Abstract
Bioaerosol characterization represents a major challenge for the risk assessment and management of exposed people. One of the most important bioaerosol sources is the organic waste collection and treatment. This work analyzed and discussed the literature with the purpose of investigating the main techniques used nowadays for bioaerosol monitoring during organic waste treatment. The discussion includes an overview on the most efficient sampling, DNA extraction, and analysis methods, including both the cultural and the bio-molecular approach. Generally, an exhaustive biological risk assessment is not applied due to the organic waste heterogeneity, treatment complexity, and unknown aerosolized emission rate. However, the application of bio-molecular methods allows a better bioaerosol characterization, and it is desirable to be associated with standardized cultural methods. Risk assessment for organic waste workers generally includes the evaluation of the potential exposition to pathogens and opportunistic pathogens or to other microorganisms as biomarkers. In most cases, Saccharopolyspora rectivirgula, Legionella spp., Aspergillus spp., and Mycobacterium spp. are included. Future perspectives are focused on identifying common composting biomarkers, on investigating the causality process between chronic bioaerosol exposure and disease onset, and finally, on defining common exposure limits.
1. Introduction
The rapid growth of the world population has led to an impressive increase of waste destined for landfill and to a resulting increase in greenhouse gas emissions. The European Union committed to reducing the total quantity of waste destined for landfill [1,2]. Wide margins for the reduction of such environmental impact can be identified, especially by the separation of the waste according to its nature [3]. Organic waste (OW) can be treated through biological transformations, by aerobic process alone, which produces an improver used as soil fertilizer, or by an anaerobic process that produces also energy. Moreover, OW can be treated by aerobic and anaerobic processes in the same plant [4]. According to ISPRA (Istituto Superiore per la Protezione e la Ricerca Ambientale) in 2018, the organic waste treatment plants (OWTPs), that integrate anaerobic digestion to the aerobic phase, are more than the sites that use only the biomethanization (35 plants against 23, respectively) [5]. Pearson et al. estimated that each ton of food waste diverted from landfill to composting plants reduces greenhouse gas emissions by the equivalent of 0.4 to 0.7 tons of carbon dioxide (CO2) [6]. For example, in 2018, 7,079,800 tons of organic waste were collected in the Italian territory, showing a 24% increase since 2014. Moreover, wide improvements could still be implemented [5].
The treatment of the OW presents though some disadvantages, among them, bioaerosol production. This is a suspension of biological pollutants also defined as primary biological airborne particles (PBAPs) with an equivalent aerodynamic diameter of 0.05–100 µm [7,8]. Bioaerosol exposure is associated with the onset of respiratory diseases as well as enteric diseases and skin and eyes inflammation processes. In 2018, the first case of hypersensitivity pneumonitis correlated with bioaerosol exposure in a composting facility was identified [9]. However, bioaerosol composition and the actual risk for human health are still unclear [10]. Nowadays, this emerging field of research is collecting interesting evidence all over the world, and the literature reports numerous papers on the bioaerosol characterization in composting facilities. Moreover, recent publications included reviews that aimed at defining the overall dose–response correlation between bioaerosol exposure and the onset of health effects. Environments such as intensive breeding plants [11] or waste water treatment plants [12,13] were especially examined. However, it is not yet possible to define shared exposure limit values. One of the main reasons for this is that nowadays results are still not comparable due to numerous factors such as the lack of data that describe the background contamination, the heterogeneity of the experimental design, the poor efficiency and the comparability of sampling methods, and above all, the lack of standardized analysis protocols. For those purposes, this work analyzed the literature with the purpose of investigating and discussing the main techniques used for the study of bioaerosol produced by OWTPs. The focus was placed on the currently most common treatments of composting alone and composting integrated with biomethanization.
2. State of the Art
The following analysis shows the background knowledge on bioaerosol contamination, composting process, anaerobic digestion, and human health risk assessment.
2.1. Bioaerosol
Bioaerosols are small airborne particles (ranging from 0.001 to 100 μm) with a biological origin and that can contain pathogenic and/or non-pathogenic microorganisms, dead or alive, as well as parts of such living organisms [14]. Bioaerosols include microorganisms such as bacteria, fungi, and viruses, as well as fragments and released molecules like endotoxin, 1-3 β-glucans, and spores [14]. Exposure to such biomolecules is a risk factor for respiratory system diseases, including aspergillosis, asthma, rhinitis, and chronic obstructive pulmonary disease (COPD). Moreover, the exposure can also cause gastrointestinal disorders and skin and eye inflammation. Human infections can be due to pathogenic microorganisms such as adenovirus (AdV), Legionella spp., Bacillus spp., Aspergillus spp., and opportunistic microorganisms such as Pseudomonas aeruginosa and Aeromonas spp. [15]. Generally, Firmicutes, Proteobacteria, and Bacteroidetes are the dominant microorganisms that can be found in compost. Moreover, the selection of sporulating species during the aerosolization could explain the dominance of Firmicutes and Actinobacteria. Finally, the most represented microorganisms in compost bioaerosol are Aspergillus spp., Penicillium spp., Bacillus spp., Thermoactinomyces spp., Thermobifida spp., Saccharomonospora spp., and Saccharopolyspora spp. [9,15,16,17,18,19].
2.2. Composting
The composting is the transformation process conducted in an aerobic environment where OW is converted into humus, an organic matter used as soil fertilizer. The compost quality strongly influences the soil quality. The latter is an important issue included in the United Nations sustainability goals [20,21]. Indeed, compost salinity prevents plants from growing in optimal conditions. To define compost quality, two parameters are observed: maturity and stability [4]. Maturity is the suitability of the compost for a specific use (e.g., ability of plants to grow) while stability is the degree of decomposition of the organic matter. There are no standard methods to measure these two values. Nowadays, the respirometric method is commonly used [22], even if it is suggested as not exhaustive for the compost quality evaluation [23].
The major influencing factors on the compost quality are oxygen intake (0.2–0.6 L/(min × Kg × Organic Matter)), temperature (45–55 °C), humidity (55–65%), pH (7–8), C/N ratio (26–35), particles magnitude, and matrix density [24,25,26]. Before the fermentation phase, a mixture preparation phase occurs. It consists of organic waste mixture and aims at obtaining a homogenous biomass suitable to air penetration, thus to the following aerobic step [27]. The degradation occurs primarily during the fermentation phase, which is divided in three minor stages: mesophilic stage, thermophilic stage, and then cooling. The microbial composition varies while the temperature is changing [28]. During the two mesophilic stages, the active microorganisms are bacteria such as Pseudomonaceae, Erythrobacteraceae, Comamonadaceae, Enterobacteriaceae, Streptomycetaceae, and Caulobacteraceae. During the thermophilic stage, the most represented microorganisms are bacteria such as Firmicutes and Actinobacteria, in particular, Thermoactinomycetaceae, Thermomonosporaceae, and Pseudonocardiaceae. During the maturation phase, bacteria concentration decreases and fungal concentration increases [29,30]. Active bacterial communities are able to adapt in relation to nutriment availability and altered environmental conditions. Moreover, their metabolic activity influences process velocity and efficiency [28]. The complex microbiota of the composting process is one of the major determinants of the compost quality.
The industrial full-scale process of composting could be mainly conducted with 3 different techniques, windrow composting, aerated static piles, and in-vessel composting [31]. Both the plant design and the variables for the compost quality also influence the generated bioaerosol composition. Table 1 shows an overview of the main published results on bioaerosol emitted in OWTPs and their involvement in risk assessment.

Table 1.
Bioaerosol studies in organic waste treatment plants (OWTPs) that presented total bacteria and/or fungi concentrations.
2.3. Biomethanization
Anaerobic digestion represents an alternative or complementary biological method for OW treatment. This technique is in expansion, as it entails many economic advantages due to the production of energy obtained from a renewable source. One of the main differences from composting is that the digestion process is conducted in a closed digester, hence reducing the bioaerosol dispersion [35]. The anaerobic process involves four stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. The final product is an energetic vector called biogas, composed primarily of methane and carbon dioxide. The purified biomethane can be used to produce energy for the partial autonomy of the plant or house heating and fuel for vehicles. Since it is obtained from the biomass, the overall balance of carbon dioxide production and greenhouse gas emissions is zero. During the four phases, the microbial composition varies in relation to the substrate availability. The most represented phyla are Firmicutes (among which the order of Clostridiales), Bacteroidetes, and Actinobacteria [36]. In addition, the methanogens belonging to the Archaea domain are active during the methanogenesis. They are characterized by a longer period of replication, by the necessity of a strictly anaerobic environment, and by the capacity of growing at high temperatures [37]. They do not include known pathogens, but they can indirectly contribute to pathogenic pathways [38]. Table 2 shows an overview of the main published results on bioaerosol emitted during biomethanization and their involvement in risk assessment.

Table 2.
Bioaerosol studies in biomethanization facilities that presented total bacteria and/or fungi concentrations.
2.4. Risk Assessment in OWTPs
The evaluation of the risk assessment is commonly divided into four stages: hazard identification, exposure assessment, dose–response assessment, and risk assessment.
The first phase requires the identification and the quantification of viable microorganisms to which humans can be exposed during the digestion process. The published studies were highly dependent on the design of the experiment and on the focus of the study. One of the major limits is the air sampling method heterogeneity and the lack of a standardized protocol analysis [44]. However, numerous pathogens classified as class 2 were detected [15].
The exposure assessment defines the quality and the quantity of air which a worker is exposed to during the shift, typically lasting 6 to 8 h. It is possible to investigate the exposure through environmental and personal samplings [34,45]. Environmental data are the most frequent. It is also possible to measure biological parameters to ideally identify exposure biomarkers [18,41,43,44,46,47]. This phase has the same limits introduced in the first phase such as lack of bioaerosol composition knowledge and heterogeneity of sampling methods. Moreover, the working-time spent in the various operating areas—also using PPE (personal protective equipment) and CPE (collective protective equipment)—is very variable, and it affects the exposure assessment substantially. The CPE can include environmental air aspiration systems and vehicle air filters.
The third phase of the evaluation requires the determination of a dose–response curve, strongly influenced by a wide number of confounding factors due both to inter-individual variability (genetics, lifestyle, habits, etc.) and bioaerosol composition. Dose–response studies are the result of two approaches: first toxicological and then epidemiological. Regarding the toxicological studies, it is still difficult to determine the effects of bioaerosol on human health in general due to the complexity of such environmental mixture. It is also necessary to describe how the induction process works (biological plausibility), and from this point of view, a large body of evidence is produced in both in vivo and in vitro models [33,42,48,49,50]. Regarding the epidemiological studies, it was possible to demonstrate a statistically significant correlation between bioaerosol exposure and the onset of health effects on human cohorts [6,15]. Unique correlation is not sufficient to demonstrate a causal link though, because there are numerous confounding factors to evaluate; therefore, the Hill’s criteria have to be verified [51]. Moreover, establishing an adequate exposure association with bioaerosol is challenging. Mbareche et al. highlighted that the majority of the studies focus their attention on the environmental quantification of the bioaerosol, while only a few studies are focused on human biomarkers for the intake evaluation [44].
The risk assessment is defined in the fourth phase, using the dose–response curve obtained in the third phase. Nowadays, the characterization of the microbial composition of bioaerosol is a primary indicator of the biological risk, as it allows to identify the airborne pathogens [6,15]. On the other hand, for example, the Global Microbial Contamination Index (GMCI) is proposed to measure the overall microbial pollution in order to define the biological risk [52]. Even if the knowledge has improved in the last years, the absence of an exhaustive bioaerosol characterization, together with the lack of experimental and epidemiological data, does not allow the determination of the effective biological risk which both workers and surrounding communities are exposed to.
Therefore, it was not possible to establish exposure limits accepted worldwide but national committees for occupational and environmental health protection, e.g., BAUA (Germany), DECOS (Noordwijk, The Netherlands), and UK Environment Agency, have determined reference values which workers and nearby residents can be exposed to. For example, in 2013, BAUA set the exposure limits to 10,000 Colony Forming Unit per cubic meter (CFU/m3) for fungi [53]. For the endotoxins exposure, the Health Council of the Netherlands defined that the maximal concentration inhaled from an operator during the 8 h shift, must not exceed 90 Endotoxin Unit per cubic meter (EU/m3) [6,40,44], while in France, the same limit is set at 200 EU/m3 [54]. Moreover, in 2018, the UK Environment Agency proposed the first environmental limits of 1000 CFU/m3 for total bacteria and 500 CFU/m3 for Aspergillus fumigatus [55].
3. Methods and Qualitative Systematic Research
The study was conducted by comparing the methods applied and described by scientific papers in the literature. A bibliographic research was conducted on 3 main different databases online: PubMed, Scopus, and ISI Web of Science. The keywords used for the research were: “bioaerosol”, “organic waste”, “composting plants”, “biomethanization”, “risk assessment”, and “human health”. Firstly, the papers were selected on the basis of the year of publication, starting with papers published from 2016 onwards. Thereafter, papers published before and available online were analyzed and included only if the reported data were significant. The papers were selected because they contained more relevant information on the state of the art for biological risk assessment, including bioaerosol exposure evaluation.
4. Results and Discussion
Bioaerosol characterization in organic waste treatment facilities is still a controversial topic, since the determination of the biological composition is strongly influenced by environmental factors, such as temperature, humidity, season, and prevalent source contamination, as well as technical factors like sampling and analysis methods. It is not yet possible to define a standardized method for bioaerosol characterization, although some identified procedures can lead to a more homogeneous research and could allow a more exhaustive bioaerosol description. The critical points of the pipeline characterization are discussed here. In Figure 1, a summary of the main preliminary steps to conduct a bioaerosol risk assessment is reported.
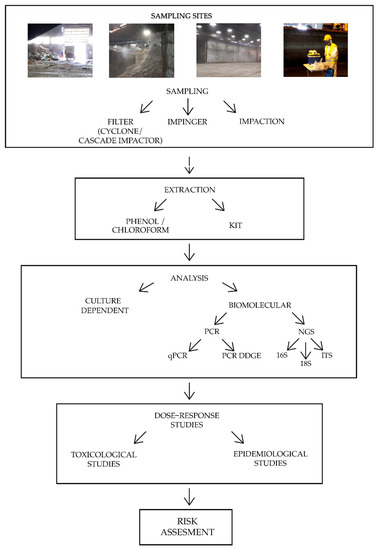
Figure 1.
Summary of the main bioaerosol risk assessment steps. The four pictures at the top represent common sampling sites in organic waste treatment plants. Note: PCR = Polymerase Chain Reaction; qPCR = quantitative Polymerase Chain Reaction; PCR DGGE = Polymerase Chain Reaction Denaturing Gradient Gel Electrophoresis; NGS = Next Generation Sequencing; 16 S = ribosomal region 16S of bacteria; 18 S = ribosomal region 18S of fungi; ITS = Internal Transcribed Spacer.
4.1. Sampling and Extraction Method
Results from exposure studies are extremely influenced by the sampling method. In the last decade, the number of samplers for bioaerosol has increased. Sampling methods commonly used and regulated by international standardized operative procedures are filtration, impaction, liquid impinger, and cyclone [56,57,58].
Filtration consists of pumping air through a porous membrane filter that captures bioaerosol particles. This method can be used both in culture-dependent and culture-independent studies. This sampler is cheap, easy to use, efficient at trapping microorganisms larger than a pore size, and the captured microorganisms remain viable [59]. Samples could be subjected to desiccation due to the flow rate and time; therefore, the spore-forming microorganisms may be preferentially recovered [60]. Generally, a personal sampler runs at a low-flow sampling rate, and only a small volume of air can be sampled on the filter. The sample of bioaerosol is not concentrated enough for an exhaustive evaluation; therefore, the environmental sampling can supply another element for risk assessment [43,61].
Impaction consists in the use of an air pump that captures the air over the surface of a Petri dish containing nutrient agar. Fungal and bacterial samples can be used for culture-dependent studies; it is also easy to use and cheap. The disadvantages are loss of viability and loss of recovery efficiency due to the lack of adherence to the surface by the microorganisms [59].
Liquid impinger captures bioaerosol in a liquid matrix. Samples can be analyzed with culture-dependent and -independent methods. These samplers are made of glass, and they can be easily broken, meaning that they are not easy to use, and they are expensive. Samples could be subjected to evaporation and violent bubbling, which could lead to a loss of sample [60].
Cyclone captures bioaerosol into a liquid matrix using centrifugal force. Samples can be analyzed with culture-dependent and -independent techniques. As observed for the impinger method, one of the main disadvantages is the possible evaporation of the liquid medium and the subsequent loss of sample. Another one is the low quantity of sample collected. Moreover, larger molecules are preferentially collected. This sampler is easy to sterilize [62,63].
Nowadays, researchers are trying to improve the real-time bioaerosol monitoring. The currently used devices only count particles but are not able to classify them. The last improvement of such technique included biochemical determination to detect biological components even if there are a lot of non-biological interferents [64]. The ideal real-time sampler should be portable, have continuous sampling and high collection efficiency, and should continuously deliver samples to the detection system. The slow development in this field is due to the difficulty in determining the concentration and the type of bioaerosols in the air stream. One of the main disadvantages is the misinterpretation due to the signal interference from non-biological particles [65].
The first phase of this study was focused on the comparison of the commonly used sampling methods (Table 3, first section referring to sampling methods), tested both on samples generated in lab and on samples from a composting site. Ferguson et al. observed that Filtration with polycarbonate (PC) filters produces better results in terms of bacterial DNA recovery than Liquid impinger with a difference of an order of magnitude [8]. This is probably due to the easier extraction of the sample from the surface of the filter or to the recovery of particles with a diameter size of less than 0.5 µm, which results extremely difficult with the impinger method. Furthermore, filters in PC are thin; hence, they completely dissolve in phenol/chloroform solution making the extraction lysis process more efficient [8]. Despite the higher DNA extraction, in filtration, the bacteria quantification was scarce and the quality of the DNA was low compared to the Impaction method (Low Melting Agar) [8]. This could be due to the not optimal extraction methods or to the strong dehydration during high flow sampling rate. It is also important to define an ideal flow rate and sampling time in order to reduce the sample loss due to the desiccation. On the other hand, too short sampling times and low flow sampling rates produce an insufficient collection of bioaerosol for proper extraction and analysis. The use of an optimized extraction kit could make this process easier, time-saving, and would also allow the use of a standardized extraction method common for all laboratories [66,67,68].

Table 3.
Sampling, extraction and analysis methods summary [8,10,60,68,69,70,71,72,73,74,75,76,77,78,79,80,81].
4.2. Analytical Methods
The commonly used methods for bioaerosol characterization are culture-dependent and quantitative Polymerase Chain Reaction (qPCR) techniques. Moreover, some studies included Next Generation Sequencing (NGS) of the regions 16S and 18S of rRNA or rDNA (Table 3).
These three methods do not exclude each other; in fact, the use of all of them allows to increase the comprehension of the bioaerosol composition. The cultural method only recognizes 1.5–15.3% of all the species that are able to create a colony, and all the microorganisms that are not viable or unable to grow are not identified [15]. In addition, a metagenomic approach could be theoretically applicable; nowadays, there are no published papers where the metagenomic method was applied to bioaerosol composting samples. Such approach could provide a more exhaustive microbiota characterization including also fungi and viruses, but on the other hand, it is still expensive. Traditional culture-dependent studies require selective culture media that allow the growth of target microorganisms limiting the undesired ones, but in the practice, the biodiversity of the matrix highlights overlapping and unexpected growth.
Bacterial and fungal quantifications are a preliminary good contamination index [15]. As previously stated, various specific targets have been identified such as total bacteria, Legionella pneumophila, Saccharopolyspora rectivirgula [40], and Thermoactinomyces vulgaris [33,80]. These targets were used to compare culture-dependent and quantitative PCR. Betelli et al. observed that there is a linear correlation between the copy number of the region 16S calculated with the qPCR and the number of CFU observed with the cultural method [32]. The culture-independent methods allow to assess the concentration of microorganisms 2 or 3 orders of magnitude higher than the culture-dependent techniques. Indeed, the cultural method estimates only about 15% of the bacteria quantification from Low Melting Agar (LMA) plates studied with qPCR [39]. Therefore, biomolecular methods seem to be more efficient than culture-dependent ones. On the contrary, Shade et al. determined the presence of a fraction of microorganisms and very low concentrations only found with the culture-dependent method [82]. The culture-independent methods allow instead to determine a concentration hundred or thousand times higher than the culture-dependent method. It also allows the identification of a large part of the microorganisms present in the sample, but it does not allow to discern the microorganism’s viability. With the Polymerase Chain Reaction (PCR), a given microorganism is identified through the amplification of a specific region of its nucleic acid. The qPCR technique, based on the measurement of the emitted fluorescence from a target during the amplification process, could also be used for a quantitative result [77].
A major disadvantage of qPCR is that it does not determine cell viability. However, a viability assay that combines qPCR with propidium monoazide (PMA-qPCR) can set alive and dead cells apart. Propidium monoazide, as well as ethidium monoazide, is a DNA dye able to bind free DNA avoiding the subsequent PCR reaction [83]. For this reason, PMA-qPCR was proposed to assess the feasibility of detecting the viability in various samples. The application on bioaerosol is very limited, but the preliminary results showed the general feasibility of PMA-qPCR in the aerobiology and the presence of a high quantity of viable but not cultivable bacteria in the air [84].
The Polymerase Chain Reaction-Denaturing Gradient Gel Electrophoresis (PCR-DGGE) is not commonly used, and it is typically replaced by Next Generation Sequencing (NGS) techniques. However, it allows a primary investigation of the numerically dominant community members, community changes, and differences. It has a scarce reproducibility and sensitivity though. NGS is one of the latest technological innovations introduced in the biomolecular practice. The main techniques used are 454 pyrosequencing and Illumina.
Pyrosequencing is based on an emulsion PCR on microbeads. Each bead covered with DNA amplicons is contained in a plot slightly larger than the beads. On the bottom of the support there is a light detector [26]. Illumina is based on bridge PCR on a glass surface, that increases both the density and number of DNAs that can be monitored at the same time [60,85]. Typically, the sequencing for bioaerosol characterization is based on the analysis of the region 16S or 18S of the rRNA for bacteria and fungi, respectively. Moreover, the ribosomal ITS (Internal Transcribed Spacer) is used as a universal marker for fungal characterization. Viruses do not share conserved genetic regions, and the virome composition is still largely undefined [76,86]. In databases like SILVA—exclusively dedicated to the collection of specific DNA regions—there are millions of full-length sequences of the genes that encode for the region 16S of the 30S subunit of the rRNA. The sequencing of the 16S is based on the study of highly variable regions (V1-V9) with particular attention to the V4-V5 regions, which encode for bacteria [76]. Moreover, it is also possible to conduct whole genome sequencing, allowing a global analysis of the genome sample. It requires high sample quantities, it is expensive, and the bioinformatic analyses are still challenging. On the other hand, it allows a global microbiome characterization (e.g., virome), including taxonomic biodiversity and biological functions information.
Few metagenomic studies on bioaerosol were published. The collection of adequate biomass is crucial for successful metagenomic analysis. Recently, metagenomic analysis of the airborne DNA and RNA were performed in a daycare center, starting from the filters of the ventilation and the air conditioning system [87]. Other studies on bioaerosol composition focused on influenza circulation, they extracted RNA from samples collected with different methods, and then they evaluated the samples with RT-PCR. The results showed viral RNA detection rates >70% but also heterogeneity between the different methods used [88]. In the waste treatment, the sample size is a limiting factor, and the published studies mainly showed only a greater complexity of the dispersed microbiota [40].
The cultural method, especially if preceded by an enrichment phase, is able to identify microorganisms that are present in the environment at very low concentrations, thus allowing the tracing of potentially pathogenic microorganisms. A culture-dependent confirmation and typing with culture-independent techniques are necessary to establish a causal link between the infectious episode and the exposure. The analysis method is truly complete only when both the biomolecular and cultural studies are combined [4].
Matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry has been proposed for microbial identification and diagnosis. This technology has some advantages because it is rapid, sensitive, and relatively economical. Nevertheless, this method has also some limitations. For example, the identification of new isolates is possible only if the spectral database contains peptide mass fingerprints of specific genera/species/subspecies/strains [71]. This is limiting especially for the bioaerosol characterization. In addition, a previous cultural phase on a plate or a pre-treatment phase of the bioaerosol sample is needed [75]. However, MALDI-TOF mass spectrometry has been already used to characterize waste workers’ exposure to bioaerosols during waste collection [74] and for the evaluation of bacterial and fungal species to assess the biological risk which waste collection workers’ are exposed to [69]. MALDI-TOF was also employed to increase our knowledge of the physicochemical and biological characteristics of bioaerosols from composting sites [70].
4.3. Risk Assessment
To perform risk assessments, some scientists decided to investigate the presence of harmful microorganisms for human health using qPCR. Dubuis et al. selected four bacterial biomarkers that have been shown in composting facilities in order to verify their presence also in biomethanization areas: Saccharopolyspora rectivirgula, Legionella spp., Legionella pneumophila, and Mycobacterium spp. [40].
S. rectivirgula is correlated with the onset of the Farmer’s lung disease [15,18]; L. pneumophila causes a serious disease, the Legionnaires’ disease [89]; Mycobacterium spp. can cause bone, skin, and lung infections.
Mbareche et al. focused their attention on the study of fungal composition of bioaerosols emitted from biomethanization sites and decided to use Aspergillus spp. and Aspergillus fumigatus as bioindicators.
The investigation of A. fumigatus is important due to its correlation with pulmonary diseases such as aspergillosis [90].
Moreover, Traversi et al. used specific bioindicators such as Pseudomonaceae to improve the risk assessment in biomethanization plants [43]. Indeed, Pseudomonas is commonly associated with pneumonia and osteoarticular infections [91,92], and it is a biofilm bioindicator, especially in water systems [43]. Nowadays, an exhaustive biological risk assessment is weakly applied because it is strongly influenced by parameters that are difficult to estimate and that are often arbitrarily defined, such as the variability of microorganisms present in the bioaerosol, the rate of bioaerosol released from the matrix, the inter-individual variability of the exposed subjects, etc. In addition, the lack of validated methodology causes a deformity in the evaluation that makes the results incomparable.
Mbareche et al. suggested the creation of a worldwide database containing all the sequences obtained from the sequencing analysis with the description of the sampling and analysis methods used. This would allow a definition of the microbial composition of bioaerosol [44].
4.4. Limitations
The sampling method based on filters is currently the most satisfying, but it requires improvement and standardization of the extraction methods. The biomolecular analysis allows a better bioaerosol characterization, but this technique does not distinguish between viable or non-viable microorganisms, potentially leading to an overestimation. Another important issue for qPCR application is that microorganisms could have more than one copy of the region 16S, as previously demonstrated in the literature [40]. Therefore, the data elaboration has to take into account such evidence whenever there are quantitative purposes.
5. Conclusions
OWTPs produce bioaerosol that represents a not negligible risk for human health. The management systems have to reduce such risk through mitigation strategies. The first step is the monitoring and the real and up-to-date knowledge of the plant contamination, especially for the areas at a high risk of contamination and where the workers could be more exposed. An important issue is how to produce reliable monitoring. The analysis suggests that there is a high heterogeneity among existing methods. The filtration with supporting filters in polycarbonate and the addition of biomolecular methods allow a better characterization of the bioaerosol from organic waste treatment facilities. On the other hand, a culture-dependent confirmation is still essential for pathogens. The DNA extraction is desirably conducted using commercial kits to standardize the method, even though a phenol/chloroform use results in a better lysis process. The biomolecular method allows a better characterization of the bioaerosol from organic waste treatment facilities.
The use of synthetic bioindicators—suggested to date by some European countries—such as fungi, total bacteria, or endotoxins, as well as the evaluation of only organic dust, seems to be a risky choice. A deeper evaluation on robust and reliable bioindicators has to be conducted. It is possible to determine some microbiological targets identified during the composting stages, essentially including total bacteria and total fungi. Moreover, specific targets seem to represent the OWTP bioaerosol risk, such as Aspergillus fumigatus, Legionella pneumophila, and endotoxins [6]. In addition, valuable bioindicators in such environments are Bacillus spp. Bacillus subtilis is one of the most common Bacillus in OWTPs, but more listed microorganisms (at least biohazard 2) can also be identified [93]. Particular attention has to be dedicated to the Enterobacteriaceae when the plant treats risky organic materials such as slaughter wastes and to the Clostridiales when anaerobic digestion is conducted.
Considering the OWTP features, the thermophilic bacteria Thermoactinomycetaceae and Saccharopolyspora rectivirgula can be included in the monitoring. These two bacteria targets grow fast during the fermentation phase, and their concentration is estimated to be lower in the background. Moreover, they are easily aerosolized from the organic biomasses [1].
A global study with a wide metacentric and international activity with the participation of several research groups and organic waste treatment facilities would be desirable using shared techniques for bioaerosol evaluation. The application of standardized protocols that use the most efficient sampling and analysis methods would also be preferred, in order to produce comparable data. Such improvement is necessary to identify a proper set of microorganisms present in all the organic waste treatment sites and to set common exposure limits for the protection of human health, starting from the analysis of the results of toxicological and epidemiological studies. The definition of those parameters would be important for everyone working in organic waste treatment plants.
Nowadays, the actual risk for human health correlated with bioaerosol exposure is still underestimated. In the near future, the use of the metagenomic approach on environmental samples will increase our knowledge, especially on the virome component of the bioaerosol. The viral component is still a black box, and this increases the prospect of comprehension on the disease causative process.
A more widespread monitoring with the collection and the elaboration of the data coming from different OWTPs could enhance the knowledge and contribute to the elaboration of reliable management guidelines as risk mitigation strategies.
Author Contributions
Conceptualization, Methodology, Software, Validation: D.T.; Formal analysis, Investigation: D.T., E.A., E.F. (Elena Franchitti), E.P.; Resources, data curation: D.T.; Writing—original draft preparation: E.F. ( Elena Franchitti); Writing—review and editing: D.T., E.A., E.F. (Elisabetta Fea), E.P.; Project administration, Supervision and funding acquisition: D.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors wish to thank the University of Torino for the support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Robertson, S.; Douglas, P.; Jarvis, D.; Marczylo, E. Bioaerosol exposure from composting facilities and health outcomes in workers and in the community: A systematic review update. Int. J. Hyg. Environ. Health 2019, 222, 364–386. [Google Scholar] [PubMed]
- Council Directive 1999/31/EC on the Landfill. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31999L0031 (accessed on 28 April 2020).
- European Environment Agency. Waste Prevention in Europe: Policies, Status and Trends in 2017; European Environment Agency: Copenhagen, Denmark, 2018. [Google Scholar]
- Cerda, A.; Artola, A.; Font, X.; Barrena, R.; Gea, T.; Sánchez, A. Composting of food wastes: Status and challenges. Bioresour. Technol. 2018, 248, 57–67. [Google Scholar] [CrossRef]
- ISPRA. Municipal Waste Report—313/2019; ISPRA: Rome, Italy, 2019; Volume 313, ISBN 978-88-448-0971-3. [Google Scholar]
- Pearson, C.; Littlewood, E.; Douglas, P.; Robertson, S.; Gant, T.W.; Hansell, A.L. Exposures and Health Outcomes in Relation to Bioaerosol Emissions From Composting Facilities: A Systematic Review of Occupational and Community Studies. J. Toxicol. Environ. Heal. Part. B 2015, 18, 43–69. [Google Scholar]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar]
- Ferguson, R.M.W.; Garcia-Alcega, S.; Coulon, F.; Dumbrell, A.J.; Whitby, C.; Colbeck, I. Bioaerosol biomonitoring: Sampling optimization for molecular microbial ecology. Mol. Ecol. Resour. 2019, 19, 672–690. [Google Scholar] [CrossRef]
- Lal, A.; Akhtar, J.; Pinto, S.; Grewal, H.; Martin, K. Recurrent Pulmonary Embolism and Hypersensitivity Pneumonitis Secondary to Aspergillus, in a Compost Plant Worker: Case Report and Review of Literature. Lung 2018, 196, 553–560. [Google Scholar]
- Duquenne, P. On the identification of culturable microorganisms for the assessment of biodiversity in bioaerosols. Ann. Work Expo. Heal. 2018, 62, 139–146. [Google Scholar]
- Douglas, P.; Hayes, E.T.; Williams, W.B.; Tyrrel, S.F.; Kinnersley, R.P.; Walsh, K.; O’Driscoll, M.; Longhurst, P.J.; Pollard, S.J.T.; Drew, G.H. Use of dispersion modelling for Environmental Impact Assessment of biological air pollution from composting: Progress, problems and prospects. Waste Manag. 2017, 70, 22–29. [Google Scholar]
- Kozajda, A.; Jeżak, K.; Kapsa, A. Airborne Staphylococcus aureus in different environments—A review. Environ. Sci. Pollut. Res. 2019, 26, 34741–34753. [Google Scholar]
- Polus, M.; Mucha, Z. Microbiological hazards in closed facilities at sewage treatment plants. Arch. Environ. Prot. 2019, 45, 58–65. [Google Scholar]
- Kim, K.; Kabir, E.; Jahan, S.A. Airborne bioaerosols and their impact on human health. J. Environ. Sci. 2018, 67, 23–35. [Google Scholar]
- Wéry, N. Bioaerosols from composting facilities—A review. Front. Cell. Infect. Microbiol. 2014, 4, 1–9. [Google Scholar]
- Steger, K.; Sjögren, Å.M.; Jarvis, Å.; Jansson, J.K.; Sundh, I. Development of compost maturity and Actinobacteria populations during full-scale composting of organic household waste. J. Appl. Microbiol. 2007, 103, 487–498. [Google Scholar] [PubMed]
- Williams, B.; Douglas, P.; Roca Barcelo, A.; Hansell, A.L.; Hayes, E. Estimating Aspergillus fumigatus exposure from outdoor composting activities in England between 2005 and 2014. Waste Manag. 2019, 84, 235–244. [Google Scholar] [PubMed]
- Le Goff, O.; Godon, J.J.; Milferstedt, K.; Bacheley, H.; Steyer, J.P.; Wéry, N. A new combination of microbial indicators for monitoring composting bioaerosols. Atmos. Environ. 2012, 61, 428–433. [Google Scholar]
- Gutarowska, B.; Skóra, J.; Stępień, Ł.; Szponar, B.; Otlewska, A.; Pielech-Przybylska, K. Assessment of microbial contamination within working environments of different types of composting plants. J. Air Waste Manag. Assoc. 2014, 65, 466–478. [Google Scholar]
- Resende, J.A.; Silva, V.L.; de Oliveira, T.L.R.; de Oliveira Fortunato, S.; da Costa Carneiro, J.; Otenio, M.H.; Diniz, C.G. Prevalence and persistence of potentially pathogenic and antibiotic resistant bacteria during anaerobic digestion treatment of cattle manure. Bioresour. Technol. 2014, 153, 284–291. [Google Scholar] [CrossRef]
- United Nations. The Sustainable Development Goals; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Barrena Gómez, R.; Vázquez Lima, F.; Sánchez Ferrer, A. The use of respiration indices in the composting process: A review. Waste Manag. Res. 2006, 24, 37–47. [Google Scholar] [CrossRef]
- Oviedo-Ocaña, E.R.; Torres-Lozada, P.; Marmolejo-Rebellon, L.F.; Hoyos, L.V.; Gonzales, S.; Barrena, R.; Komilis, D.; Sanchez, A. Stability and maturity of biowaste composts derived by small municipalities: Correlation among physical, chemical and biological indices. Waste Manag. 2015, 44, 63–71. [Google Scholar]
- Juárez, M.F.; Prähauser, B.; Walter, A.; Insam, H.; Franke-Whittle, I.H. Co-composting of biowaste and wood ash, influence on a microbially driven-process. Waste Manag. 2015, 46, 155–164. [Google Scholar] [CrossRef]
- Kumar, S. Composting of municipal solid waste. Crit. Rev. Biotechnol. 2011, 31, 112–136. [Google Scholar] [PubMed]
- Li, Z.; Lu, H.; Ren, L.; He, L. Experimental and modeling approaches for food waste composting: A review. Chemosphere 2013, 93, 1247–1257. [Google Scholar] [PubMed]
- Gil, M.V.; Carballo, M.T.; Calvo, L.F. Fertilization of maize with compost from cattle manure supplemented with additional mineral nutrients. Waste Manag. 2008, 28, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.; López, M.J.; Suárez-Estrella, F.; Vargas-García, M.C.; López-González, J.A.; Moreno, J. Exploiting composting biodiversity: Study of the persistent and biotechnologically relevant microorganisms from lignocellulose-based composting. Bioresour. Technol. 2014, 162, 283–293. [Google Scholar] [CrossRef]
- Insam, H.; de Bertoldi, M. Microbiology of the Composting Process. In Compost Science and Technology; Elsevier Waste Management Series 8; Elsevier: Amsterdam, The Netherlands, 2007; pp. 32–34. [Google Scholar]
- Sánchez, Ó.J.; Ospina, D.A.; Montoya, S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manag. 2017, 69, 136–153. [Google Scholar] [CrossRef]
- ANPA (Agenzia Nazionale per la Protezione dell’Ambiente). Il Recupero di Sostanza Organica dai Rifiuti per la Produzione di Ammendanti di Qualità; ANPA: Rome, Italy, 2002; Volume 7. [Google Scholar]
- Betelli, L.; Duquenne, P.; Grenouillet, F.; Simon, X.; Scherer, E.; Géhin, E.; Hartmann, A. Development and evaluation of a method for the quantification of airborne Thermoactinomyces vulgaris by real-time PCR. J. Microbiol. Methods 2013, 92, 25–32. [Google Scholar] [CrossRef]
- Chang, M.W.; Lee, C.R.; Hung, H.F.; Teng, K.S.; Huang, H.; Chuang, C.Y. Bioaerosols from a food waste composting plant affect human airway epithelial cell remodeling genes. Int. J. Environ. Res. Public Health 2014, 11, 337–354. [Google Scholar]
- Schlosser, O.; Robert, S.; Debeaupuis, C.; Huyard, A. Inhalable dust as a marker of exposure to airborne biological agents in composting facilities. Waste Manag. 2018, 81, 78–87. [Google Scholar]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar]
- Carballa, M.; Regueiro, L.; Lema, J.M. Microbial management of anaerobic digestion: Exploiting the microbiome-functionality nexus. Curr. Opin. Biotechnol. 2015, 33, 103–111. [Google Scholar]
- Suryawanshi, P.C.; Chaudhari, A.B.; Kothari, R.M. Thermophilic anaerobic digestion: The best option for waste treatment. Crit. Rev. Biotechnol. 2010, 30, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.P.; Conway, P.L.; Schlundt, J. Methanogens in humans: Potentially beneficial or harmful for health. Appl. Microbiol. Biotechnol. 2018, 102, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Anedda, E.; Carletto, G.; Gilli, G.; Traversi, D. Monitoring of Air Microbial Contaminations in Different Bioenergy Facilities Using Cultural and Biomolecular Methods. Int. J. Environ. Res. Public Health 2019, 16, 2546. [Google Scholar] [CrossRef] [PubMed]
- Dubuis, M.E.; M’Bareche, H.; Veillette, M.; Bakhiyi, B.; Zayed, J.; Lavoie, J.; Duchaine, C. Bioaerosols concentrations in working areas in biomethanization facilities. J. Air Waste Manag. Assoc. 2017, 67, 1258–1271. [Google Scholar] [CrossRef]
- Madsen, A.M.; Mårtensson, L.; Schneider, T.; Larsson, L. Microbial dustiness and particle release of different biofuels. Ann. Occup. Hyg. 2004, 48, 327–338. [Google Scholar]
- Timm, M.; Madsen, A.M.; Hansen, J.V.; Moesby, L.; Hansen, E.W. Assessment of the total inflammatory potential of bioaerosols by using a granulocyte assay. Appl. Environ. Microbiol. 2009, 75, 7655–7662. [Google Scholar]
- Traversi, D.; Gorrasi, I.; Pignata, C.; Degan, R.; Anedda, E.; Carletto, G.; Vercellino, G.; Fornasero, S.; Bertino, A.; Filippi, F.; et al. Aerosol exposure and risk assessment for green jobs involved in biomethanization. Environ. Int. 2018, 114, 202–211. [Google Scholar] [CrossRef]
- Mbareche, H.; Morawska, L.; Duchaine, C. On the interpretation of bioaerosol exposuremeasurements and impacts on health. J. Air Waste Manag. Assoc. 2019, 69, 789–804. [Google Scholar]
- Eduard, W.; Heederik, D.; Duchaine, C.; Green, B.J. Bioaerosol exposure assessment in the workplace: The past, present and recent advances. J. Environ. Monit. 2012, 14, 334–339. [Google Scholar] [CrossRef]
- Schlosser, O.; Robert, S.; Debeaupuis, C. Aspergillus fumigatus and mesophilic moulds in air in the surrounding environment downwind of non-hazardous waste landfill sites. Int. J. Hyg. Environ. Health 2016, 219, 239–251. [Google Scholar] [CrossRef]
- Wouters, I.M.; Spaan, S.; Douwes, J.; Doekes, G.; Heederik, D. Overview of Personal Occupational Exposure Levels to Inhalable Dust, Endotoxin, β(1→3)-Glucan and Fungal Extracellular Polysaccharides in the Waste Management Chain. Ann. Occup. Hyg. 2005, 50, 39–53. [Google Scholar] [PubMed]
- Lakey, P.S.J.; Berkemeier, T.; Tong, H.; Arangio, A.M.; Lucas, K.; Pöschl, U.; Shiraiwa, M. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Sci. Rep. 2016, 6, 1–6. [Google Scholar]
- Nayak, A.P.; Green, B.J.; Lemons, A.R.; Marshall, N.B.; Goldsmith, W.T.; Kashon, M.L.; Anderson, S.E.; Germolec, D.R.; Beezhold, D.H. Subchronic exposures to fungal bioaerosols promotes allergic pulmonary inflammation in naïve mice. Clin. Exp. Allergy 2016, 46, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.; Saber, A.T.; Nordly, P.; Kumar, A.; Wallin, H.; Vogel, U. Inflammation but no DNA (deoxyribonucleic acid) damage in mice exposed to airborne dust from a biofu. Scand. J. Work Environ. Health 2008, 34, 278–287. [Google Scholar] [CrossRef]
- Asokan, G.V.; Asokan, V. Bradford Hill’s criteria, emerging zoonoses, and One Health. J. Epidemiol. Glob. Health 2016, 6, 125–129. [Google Scholar]
- Dacarro, C.; Grignani, E.; Lodola, L.; Grisoli, P.; Cottica, D. Proposed microbiological indexes for the assessment of air quality in buildings. G. Ital. Med. Lav. Ergon. 2010, 22, 229–235. [Google Scholar]
- TRBA 400 Handlungsanleitung zur Gefährdungsbeurteilung und für die Unterrichtung der Beschäftigten bei Tätigkeiten mit Biologischen Arbeitsstoffen. Available online: https://www.baua.de/DE/Angebote/Rechtstexte-und-Technische-Regeln/Regelwerk/TRBA/TRBA-400.html (accessed on 28 April 2020).
- Balty, I.; Bertrand, N.; David, C.; Burzoni, S.; Clerc, F.; Duquenne, P.; Simon, X.; Caron, V.; Facon, B.; Renevot, V. Valeurs guides endotoxines. Interprétation des résultats de métrologie des bioaérosols. Hyg. Sécur. Trav. 2015, 239, 46–51. [Google Scholar]
- Environment Agency UK. Bioaerosol Monitoring at Regulated Facilities—Use of M9: RPS 209; Environmental Agency UK: Rotheram, UK, 2018. [Google Scholar]
- EN 13098. Guidelines for Measurement of Airborne Microorganisms and Endotoxin. Available online: https://shop.bsigroup.com/ProductDetail/?pid=000000000030037644 (accessed on 28 April 2020).
- CEN/TS 16115-1. Measurement of Bioaerosols. Part. 1: Determination of Moulds Using Filter Sampling Systems and Culture-Based Analyses. Available online: https://www.sis.se/en/produkter/environment-health-protection-safety/air-quality/ambient-atmospheres/siscents1611512011/ (accessed on 28 April 2020).
- EN 1403. Determination of Airborne Endotoxins. Available online: http://store.uni.com/catalogo/en-14031-2003?josso_back_to=http://store.uni.com/josso-security-check.php&josso_cmd=login_optional&josso_partnerapp_host=store.uni.com/ (accessed on 28 April 2020).
- Griffin, D.W. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin. Microbiol. Rev. 2007, 20, 459–477. [Google Scholar] [CrossRef]
- Yoo, K.; Lee, T.K.; Choi, E.J.; Yang, J.; Shukla, S.K.; Hwang, S.I.L.; Park, J. Molecular approaches for the detection and monitoring of microbial communities in bioaerosols: A review. J. Environ. Sci. 2017, 51, 234–247. [Google Scholar] [CrossRef]
- Gong, J.; Qi, J.; Beibei, E.; Yin, Y.; Gao, D. Concentration, viability and size distribution of bacteria in atmospheric bioaerosols under different types of pollution. Environ. Pollut. 2020, 257, 113485. [Google Scholar]
- Ghosh, B.; Lal, H.; Srivastava, A. Review of bioaerosols in indoor environment with special reference to sampling, analysis and control mechanisms. Environ. Int. 2015, 85, 254–272. [Google Scholar] [PubMed]
- Haig, C.W.; Mackay, W.G.; Walker, J.T.; Williams, C. Bioaerosol sampling: Sampling mechanisms, bioefficiency and field studies. J. Hosp. Infect. 2016, 93, 242–255. [Google Scholar] [PubMed]
- Tian, J.H.; Yan, C.; Nasir, Z.A.; Alcega, S.G.; Tyrrel, S.; Coulon, F. Real time detection and characterisation of bioaerosol emissions from wastewater treatment plants. Sci. Total Environ. 2020, 721, 137629. [Google Scholar] [CrossRef]
- Cho, Y.S.; Hong, S.C.; Choi, J.; Jung, J.H. Development of an automated wet-cyclone system for rapid, continuous and enriched bioaerosol sampling and its application to real-time detection. Sens. Actuators B Chem. 2019, 284, 525–533. [Google Scholar] [PubMed]
- Pilote, J.; Létourneau, V.; Girard, M.; Duchaine, C. Quantification of airborne dust, endotoxins, human pathogens and antibiotic and metal resistance genes in Eastern Canadian swine confinement buildings. Aerobiologia 2019, 35, 283–296. [Google Scholar] [CrossRef]
- Mbareche, H.; Veillette, M.; Bilodeau, G.J.; Duchaine, C. Bioaerosol sampler choice should consider efficiency and ability of samplers to cover microbial diversity. Appl. Environ. Microbiol. 2018, 84, 1–22. [Google Scholar]
- Jiang, W.; Liang, P.; Wang, B.; Fang, J.; Lang, J.; Tian, G.; Jiang, J.; Zhu, T.F. Optimized DNA extraction and metagenomic sequencing of airborne microbial communities. Nat. Protoc. 2015, 10, 768–779. [Google Scholar]
- Madsen, A.M.; Frederiksen, M.W.; Jacobsen, M.H.; Tendal, K. Towards a risk evaluation of workers’ exposure to handborne and airborne microbial species as exemplified with waste collection workers. Environ. Res. 2020, 183, 109177. [Google Scholar]
- Nasir, Z.A.; Rolph, C.; Collins, S.; Stevenson, D.; Gladding, T.L.; Hayes, E.; Williams, B.; Khera, S.; Jackson, S.; Bennett, A.; et al. A controlled study on the characterisation of bioaerosols emissions from compost. Atmosphere 2018, 9, 1–15. [Google Scholar]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 1–16. [Google Scholar]
- Madsen, A.M.; Zervas, A.; Tendal, K.; Nielsen, J.L. Microbial diversity in bioaerosol samples causing ODTS compared to reference bioaerosol samples as measured using Illumina sequencing and MALDI-TOF. Environ. Res. 2015, 140, 255–267. [Google Scholar] [PubMed]
- Cox, J.; Mbareche, H.; Lindsley, W.G.; Duchaine, C. Field sampling of indoor bioaerosols. Aerosol Sci. Technol. 2019, 54, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.; Thilsing, T.; Bælum, J.; Garde, A.H.; Vogel, U. Occupational exposure levels of bioaerosol components are associated with serum levels of the acute phase protein Serum Amyloid A in greenhouse workers. Environ. Health 2016, 15, 1–9. [Google Scholar]
- Druckenmüller, K.; Gärtner, A.; Jäckel, U.; Klug, K.; Schiffels, J.; Günther, K.; Elbers, G. Development of a methodological approach for the characterization of bioaerosols in exhaust air from pig fattening farms with MALDI-TOF mass spectrometry. Int. J. Hyg. Environ. Health 2017, 220, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Núñez, A.; de Paz, G.A.; Rastrojo, A.; García, A.M.; Alcamí, A.; Montserrat Gutiérrez-Bustillo, A.; Moreno, D.A. Monitoring of airborne biological particles in outdoor atmosphere. Part 2: Metagenomics applied to urban environments. Int. Microbiol. 2016, 19, 69–80. [Google Scholar] [PubMed]
- Heid, C.A.; Stevens, J.; Livak, K.J.; Williams, P.M. Real time quantitative PCR. Genome Res. 1996, 6, 986–994. [Google Scholar]
- Mbareche, H.; Brisebois, E.; Veillette, M.; Duchaine, C. Bioaerosol sampling and detection methods based on molecular approaches: No pain no gain. Sci. Total Environ. 2017, 599–600, 2095–2104. [Google Scholar]
- Shendure, J.; Ji, H. Next-generation DNA sequencing. Nat. Biotechnol. 2008, 26, 1135–1145. [Google Scholar]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.I.; McDonald, D.; et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 2018, 16, 410–422. [Google Scholar]
- Torsvik, V.; Daae, F.L.; Sandaa, R.A.; Øvreås, L. Novel techniques for analysing microbial diversity in natural and perturbed environments. J. Biotechnol. 1998, 64, 53–62. [Google Scholar]
- Shade, A.; Hogan, C.S.; Klimowicz, A.K.; Linske, M.; Mcmanus, P.S.; Handelsman, J. Culturing captures members of the soil rare biosphere. Environ. Microbiol. 2012, 14, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Emerson, J.B.; Adams, R.I.; Román, C.M.B.; Brooks, B.; Coil, D.A.; Dahlhausen, K.; Ganz, H.H.; Hartmann, E.M.; Hsu, T.; Justice, N.B.; et al. Schrödinger’s microbes: Tools for distinguishing the living from the dead in microbial ecosystems. Microbiome 2017, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Hung, N.T.; Chen, N.T. Optimization and application of propidium monoazide-quantitative PCR method for viable bacterial bioaerosols. J. Aerosol Sci. 2017, 104, 90–99. [Google Scholar]
- Kircher, M.; Kelso, J. High-throughput DNA sequencing—Concepts and limitations. Bioessays 2010, 32, 524–536. [Google Scholar] [CrossRef]
- Prussin, A.J.; Marr, L.C.; Bibby, K.J. Challenges of studying viral aerosol metagenomics and communities in comparison with bacterial and fungal aerosols. FEMS Microbiol. Lett. 2014, 357, 1–9. [Google Scholar]
- Prussin, A.J.; Torres, P.J.; Shimashita, J.; Head, S.R.; Bibby, K.J.; Kelley, S.T.; Marr, L.C. Seasonal dynamics of DNA and RNA viral bioaerosol communities in a daycare center. Microbiome 2019, 7, 1–14. [Google Scholar]
- Prost, K.; Kloeze, H.; Mukhi, S.; Bozek, K.; Poljak, Z.; Mubareka, S. Bioaerosol and surface sampling for the surveillance of influenza A virus in swine. Transbound. Emerg. Dis. 2019, 66, 1210–1217. [Google Scholar]
- Casati, S.; Conza, L.; Bruin, J.; Gaia, V. Compost facilities as a reservoir of Legionella pneumophila and other Legionella species. Clin. Microbiol. Infect. 2010, 16, 945–947. [Google Scholar]
- Mbareche, H.; Veillette, M.; Dubuis, M.È.; Bakhiyi, B.; Marchand, G.; Zayed, J.; Lavoie, J.; Bilodeau, G.J.; Duchaine, C. Fungal bioaerosols in biomethanization facilities. J. Air Waste Manag. Assoc. 2018, 68, 1198–1210. [Google Scholar]
- Ribera, A.; Benavent, E.; Lora-Tamayo, J.; Tubau, F.; Pedrero, S.; Cabo, X.; Ariza, J.; Murillo, O. Osteoarticular infection caused by MDR Pseudomonas aeruginosa: The benefits of combination therapy with colistin plus β-lactams. J. Antimicrob. Chemother. 2015, 70, 3357–3365. [Google Scholar]
- Park, S.Y.; Park, H.J.; Moon, S.M.; Park, K.H.; Chong, Y.P.; Kim, M.N.; Kim, S.H.; Lee, S.O.; Kim, Y.S.; Woo, J.H.; et al. Impact of adequate empirical combination therapy on mortality from bacteremic Pseudomonas aeruginosa pneumonia. BMC Infect. Dis. 2012, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Ecker, D.J.; Sampath, R.; Willett, P.; Wyatt, J.R.; Samant, V.; Massire, C.; Hall, T.A.; Hari, K.; McNeil, J.A.; Büchen-Osmond, C.; et al. The Microbial Rosetta Stone Database: A compilation of global and emerging infectious microorganisms and bioterrorist threat agents. BMC Microbiol. 2005, 5, 1–17. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).