was also determined. At both sites, the most abundant compound was succinic acid (C4), followed by glutaric acid (C5) and malonic acid (C3). Again, it is observed that the CIQ site presents slightly higher concentrations than the Centro site. When evaluating the behavior of the concentrations, it is observed that the maximum values were presented in the period (April–May) in which the greatest number of fires were registered, which is congruent with the observed behavior of the concentration of PM2.5 and Levoglucosan.
The total average concentration of organic acids (C3, C4 and C5) was 178.1 ng m−3 at the Centro site and 211.8 ng m−3 at the CIQ site. The excess concentration of succinic acid over malonic acid indicates that emissions can come from burning biomass, fossil fuel combustion and vehicular emissions [31,32]. Kawamura and Ikushima (1993) indicate that malonic acid is partly produced from the incomplete combustion of fossil fuels and biomass burning, but is mainly due to photochemical oxidation of succinic acid in the atmosphere [15]. On the other hand, Kawamura et al. (1996a) proposed that succinic acid can also be generated via the photo-oxidation of unsaturated fatty acids from terrestrial higher plants and domestic cooking [33].
Moreover, Kawamura and Sakaguchi (1999) proposed that the C3/C4 ratio is a good indicator to determine if the presence of dicarboxylic acids in the atmosphere corresponds to primary sources or oxidation processes [31]. Kawamura and Kaplan (1987) reported that lower C3/C4 ratios (0.25–0.44) correspond mainly to vehicular emissions, while values above that correspond to oxidation processes in the atmosphere [16]. The C3/C4 ratio obtained in this study, ranging from 0.5 to 1.2, with an average of 0.8, suggests that, in the urban area of Cuernavaca, photochemical processes regulate, to a large extent, the presence of the dicarboxylic acids studied.
3.2.3. Comparisons of the Observed Concentration for Levoglucosan and Dicarboxylic Acids with Other Studies
These comparisons should be analyzed with caution, since the results were obtained with different analytical methodologies and under different environmental conditions.
The average concentration of levoglucosan was lower than those reported in samples collected in Elverum, Norway (605 ng m−3), during the winter of 2002, in a suburban atmosphere, and the 713.0 ng m−3 recorded at Tengchon, China, during the spring of 2004, in a rural site, suggesting that the main sources of levoglucosan in such places can be attributed to the combustion of firewood and agricultural and garden-waste burning [34,35]. However, the concentrations of levoglucosan found in the present study were significantly higher than those observed in the rural atmosphere in K-puszta, Hungary (12.3 ng m−3) during the summer of 2003 (which was characterized by intense solar radiation) [36]; in an urban zone of Copenhagen, Denmark, by Oliveira et al. (2007) (40 ng m−3) during the winter season [37]; and in Barcelona, Spain, during the Winter season, in an urban atmosphere, by Reche et al. (2012) (60 ng m−3) [38] (Table 6). Regarding the observed differences in levoglucosan concentration, Liu et al. (2005) mentioned that it is also important to consider different emission fluxes for different biomass combustion types, such as fuel types, oven types, agricultural fires and forest fires, among others [39].
Likewise, comparisons of dicarboxylic acid concentrations observed in this study with other studies conducted in other urban areas showed that the average concentration of dicarboxylic acids (70.3 ng m−3) was higher than that reported by Balla et al. (2018) (21.0 ng m−3) in a suburban area of the city of Thessaloniki, Greece [40], Pietrogrande and Bacco (2011) (12.6 ng m−3) in an urban area of the city of Ferrara, Italy [41], and Ho et al. (2006) (60.3 ng m−3) in an urban zone of Hong Kong, China [42]. Conversely, it is lower than the reported results by Wang et al. (2002) in urban atmosphere from Nanging, China (128.2 ng m−3) [43], and Choi et al. (2016) in an urban zone of the city of Seoul, S. Korea (99.2 ng m−3) [44]. Such differences can be attributed to an increase in burning for residential heating in cold periods [19]. However, other studies reported that some of the DCAs tend to be higher during the warm season in urban areas, mainly due to photochemical activity [45]. In this sense, it is important to highlight that the period in which the present study was carried out was a warm season with the presence of many forest fire events (Table 6).

Table 6.
Comparison with other studies carried out in other cities of the world, average concentration (ng m−3).
Table 6.
Comparison with other studies carried out in other cities of the world, average concentration (ng m−3).
| Reference | Site | Background | Date | Size Particle | Levoglucosan | Glutaric | Malonic | Succinic |
|---|---|---|---|---|---|---|---|---|
| Yttri et al., 2007 [34] | Elverum, Norway | Suburban | September–October 2002 | PM10 | 605 | nr | nr | nr |
| Engling et al., 2011 [35] | Tengchon, China | Rural | April–May 2004 | PM2.5 | 713 | nr | nr | nr |
| Stone et al., 2008 [23] | México City, México | Urban | 16–30 March 2006 | PM2.5 | 151.3 | nr | nr | nr |
| Ion et al., 2005 [36] | K-puszta, Hungary | Rural | June–July 2003 | PM2.5 | 12.3 | nr | nr | nr |
| Oliveira et al., 2007 [37] | Copenhagen, Denmark | Urban | February–March (2004) | PM2.5 | 40 | nr | nr | nr |
| Mancilla et al., 2016 [24] | Monterrey, México | Urban | May–June 2011 | PM2.5 | 54.3 | nr | nr | nr |
| Reche et al., 2012 [38] | Barcelona, Spain | Urban | February–March (2009) | PM2.5 | 60 | nr | nr | nr |
| Balla et al., 2018 [40] | Thessaloniki, Greek | Suburban | November–December 2014 and May 2015 | PM2.5 | nr | 26 | nr | 16 |
| Pietrogrande and Bacco, 2011 [41] | Ferrara, Italy | Urban | 2011–2014 | PM2.5 | 254 | 14.7 | 4.3 | 18.9 |
| Wang et al., 2002 [43] | Nanjing, China | Urban | February–March 2001 | PM2.5 | nr | 64.1 | 125.5 | 195 |
| Choi et al., 2016 [44] | Seoul, South Korea | Urban | April 2010–April 2011) | PM10 | nr | 10.6 | 191.8 | 95.3 |
| Ho et al., 2006 [42] | Hong Kong, China | Urban | December 2003–February 2004 | PM2.5 | nr | 20 | 89.1 | 71.9 |
| This study | Cuernavaca, México | Urban | February–August 2016 | PM2.5 | 367.6 | 63.2 | 46.9 | 101.7 |
nr: not reported.
3.2.4. Water-Soluble Potassium (K+)
To evaluate the degree of association between water-soluble potassium and levoglucosan, both considered as indicators of biomass burning, a time-series analysis was performed with the observed concentrations during the study period. Figure 5 reveals two peaks on the days when the highest number of forest fires occurred in the state of Morelos (April–May 2016), confirming the hypothesis that both are indicators of biomass burning. This behavior is consistent with that observed by Zhang et al. (2010) in a study conducted in the urban areas of some cities in the Southwestern United States during winter, when there was an increase in biomass burning [46]. Likewise, Reche et al. (2012) observed similar behavior in the urban area of Barcelona, in biomass burning scenarios [38]. Locker (1988) established the K+/levoglucosan relationship to determine if the combustion processes correspond to ovens or fireplaces that use wood, or open fires. If the K+/levoglucosan ratio is <0.2, it may indicate the prevalence of domestic heating with wood, while a K+/levoglucosan ratio around 0.5 may indicate open fires or combustion of fuels such as slash, or straw [47]. In the present study, the value was 0.44, probably indicating that open fires are the main source of these tracers in the study region.
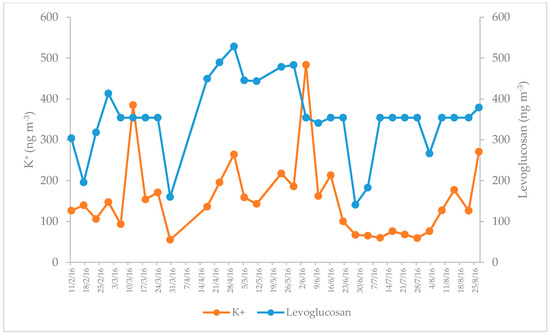
Figure 5.
Association between water-soluble potassium and levoglucosan.
3.3. Sources of Molecular Markers
In order to investigate possible correlations between the studied compounds, meteorological data and criteria pollutants, the Spearman correlation coefficients were calculated (Table 7).

Table 7.
Correlations between meteorological parameters, criteria air pollutants and organic pollutants evaluated in Cuernavaca (Site 1) in 2016.
Levoglucosan positively correlated with glutaric and malonic acids and K+, probably indicating common sources, i.e., from biomass burning and mainly cellulose pyrolysis [48,49]. The positive correlation observed between temperature and O3 with glutaric and malonic acids and levoglucosan suggests the incidence of photochemical activity. Specifically, for the observed correlation between temperature and malonic and glutaric acids, Ho et al. (2006) indicates that this behavior is associated with secondary photochemical processes in the atmosphere rather than primary emissions from vehicular exhaust [19]. Other studies suggest that glutaric acid has a mechanism of formation through secondary photochemical reactions in the atmosphere, from vehicle emissions [33,50]. The positive correlation observed between temperature and levoglucosan can be attributed to the large number of forest fires that occurred in the study season in the state of Morelos. The positive correlation between PM2.5 and glutaric and malonic acids and levoglucosan suggests that such compounds are contributing to secondary organic aerosol particles’ formation. Gaseous pollutants (NOx, CO and SO2) did not show a correlation with levoglucosan, as these compounds are mainly generated by exhaust emissions from local sources.
Finally taking into account the recommendations established in the European guide for estimating sources of air pollution, a Factor Analysis with Varimax rotation was performed, only to determine a preliminary overview of the possible sources of the compounds studied for the Centro site [51].
Three factors were extracted that explained 84.7% of the total variance (Table 8). The first factor, accounting for 30.7% of the variance, was mainly constituted by glutaric, malonic, and succinic acids and temperature, suggesting photochemical formation, possibly by precursors from vehicle exhausts. The second component explained 25.45% of the variance, clearly indicating local vehicle emissions. The third factor explained 28.55%, conformed mainly by K+ and levoglucosan, indicating the incidence of common sources, i.e., the burning of biomass (Table 8).

Table 8.
Factor analysis with Varimax rotation realized for the Centro site.
4. Conclusions
This is the first study carried out in Cuernavaca in which the concentration of molecular markers associated with the burning of biomass was determined. It is clearly observed how, in the fire season, the concentration of such compounds is significantly increased, which contributes significantly to the deterioration of air quality in this zone and has possible implications for climate change.
The impact of biomass burning was observed by means of tracers such as levoglucosan and K+. The results showed that the high PM2.5 concentrations and molecular tracers coincided with the fire season. The C3/C4 ratio observed in this study suggested that, in the urban area of Cuernavaca, photochemical processes regulate, to a large extent, the presence of the dicarboxylic acids studied. Likewise, the K+/levoglucosan ratio reveals the relevance of open fires in the region’s air quality.
The Factor Analysis gave us an overview of the possible contribution of sources of the compounds studied in the region, however, to make a more precise estimate of the possible sources, it is required to apply more advanced models, such as PMF and CMB. These software programs will be applied in the next stage of the project, in which more sampling sites and more samples will be considered.
Finally, these results constitute a call to the environmental authorities for attention to the implementation of environmental education campaigns, as well as for the generation of strategies that allow for prevention, to a large extent, of the indiscriminate burning of plant material.
Author Contributions
Conceptualization, H.S.-N. and M.I.A.-M.; methodology, J.A.G.-A., P.G.R. and S.M.-P.; investigation, J.V.-S., F.R.-Q. and M.A.M.-T.; writing—original draft preparation, H.S.-N. and R.L.-M. All authors have read and agreed to the published version of the manuscript.
Funding
Authors would like to thank Consejo Nacional de Ciencia y Tecnología (CONACYT) for the studentship and Laboratorio Nacional de Estructura de Macromoléculas (LANEM CONACyT/251613).
Acknowledgments
The authors wish to thank the Program for the Development of Teachers (PRODEP, by its acronym in Spanish) for its support in the financing of this publication and the National Laboratory of Macromolecule Structure (LANEM, by its acronym in Spanish) for allowing us to use its laboratories and infrastructure.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lelieveld, J.O.; Crutzen, P.J.; Ramanathan, V.; Andreae, M.O.; Brenninkmeijer, C.A.; Campos, T.; Cass, G.R.; Dickerson, R.R.; Fischer, H.; De Gouw, J.A.; et al. The Indian Ocean Experiment: Widespread air pollution from South and Southeast Asia. Science 2001, 291, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Lighty, J.S.; Veranth, J.M.; Sarofim, A.F. Combustion aerosols: Factors governing their size and composition and implications to human health. J. Air Waste Manag. Assoc. 2000, 50, 1565–1618. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, P.V.; Reid, J.S.; Kotchenruther, R.A.; Ferek, R.J.; Weiss, R. Direct radiative forcing by smoke from biomass burning. Science 1997, 275, 1776–1778. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, E.; Johansson, C.; Johansson, L.; Swietlicki, E.; Brorström-Lundén, E. Is levoglucosan a suitable quantitative tracer for wood burning? Comparison with receptormodeling on trace elements in Lycksele, Sweden. J. Air Waste Manag. 2006, 56, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Saarikoski, S.K.; Sillanpää, M.K.; Saarnio, K.M.; Hillamo, R.E.; Pennanen, A.S.; Salonen, R.O. Impact of biomass combustion on urban fine particulate matter in Central and Northern Europe. Water Air Soil Pollut. 2008, 191, 265–277. [Google Scholar] [CrossRef]
- Graham, B.; Bracero, M.O.L.; Guyon, P.; Roberts, G.C.; Decesari, S.; Facchini, M.C.; Artaxo, P.; Maenhaut, W.; Koll, P.; Andreae, M.O. Watersoluble organic compounds in biomass burning aerosols over Amazonia 1. characterization by NMR and GC–MS. J. Geophys. Res. Atmos. 2002, 107, 14–16. [Google Scholar] [CrossRef]
- Robinson, A.L.; Donahue, N.M.; Shrivastava, M.K.; Weitkamp, E.A.; Sage, A.M.; Grieshop, A.P.; Lane, T.E.; Pierce, J.R.; Pandis, S.N. Rethinking organic aerosols: Semivolatile emissions and photochemical aging. Science 2007, 315, 1259–1262. [Google Scholar] [CrossRef]
- Bracero, M.O.L.; Guyon, P.; Graham, B.; Roberts, G.; Andreae, M.O.; Decesari, S.; Facchini, M.C.; Fuzzi, S.; Artaxo, P. Water-soluble organic compounds in biomass burning aerosols over Amazonia. 2. Apportionment of the chemical composition and importance of the polyacidic fraction. J. Geophys. Res. 2002, 107, 8091. [Google Scholar] [CrossRef]
- Gao, S.; Hegg, D.A.; Hobbs, P.V.; Kirchstetter, T.W.; Magi, B.I.; Sadilek, M. Water-soluble organic components in aerosols associated with savanna fires in Southern Africa: Identification, evolution, and distribution. J. Geophys. Res. Atmos. 2003, 108, D13. [Google Scholar] [CrossRef]
- Agarwal, S.; Aggarwal, S.G.; Okuzawa, K.; Kawamura, K. Size distributions of dicarboxylic acids, ketoacids, α-dicarbonyls, sugars, WSOC, OC, EC and inorganic ions in atmospheric particles over northern Japan: Implication for long-range transport of Siberian biomass burning and East Asian polluted aerosols. Atmos. Chem. Phys. 2010, 10, 5839–5858. [Google Scholar] [CrossRef]
- Tsai, Y.I.; Wu, P.L.; Hsu, Y.T.; Yang, C.R. Anhydrosugar and sugar alcohol organic markers associated with carboxylic acids in particulate matter from incense burning. Atmos. Environ. 2010, 44, 3708–3718. [Google Scholar] [CrossRef]
- Chang, C.H.; Liu, C.C.; Tseng, P.Y. Emissions inventory for rice Straw open burning in Taiwan based on burned area classification and mapping using formosat-2 satellite imagery. Aerosol. Air Qual. Res. 2013, 13, 474–487. [Google Scholar] [CrossRef]
- Simoneit, B.R. A review of biomarker compounds as source indicators and tracers for air pollution. Environ. Sci. Pollut. Res. 1999, 6, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Engling, G.; Lee, J.J.; Tsai, Y.W.; Lung, S.C.C.; Chou, C.C.K.; Chan, C.Y. Size-resolved anhydrosugar composition in smoke aerosol from controlled field burning of rice straw. Aerosol. Sci. Technol. 2009, 43, 662–672. [Google Scholar] [CrossRef]
- Kawamura, K.; Ikushima, K. Seasonal changes in the distribution of dicarboxylic acids in the urban atmosphere. Environ. Sci. Technol. 1993, 27, 2227–2235. [Google Scholar] [CrossRef]
- Kawamura, K.; Kaplan, I.R. Motor exhaust emissions as a primary source for dicarboxylic acids in Los Angeles ambient air. Environ. Sci. Technol. 1987, 21, 105–110. [Google Scholar] [CrossRef]
- Wang, Z.; Bi, X.; Sheng, G.; Fu, J. Characterization of organic compounds and molecular tracers from biomass burning smoke in south China I: Broad-leaf trees and shrubs. Atmos. Environ. 2009, 43, 3096–3102. [Google Scholar] [CrossRef]
- Popovicheva, O.; Kistler, M.; Kireeva, E.; Persiantseva, N.; Timofeev, M.; Kopeikin, V.; Kasper-Giebl, A. Physicochemical characterization of smoke aerosol during large-scale wildfires: Extreme event of August 2010 in Moscow. Atmos. Environ. 2014, 96, 405–414. [Google Scholar] [CrossRef]
- Ho, K.F.; Lee, S.C.; Cao, J.J.; Kawamura, K.; Watanabe, T.; Cheng, Y.; Chow, J.C. Dicarboxylic acids, ketocarboxylic acids and dicarbonyls in the urban roadside area of Hong Kong. Atmos. Environ. 2006, 40, 3030–3040. [Google Scholar] [CrossRef]
- Andreae, M.O. Soot Carbon and Excess Fine Potassium: Long-Range Transport of Combustion-Derived Aerosols. Science 1993, 220, 1148–1151. [Google Scholar] [CrossRef]
- Waston, J.G.; Chow, J.C.; Houck, J.E. PM2.5 Chemical Source Profiles for Vehicle Exhaust, Vegetative Burning, Geological Materials, and Coal Burning in Northwestern Colorado During 1995. Chemosphere 2001, 43, 1141–1151. [Google Scholar]
- Fondo Sectorial para la Investigación, el Desarrollo y la Innovación Tecnológica Forestal. CONAFOR-CONACyT. 2016–2018. Available online: https://www.conacyt.gob.mx/index.php/fondos-sectoriales-constituidos2/item/conafor-conacyt (accessed on 20 November 2019).
- Stone, E.A.; Snyder, D.C.; Sheesley, R.J.; Sullivan, A.P.; Weber, R.J.; Schauer, J.J. Source apportionment of fine organic aerosol in Mexico City during the MILAGRO experiment. Atmos. Chem. Phys. 2006, 8, 1249–1259. [Google Scholar] [CrossRef]
- Mancilla, Y.; Mendoza, A.; Fraser, M.P.; Herckes, P. Organic composition and source apportionment of fine aerosol at Monterrey, Mexico, based on organic markers. Atmos. Chem. Phys. 2016, 16, 953–970. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística y Geografía. Censo de Población y Vivienda. 2010. Available online: https://www.inegi.org.mx/contenidos/programas/ccpv/2010/doc/cpv2010_cuest_basico.pdf (accessed on 18 September 2019).
- Appendix A to Part 58–Quality Assurance Requirements for Monitors used in Evaluations of National Ambient Air Quality Standards. Available online: https://www3.epa.gov/ttnamti1/files/ambient/longpath/fropenph.pdf (accessed on 11 September 2019).
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education: Harlow, UK, 2010; pp. 124–126. [Google Scholar]
- Draxler, R.R.; Rolph, G.D. HYSPLIT—Hybrid Single-Particle Lagrangian Integrated Trajectory Model. Available online: http://www.arl.noaa.gov/HYSPLIT.php (accessed on 11 March 2019).
- Saffari, A.; Daher, N.; Samara, C.; Voutsa, D.; Kouras, A.; Manoli, E.; Karagkiozidou, O.; Vlachokostas, C.; Moussiopoulos, N.; Shafer, M.M.; et al. Increased biomass burning due to the economic crisis in Greece and its adverse impact on wintertime air quality in Thessaloniki. Environ. Sci. Technol. 2013, 47, 13313–13320. [Google Scholar] [CrossRef] [PubMed]
- Mikuska PKubatkov, N.; Krumal, K.K.; Vecera, Z. Seasonal variability of monosaccharide anhydrides, resin acids, methoxyphenols and saccharides in PM2.5 in Brno, the Czech Republic. Atmos. Pollut. Res. 2017, 8, 576–586. [Google Scholar] [CrossRef]
- Kawamura, K.; Sakaguchi, F. Molecular distribution of water soluble dicarboxylic acids in marine aerosols over the Pacific Ocean including tropics. J. Geophys. Res. 1999, 104, 3501–3509. [Google Scholar] [CrossRef]
- Zhao, W.; Kawamura, K.; Yue, S.; Wei, L.; Ren, H.; Yan, Y.; Kang, M.; Li, L.; Ren, L.; Lai, S.; et al. Molecular distribution and compound-specific stable carbon isotopic composition of dicarboxylic acids, oxocarboxylic acids and α-dicarbonyls in PM2.5 from Beijing, China. Atmos. Chem. Phys. 2018, 18, 2749–2767. [Google Scholar] [CrossRef]
- Kawamura, K.; Kasukabe, H.; Barrie, L.A. Source and reaction pathways of dicarboxylic acids, ketoacids and dicarbonyls in arctic aerosols: One year of observations. Atmos. Environ. 1996, 30, 1709–1722. [Google Scholar] [CrossRef]
- Yttri, K.E.; Dye, C.; Kiss, G. Ambient aerosol concentrations of sugars and sugars-alcohols at four different sites in Norway. Atmos. Chem. Phys. 2007, 7, 4267–4279. [Google Scholar] [CrossRef]
- Engling, G.; Zhang, Y.N.; Chan, C.Y.; Sang, X.F.; Lin, M.; Ho, K.F.; Li, Y.S.; Lin, C.Y.; Lee, J.J. Characterization and sources of aerosol particles over the southeastern Tibetan Plateau during the Southeast Asia biomass-burning season. Tellus B 2011, 63, 117–128. [Google Scholar] [CrossRef]
- Ion, A.C.; Vermeylen, R.; Kourtchev, I.; Cafmeyer, J.; Chi, X.; Gelencsér, A.; Maenhaut, W.; Claeys, M. Polar organic compounds in rural PM2.5 aerosols from K-puszta, Hungary, during a 2003 summer field campaign: Sources and diel variations. Atmos. Chem. Phys. 2005, 5, 1805–1814. [Google Scholar] [CrossRef]
- Oliveira, C.; Pio, C.; Alves, C.; Evtyugina, M.; Santos, P.; Gonçalves, V.; Nunes, T.; Silvestre, A.J.; Palmgren, F.; Wåhlin, P.; et al. Seasonal distribution of polar organic compounds in the urban atmosphere of two large cities from the north and south of Europe. Atmos. Environ. 2007, 41, 5555–5570. [Google Scholar] [CrossRef]
- Reche, C.; Viana, M.; Amato, F.; Alastuey, A.; Moreno, T.; Hillamo, R.; Teinilä, K.; Saarnio, K.; Seco, R.; Peñuelas, J.; et al. Biomass burning contributions to urban aerosols in a coastal Mediterranean City. Sci. Total Environ. 2012, 427–428, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Russell, A.; Edgerton, E.S. Atmosphericaerosol over two urban–rural pairs in the southeastern United States: Chemical composition and possible sources. Atmos. Environ. 2005, 39, 4453–4470. [Google Scholar] [CrossRef]
- Balla, D.; Voutsa, D.; Samara, C. Study of polar organic compounds in airborne particulate matter of a coastal urban city. Environ. Sci. Pollut. Res. 2018, 25, 12191–12205. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Bacco, D. GC–MS analysis of water-soluble organics in atmospheric aerosol: Response surface methodology for optimizing silyl-derivatization for simultaneous analysis of carboxylic acids and sugars. Anal. Chim. Acta 2011, 689, 257–264. [Google Scholar] [CrossRef]
- Ho, K.F.; Lee, S.C.; Cao, J.J.; Li, Y.S.; Chow, J.C.; Watson, J.G.; Fung, K. Variability of organic and elemental carbon, water soluble organic carbon, and isotopes in Hong Kong. Atmos. Chem. Phys. 2006, 6, 4569–4576. [Google Scholar] [CrossRef]
- Wang, G.; Huang, L.; Gao, S.; Gao, S.; Wang, L. Characterization of water-soluble species of PM10 and PM2.5 aerosols in urban area in Nanjing, China. Atmos. Environ. 2002, 36, 1299–1307. [Google Scholar] [CrossRef]
- Choi, N.R.; Lee, S.P.; Lee, J.Y.; Jung, C.H.; Kim, Y.P. Speciation and source identification of organic compounds in PM 10 over Seoul, South Korea. Chemosphere 2016, 144, 1589–1596. [Google Scholar] [CrossRef]
- Wagener, S.; Langner, M.; Hansen, U.; Moriske, H.J.; Endlicher, W.R. Spatial and seasonal variations of biogenic tracer compounds in ambient PM10 and PM1 samples in Berlin, Germany. Atmos. Environ. 2012, 47, 33–42. [Google Scholar] [CrossRef]
- Zhang, X.; Hecobian, A.; Zheng, M.; Frank, N.H.; Weber, R.J. Biomass burning impact on PM 2.5 over the southeastern US during 2007: Integrating chemically speciated FRM filter measurements, MODIS fire counts and PMF analysis. Atmos. Chem. Phys. 2010, 10, 6839–6853. [Google Scholar] [CrossRef]
- Locker, H.B. The Use of Levoglucosan to Assess the Environmental Impact of Residential Wood Burning on Air Quality. Ph.D. Thesis, Dartmouth College, Hanover, NH, USA, 1988; p. 137. [Google Scholar]
- Simoneit, B.R.T.; Elias, V.O.; Kobayashi, M.; Kawamura, K.; Rushdi, A.I.; Medeiros, P.M.; Rogge, W.F.; Didyk, B.M. Sugars—Dominant water-soluble organic compounds in soils and characterization as tracers in atmospheric participate matter. Environ. Sci. Technol. 2004, 38, 5939–5949. [Google Scholar] [CrossRef] [PubMed]
- Urban, R.C.; Alves, C.A.; Allen, A.G.; Cardoso, A.A.; Queiroz, M.E.; Campos, M.L. Sugar markers in aerosol particles froman agro-industrial region in Brazil. Atmos. Environ. 2014, 90, 106–112. [Google Scholar] [CrossRef]
- Kawamura, K.; Yasui, O. Diurnal changes in the distribution of dicarboxylic acids, ketocarboxylic acids and dicarbonyls in the urban Tokyo atmosphere. Atmos. Environ. 2005, 39, 1945–1960. [Google Scholar] [CrossRef]
- Belis, C.A.; Favez, O.; Mircea, M.; Diapouli, E.; Manousakas, M.-I.; Vratolis, S.; Gilardoni, S.; Paglione, M.; Decesari, S.; Mocnik, G.; et al. European Guide on Air Pollution Source Apportionment with Receptor Models–Revised Version 2019, EUR 29816 EN; Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-76-09001-4. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).