Significant Contribution of Primary Sources to Water-Soluble Organic Carbon During Spring in Beijing, China
Abstract
1. Introduction
2. Methods
2.1. Sampling Site and High Time-Resolved Measurement
2.2. Source Apportionment of WSOC by Positive Matrix Factorization (PMF) Model
2.3. Estimation of Secondary Organic Carbon (SOC) by the EC-Tracer Method
3. Results and Discussion
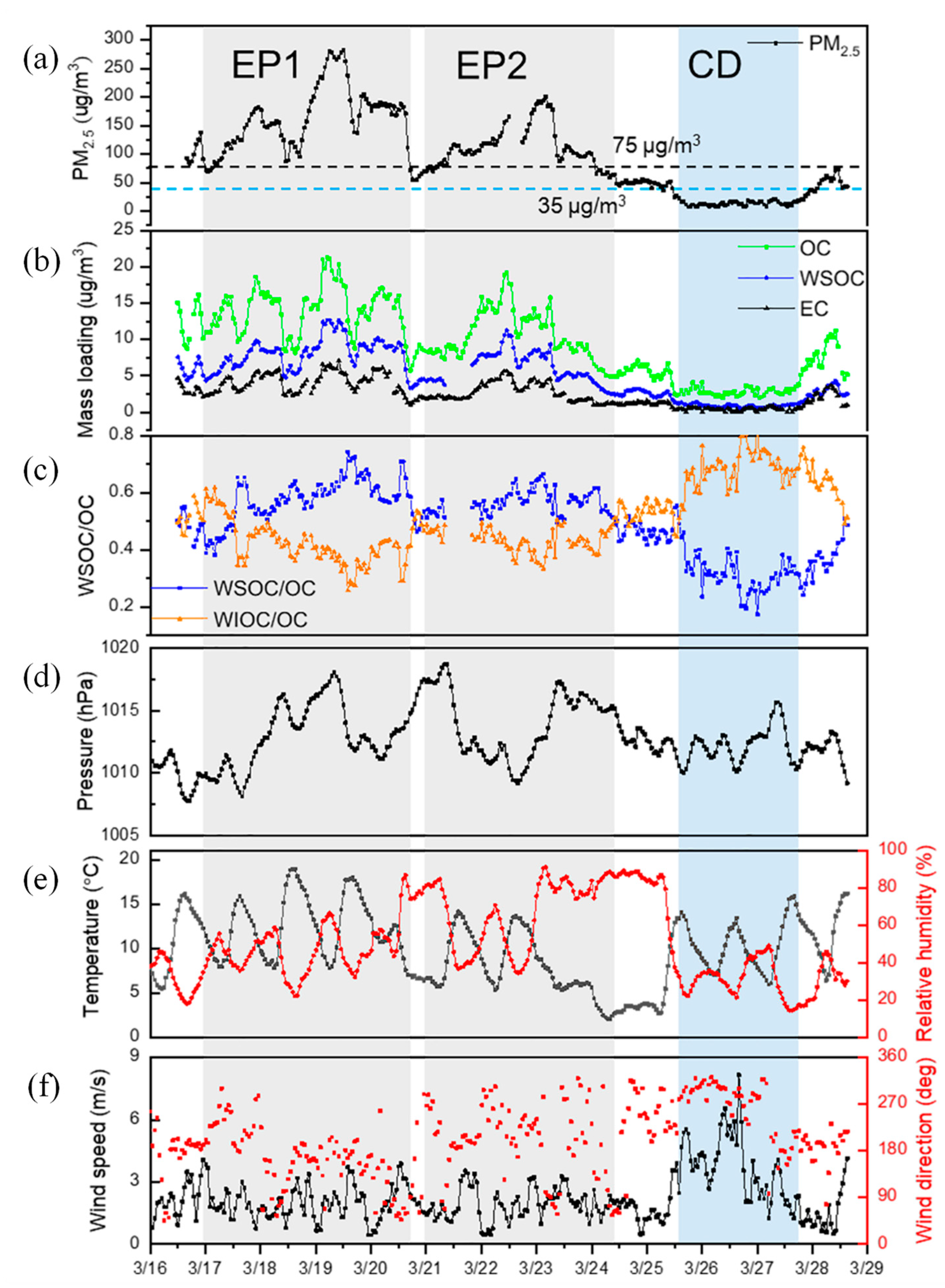
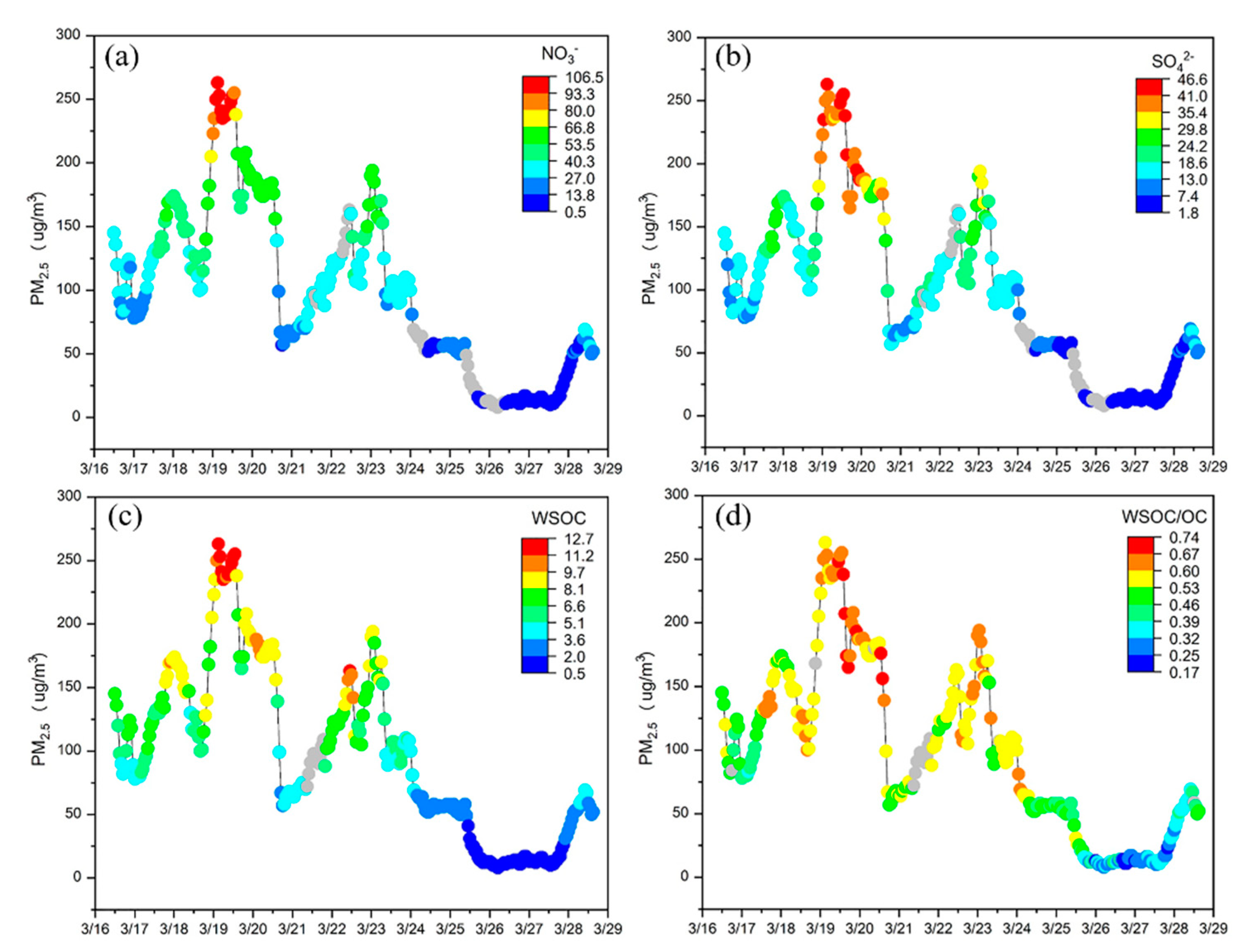
3.1. Characteristics of WSOC During the Study Period
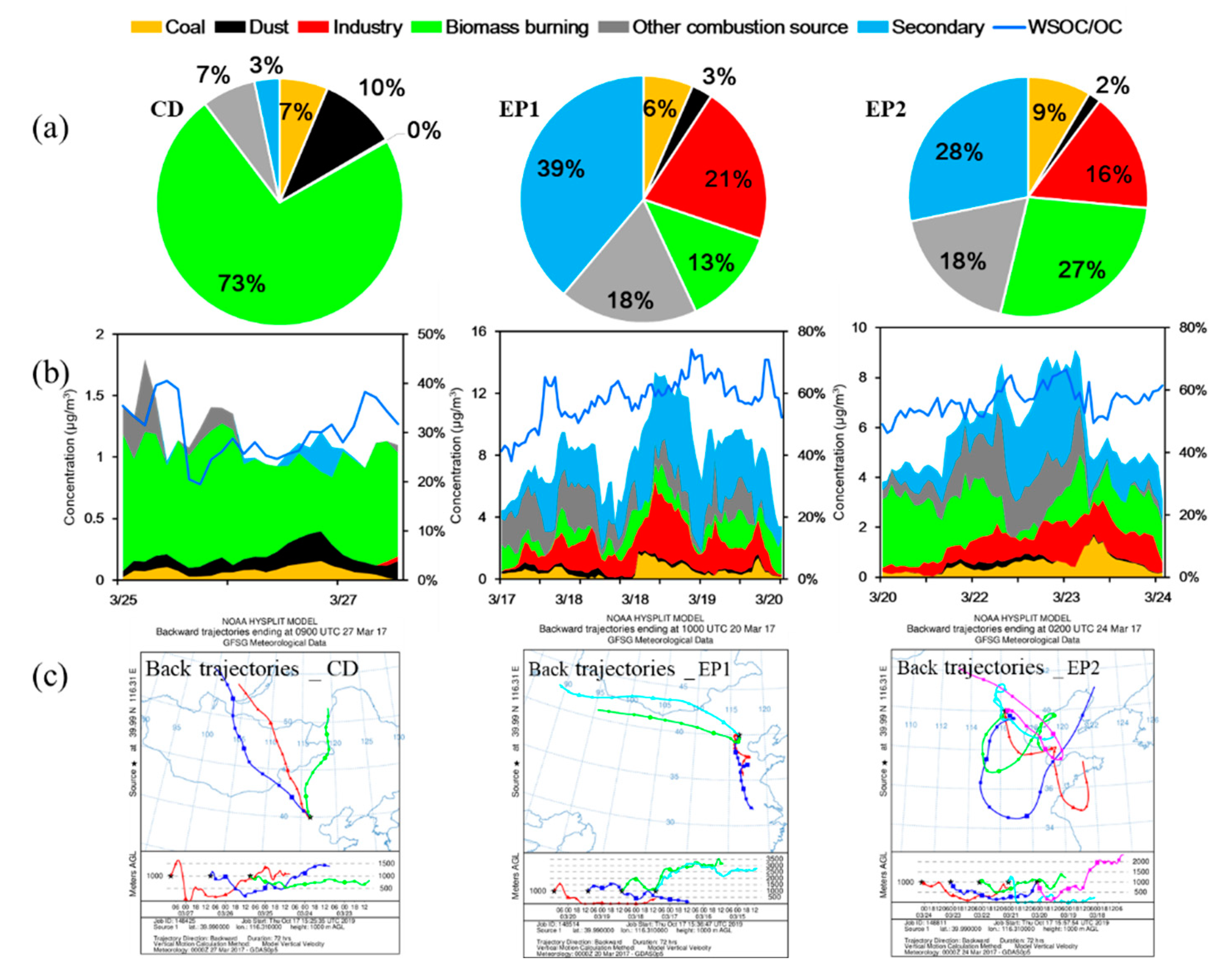
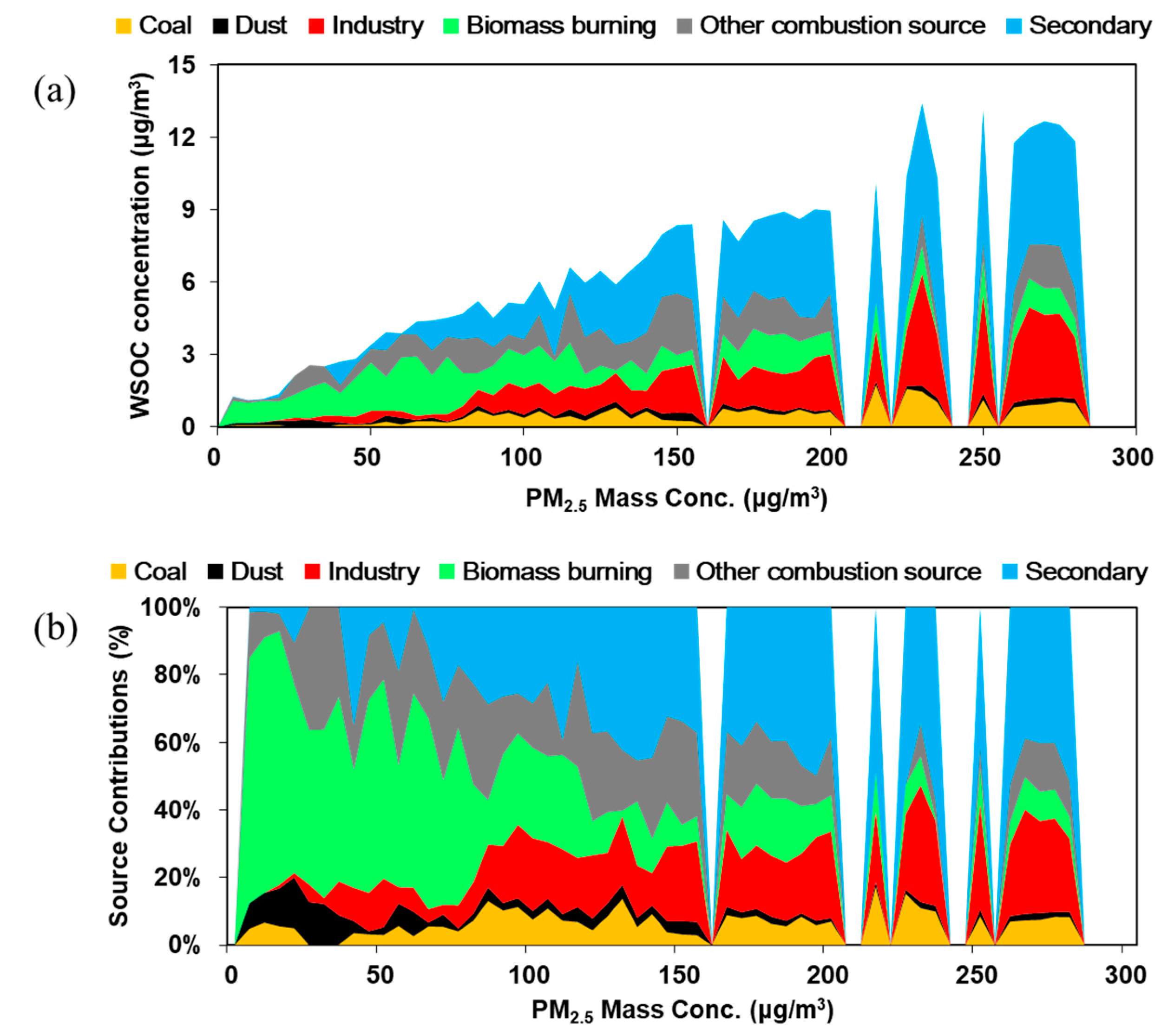
3.2. Significant Contribution of Primary Sources to WSOC
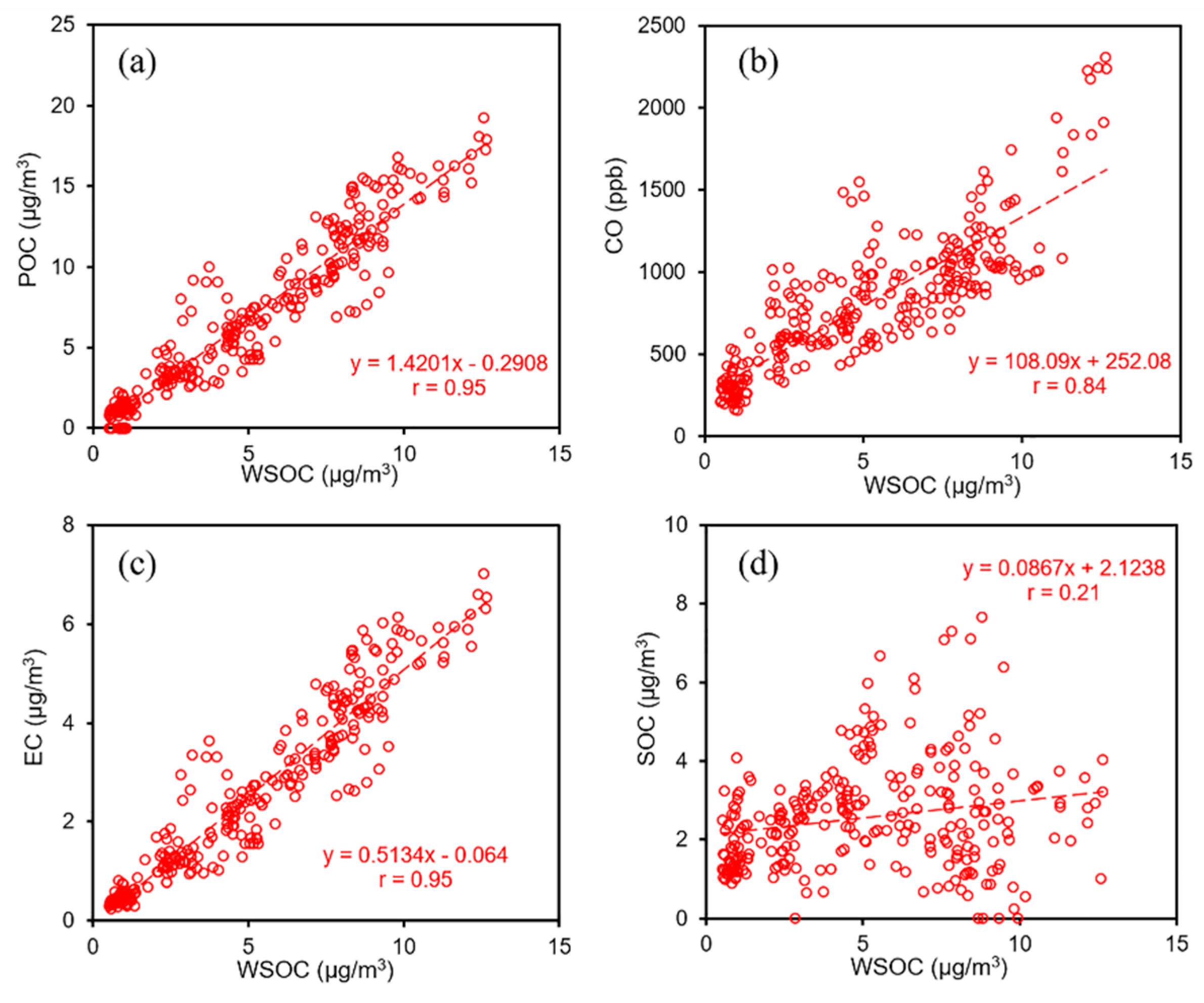
3.2.1. WSOC Highly Correlated with Primary Tracers
3.2.2. Primary Sources of WSOC Determined by the PMF Model
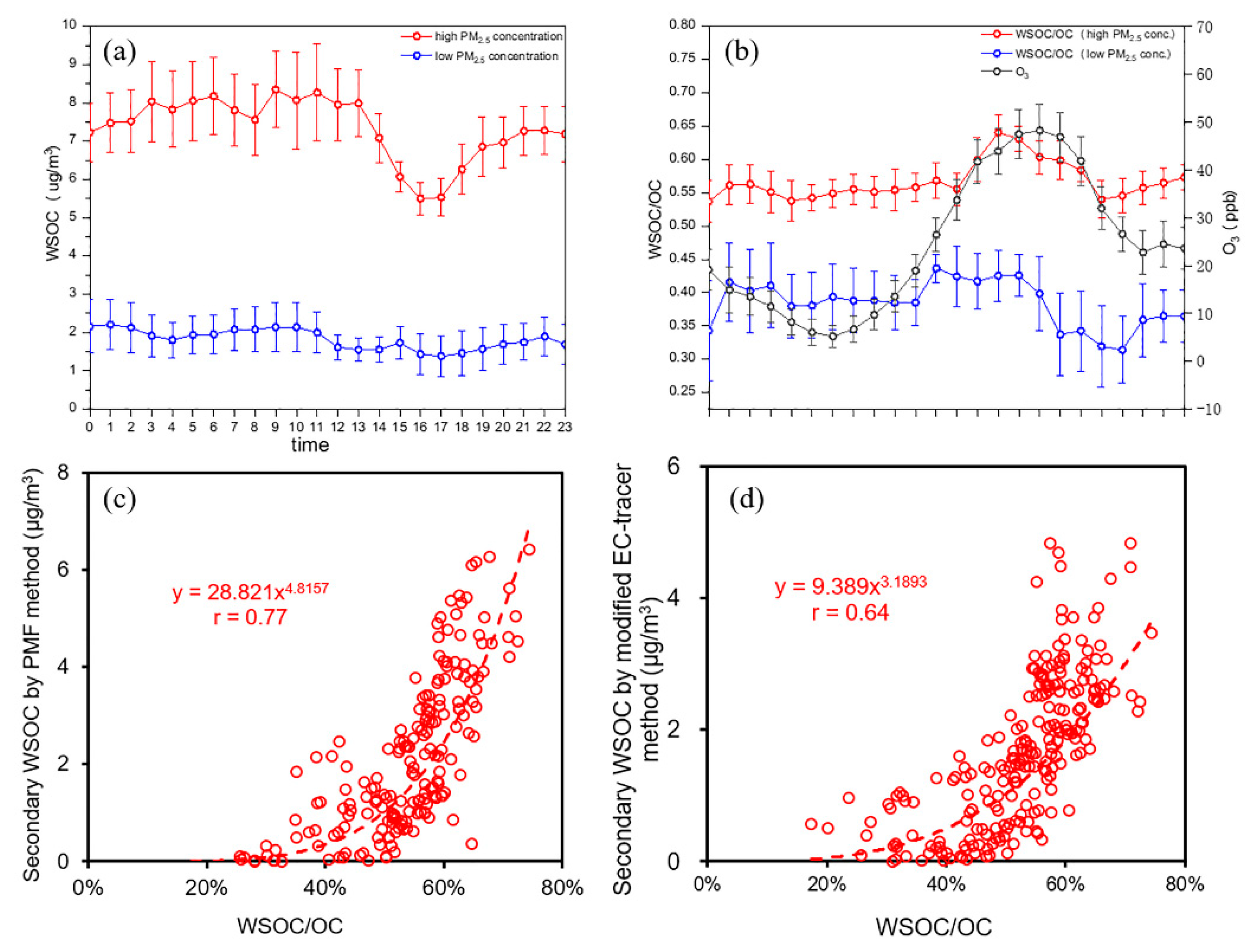
3.3. Increased Secondary Source of WSOC during Haze Episodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kleeman, M.J.; Cass, G.R.; Eldering, A. Modeling the airborne particle complex as a source-oriented external mixture. J. Geophys. Res. Atmos. 1997, 102, 21355–21372. [Google Scholar] [CrossRef]
- Zhang, Q.; Jimenez, J.-L.; Canagaratna, M.R.; Allan, J.D.; Coe, H.; Ulbrich, I.; Alfarra, M.R.; Takami, A.; Middlebrook, A.; Sun, Y.; et al. Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes. Geophys. Res. Lett. 2007, 34, L13801. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, J.; Wang, S.; He, K.; Zheng, M. Review of receptor-based source apportionment research of fine particulate matter and its challenges in China. Sci. Total Environ. 2017, 586, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, P.; Kang, S.; Yan, F.; Hu, Z.; Qu, B.; Sillanpää, M. Concentrations and light absorption characteristics of carbonaceous aerosol in PM 2.5 and PM 10 of Lhasa city, the Tibetan Plateau. Atmos. Environ. 2016, 127, 340–346. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, X.; Wang, Z.; Ci, Z. Water-soluble organic carbon (WSOC) and its temperature-resolved carbon fractions in atmospheric aerosols in Beijing. Atmos. Res. 2016, 181, 200–210. [Google Scholar] [CrossRef]
- Xiang, P.; Zhou, X.; Duan, J.; Tan, J.; He, K.; Yuan, C.; Ma, Y.; Zhang, Y. Chemical characteristics of water-soluble organic compounds (WSOC) in PM2.5 in Beijing, China: 2011–2012. Atmos. Res. 2017, 183, 104–112. [Google Scholar] [CrossRef]
- Asa-Awuku, A.; Engelhart, G.J.; Lee, B.H.; Pandis, S.N.; Nenes, A. Relating CCN activity, volatility, and droplet growth kinetics of β-caryophyllene secondary organic aerosol. Atmos. Chem. Phys. 2009, 9, 795–812. [Google Scholar] [CrossRef]
- Boreddy, S.K.R.; Kawamura, K.; Mkoma, S.; Fu, P.Q. Hygroscopic behavior of water-soluble matter extracted from biomass burning aerosols collected at a rural site in Tanzania, East Africa. J. Geophys. Res. Atmos. 2014, 119, 12233–12245. [Google Scholar] [CrossRef]
- Hallar, A.G.; Lowenthal, D.H.; Clegg, S.L.; Samburova, V.; Taylor, N.; Mazzoleni, L.R.; Zielinska, B.; Kristensen, T.B.; Chirokova, G.; McCubbin, I.B.; et al. Chemical and hygroscopic properties of aerosol organics at Storm Peak Laboratory. J. Geophys. Res. Atmos. 2013, 118, 4767–4779. [Google Scholar] [CrossRef]
- Padró, L.T.; Tkacik, D.; Lathem, T.; Hennigan, C.J.; Sullivan, A.P.; Weber, R.J.; Huey, L.G.; Nenes, A.; Hennigan, C. Investigation of cloud condensation nuclei properties and droplet growth kinetics of the water-soluble aerosol fraction in Mexico City. J. Geophys. Res. Space Phys. 2010, 115, 115. [Google Scholar] [CrossRef]
- Du, Z.; He, K.; Cheng, Y.; Duan, F.; Ma, Y.; Liu, J.; Zhang, X.; Zheng, M.; Weber, R.J. A yearlong study of water-soluble organic carbon in Beijing I: Sources and its primary vs. secondary nature. Atmos. Environ. 2014, 92, 514–521. [Google Scholar] [CrossRef]
- Liu, J.; Mo, Y.; Ding, P.; Li, J.; Shen, C.; Zhang, G. Dual carbon isotopes ((14)C and (13)C) and optical properties of WSOC and HULIS-C during winter in Guangzhou, China. Sci. Total Environ. 2018, 633, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Devi, J.J.; Bergin, M.H.; McKenzie, M.; Schauer, J.J.; Weber, R.J. Contribution of particulate brown carbon to light absorption in the rural and urban Southeast US. Atmos. Environ. 2016, 136, 95–104. [Google Scholar] [CrossRef]
- Hamad, S.H.; Shafer, M.M.; Kadhim, A.K.H.; Al-Omran, S.M.; Schauer, J.J. Seasonal trends in the composition and ROS activity of fine particulate matter in Baghdad, Iraq. Atmos. Environ. 2015, 100, 102–110. [Google Scholar] [CrossRef]
- Verma, V.; Fang, T.; Guo, H.; King, L.; Bates, J.T.; Peltier, R.E.; Edgerton, E.; Russell, A.; Weber, R.J. Reactive oxygen species associated with water-soluble PM2.5 in the southeastern United States: Spatiotemporal trends and source apportionment. Atmos. Chem. Phys. Discuss. 2014, 14, 12915–12930. [Google Scholar] [CrossRef]
- Kondo, Y.; Miyazaki, Y.; Takegawa, N.; Miyakawa, T.; Weber, R.J.; Jimenez, J.-L.; Zhang, Q.; Worsnop, D.R. Oxygenated and water-soluble organic aerosols in Tokyo. J. Geophys. Res. Space Phys. 2007, 112, 112. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Zhang, R.; Wu, Y.; Zhang, Z.; Zhang, X.; Tang, Y.; Cao, J.; Zhang, Y. Uncertainty assessment of source attribution of PM2.5 and its water-soluble organic carbon content using different biomass burning tracers in positive matrix factorization analysis—A case study in Beijing, China. Sci. Total Environ. 2016, 543, 326–335. [Google Scholar] [CrossRef]
- Villalobos, A.; Amonov, M.O.; Shafer, M.M.; Devi, J.J.; Gupta, T.; Tripathi, S.N.; Rana, K.S.; McKenzie, M.; Bergin, M.H.; Schauer, J.J. Source apportionment of carbonaceous fine particulate matter (PM 2.5) in two contrasting cities across the Indo–Gangetic Plain. Atmos. Pollut. Res. 2015, 6, 398–405. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Hecobian, A.; Zheng, M.; Frank, N.H.; Edgerton, E.S.; Weber, R.J. Spatial and seasonal variations of fine particle water-soluble organic carbon (WSOC) over the southeastern United States: Implications for secondary organic aerosol formation. Atmos. Chem. Phys. Discuss. 2012, 12, 6593–6607. [Google Scholar] [CrossRef]
- Weber, R.J.; Sullivan, A.P.; Peltier, R.E.; Russell, A.; Yan, B.; Zheng, M.; De Gouw, J.; Warneke, C.; Brock, C.; Holloway, J.S.; et al. A study of secondary organic aerosol formation in the anthropogenic-influenced southeastern United States. J. Geophys. Res. Space Phys. 2007, 112, D13302. [Google Scholar] [CrossRef]
- Kirillova, E.N.; Andersson, A.; Han, J.; Lee, M.; Gustafsson, Ö. Sources and light absorption of water-soluble organic carbon aerosols in the outflow from northern China. Atmos. Chem. Phys. 2014, 14, 1413–1422. [Google Scholar] [CrossRef]
- Yan, C.; Zheng, M.; Bosch, C.; Andersson, A.; Desyaterik, Y.; Sullivan, A.P.; Collett, J.; Zhao, B.; Wang, S.; He, K.; et al. Important fossil source contribution to brown carbon in Beijing during winter. Sci. Rep. 2017, 7, 43182. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; El-Haddad, I.; Huang, R.-J.; Ho, K.F.; Cao, J.; Han, Y.; Zotter, P.; Bozzetti, C.; Daellenbach, K.R.; Slowik, J.G.; et al. Large contribution of fossil fuel derived secondary organic carbon to water soluble organic aerosols in winter haze in China. Atmos. Chem. Phys. Discuss. 2018, 18, 4005–4017. [Google Scholar] [CrossRef]
- Ni, H.; Huang, R.-J.; Cao, J.; Guo, J.; Deng, H.; Dusek, U. Sources and formation of carbonaceous aerosols in Xi’an, China: Primary emissions and secondary formation constrained by radiocarbon. Atmos. Chem. Phys. 2019, 19, 15609–15628. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Sullivan, A.P.; Weber, R.J.; Ingall, E.D. Characterization of water-soluble organic carbon in urban atmospheric aerosols using solid-state C-13 NMR spectroscopy. Environ. Sci. Technol. 2006, 40, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.P.; Hodas, N.; Turpin, B.J.; Skog, K.; Keutsch, F.N.; Gilardoni, S.; Paglione, M.; Rinaldi, M.; Decesari, S.; Facchini, M.C.; et al. Evidence for ambient dark aqueous SOA formation in the Po Valley, Italy. Atmos. Chem. Phys. Discuss. 2016, 16, 8095–8108. [Google Scholar] [CrossRef]
- Yan, C.; Zheng, M.; Sullivan, A.P.; Bosch, C.; Desyaterik, Y.; Andersson, A.; Li, X.; Guo, X.; Zhou, T.; Collett, J.L., Jr.; et al. Chemical characteristics and light-absorbing property of water-soluble organic carbon in Beijing: Biomass burning contributions. Atmos. Environ. 2015, 121, 4–12. [Google Scholar] [CrossRef]
- Biswas, S.; Verma, V.; Schauer, J.J.; Sioutas, C. Chemical speciation of PM emissions from heavy-duty diesel vehicles equipped with diesel particulate filter (DPF) and selective catalytic reduction (SCR) retrofits. Atmos. Environ. 2009, 43, 1917–1925. [Google Scholar] [CrossRef]
- Fisseha, R.; Dommen, J.; Gaeggeler, K.; Weingartner, E.; Samburova, V.; Kalberer, M.; Baltensperger, U. Online gas and aerosol measurement of water soluble carboxylic acids in Zurich. J. Geophys. Res. Space Phys. 2006, 111, 111. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, L.; Zhou, X.; Duan, J.; Li, Y.; Hu, J.; He, K. Chemical characteristics and source apportionment of PM 2.5 in Lanzhou, China. Sci. Total Environ. 2017, 601, 1743–1752. [Google Scholar] [CrossRef]
- Van Donkelaar, A.; Martin, R.V.; Brauer, M.; Kahn, R.; Levy, R.C.; Verduzco, C.; Villeneuve, P.J. Global Estimates of Ambient Fine Particulate Matter Concentrations from Satellite-Based Aerosol Optical Depth: Development and Application. Environ. Health Perspect. 2010, 118, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Chen, L.; Su, L.; Tao, J. Satellite observation of regional haze pollution over the North China Plain. J. Geophys. Res. Atmos. 2012, 117, D12203. [Google Scholar] [CrossRef]
- Ho, K.F.; Lee, S.C.; Cao, J.; Li, Y.S.; Chow, J.C.; Watson, J.; Fung, K. Variability of organic and elemental carbon, water soluble organic carbon, and isotopes in Hong Kong. Atmos. Chem. Phys. Discuss. 2006, 6, 4569–4576. [Google Scholar] [CrossRef]
- Ram, K.; Sarin, M.M. Day-night variability of EC, OC, WSOC and inorganic ions in urban environment of Indo-Gangetic Plain: Implications to secondary aerosol formation. Atmos. Environ. 2011, 45, 460–468. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, C.; Zheng, M. Source apportionment of black carbon during winter in Beijing. Sci. Total Environ. 2018, 618, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, M.; Yu, M.; Cai, X.; Du, H.; Li, J.; Zhou, T.; Yan, C.; Wang, X.; Shi, Z.; et al. High-time-resolution source apportionment of PM2.5 in Beijing with multiple models. Atmos. Chem. Phys. Discuss. 2019, 19, 6595–6609. [Google Scholar] [CrossRef]
- Sullivan, A.P.; Peltier, R.E.; Brock, C.; De Gouw, J.; Holloway, J.S.; Warneke, C.; Wollny, A.G.; Weber, R.J. Airborne measurements of carbonaceous aerosol soluble in water over northeastern United States: Method development and an investigation into water-soluble organic carbon sources. J. Geophys. Res. Space Phys. 2006, 111, D23S46. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, T.; Liu, Y.; Yan, C.; Li, X.; Yu, J.; Wang, S.; Liu, B.; Zheng, M. Comparison of water-soluble inorganic ions and trace metals in PM2.5 between online and offline measurements in Beijing during winter. Atmos. Pollut. Res. 2019, 10, 1755–1765. [Google Scholar] [CrossRef]
- Paterson, K.G.; Sagady, J.L.; Hooper, D.L. Analysis of Air Quality Data Using Positive Matrix Factorization. Environ. Sci. Technol. 1999, 33, 635–641. [Google Scholar] [CrossRef]
- Paatero, P. Least squares formulation of robust non-negative factor analysis. Chemometr. Intell. Lab. 1997, 37, 23–35. [Google Scholar] [CrossRef]
- Norris, G.; Duvall, R.; Brown, S.; Bai, S. EPA Positive Matrix Factorization (PMF) 5.0 Fundamentals and User Guide; U.S. Environmental Protection Agency: Washington, DC, USA, 2014.
- Brown, S.G.; Eberly, S.; Paatero, P.; Norris, G.A. Methods for estimating uncertainty in PMF solutions: Examples with ambient air and water quality data and guidance on reporting PMF results. Sci. Total Environ. 2015, 518–519, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Turpin, B.J.; Huntzicker, J.J. Identification of secondary organic aerosol episodes and quantitation of primary and secondary organic aerosol concentrations during SCAQS. Atmos. Environ. 1995, 29, 3527–3544. [Google Scholar] [CrossRef]
- Srivastava, D.; Favez, O.; Perraudin, E.; Villenave, E.; Albinet, A. Comparison of Measurement-Based Methodologies to Apportion Secondary Organic Carbon (SOC) in PM2.5: A Review of Recent Studies. Atmosphere 2018, 9, 452. [Google Scholar] [CrossRef]
- Wu, C.; Yu, J.Z. Determination of primary combustion source organic carbon-to-elemental carbon (OC / EC) ratio using ambient OC and EC measurements: Secondary OC-EC correlation minimization method. Atmos. Chem. Phys. 2016, 16, 5453–5465. [Google Scholar] [CrossRef]
- Wu, C.; Wu, D.; Yu, J.Z. Quantifying black carbon light absorption enhancement with a novel statistical approach. Atmos. Chem. Phys. 2018, 18, 289–309. [Google Scholar] [CrossRef]
- Rolph, G.; Stein, A.; Stunder, B. Real-time Environmental Applications and Display sYstem: READY. Environ. Modell. Softw. 2017, 95, 210–228. [Google Scholar] [CrossRef]
- Stein, A.F.; Draxler, R.R.; Rolph, G.D.; Stunder, B.J.B.; Cohen, M.D.; Ngan, F. NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. Bull. Am. Meteorol. Soc. 2015, 96, 2059–2077. [Google Scholar] [CrossRef]
- Xu, W.; Fu, Z.M.; Chen, J.X.; Tian, H. Ground-Based Measurement and Variation Analysis of Carbonaceous Aerosols in Wuqing. Acta Sci. Natur. Univ. Pekin. 2016, 52. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Engling, G.; Zhang, R.; Yang, Y.; Cao, J.; Zhu, C.; Wang, Q.; Luo, L. Chemical composition of PM2.5 in an urban environment in Chengdu, China: Importance of springtime dust storms and biomass burning. Atmos. Res. 2013, 122, 270–283. [Google Scholar] [CrossRef]
- Zheng, B.; Tong, D.; Li, M.; Liu, F.; Hong, C.; Geng, G.; Li, H.; Li, X.; Peng, L.; Qi, J.; et al. Trends in China’s anthropogenic emissions since 2010 as the consequence of clean air actions. Atmos. Chem. Phys. Discuss. 2018, 18, 14095–14111. [Google Scholar] [CrossRef]
- Sullivan, A.P.; Weber, R.J.; Clements, A.L.; Turner, J.R.; Bae, M.S.; Schauer, J.J. A method for on-line measurement of water-soluble organic carbon in ambient aerosol particles: Results from an urban site. Geophys. Res. Lett. 2004, 31, L13105. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, S.S. Resolving sources of water-soluble organic carbon in fine particulate matter measured at an urban site during winter. Environ. Sci. Proc. Impact 2013, 15, 524–534. [Google Scholar] [CrossRef]
- Pathak, R.K.; Wang, T.; Ho, K.F.; Lee, S.C. Characteristics of summertime PM2.5 organic and elemental carbon in four major Chinese cities: Implications of high acidity for water-soluble organic carbon (WSOC). Atmos. Environ. 2011, 45, 318–325. [Google Scholar] [CrossRef]
- Vejahati, F.; Xu, Z.; Gupta, R. Trace elements in coal: Associations with coal and minerals and their behavior during coal utilization—A review. Fuel 2010, 89, 904–911. [Google Scholar] [CrossRef]
- Xu, H.; Cao, J.; Ho, K.F.; Ding, H.; Han, Y.; Wang, G.; Chow, J.; Watson, J.; Khol, S.; Qiang, J.; et al. Lead concentrations in fine particulate matter after the phasing out of leaded gasoline in Xi’an, China. Atmos. Environ. 2012, 46, 217–224. [Google Scholar] [CrossRef]
- Amato, F.; Schaap, M.; Van Der Gon, H.D.; Pandolfi, M.; Alastuey, A.; Keuken, M.; Querol, X. Short-term variability of mineral dust, metals and carbon emission from road dust resuspension. Atmos. Environ. 2013, 74, 134–140. [Google Scholar] [CrossRef]
- Yu, L. Characterization and Source Apportionment of PM2.5 in an Urban Environment in Beijing. Aerosol Air Qual. Res. 2013, 13, 574–583. [Google Scholar] [CrossRef]
- Duan, F.; Liu, X.; Yu, T.; Cachier, H. Identification and estimate of biomass burning contribution to the urban aerosol organic carbon concentrations in Beijing. Atmos. Environ. 2004, 38, 1275–1282. [Google Scholar] [CrossRef]
- Kim, E.; Hopke, P.K.; Edgerton, E.S. Source Identification of Atlanta Aerosol by Positive Matrix Factorization. J. Air Waste Manag. Assoc. 2003, 53, 731–739. [Google Scholar] [CrossRef]
- Gao, J.; Peng, X.; Chen, G.; Xu, J.; Shi, G.-L.; Zhang, Y.-C.; Feng, Y.-C. Insights into the chemical characterization and sources of PM2.5 in Beijing at a 1-h time resolution. Sci. Total. Environ. 2016, 542, 162–171. [Google Scholar] [CrossRef]
- Ashrafi, K.; Fallah, R.; Hadei, M.; Yarahmadi, M.; Shahsavani, A. Source Apportionment of Total Suspended Particles (TSP) by Positive Matrix Factorization (PMF) and Chemical Mass Balance (CMB) Modeling in Ahvaz, Iran. Arch. Environ. Contam. Toxicol. 2018, 75, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Santoso, M.; Hopke, P.K.; Hidayat, A.; Diah Dwiana, L. Sources identification of the atmospheric aerosol at urban and suburban sites in Indonesia by positive matrix factorization. Sci. Total Environ. 2008, 397, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Shi, Y.; Liu, Y.; Zhang, C.; Wang, X.; Cao, Q.; Li, S.; Zhang, F. Emission characteristics of Cd, Pb and Mn from coal combustion: Field study at coal-fired power plants in China. Fuel Process. Technol. 2014, 126, 469–475. [Google Scholar] [CrossRef]
- Tian, H.; Wang, Y.; Xue, Z.G.; Cheng, K.; Qu, Y.P.; Chai, F.H.; Hao, J.M. Trend and characteristics of atmospheric emissions of Hg, As, and Se from coal combustion in China, 1980–2007. Atmos. Chem. Phys. Discuss. 2010, 10, 11905–11919. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, Y.; Yan, C.; Fu, H.; Niu, H.; Huang, K.; Fu, Q. Establishing PM2.5 industrial source profiles in Shanghai. China Environ. Sci. 2013, 33, 1354–1359. [Google Scholar]
- Wen, J.; Shi, G.; Tian, Y.; Chen, G.; Liu, J.; Huang-Fu, Y.; Ivey, C.E.; Feng, Y. Source contributions to water-soluble organic carbon and water-insoluble organic carbon in PM2.5 during Spring Festival, heating and non-heating seasons. Ecotoxicol. Environ. Saf. 2018, 164, 172–180. [Google Scholar] [CrossRef]
- Jugder, D.; Sugimoto, N.; Shinoda, M.; Kimura, R.; Matsui, I.; Nishikawa, M. Dust, biomass burning smoke, and anthropogenic aerosol detected by polarization-sensitive Mie lidar measurements in Mongolia. Atmos. Environ. 2012, 54, 231–241. [Google Scholar] [CrossRef]
- Lee, K.Y.; Batmunkh, T.; Joo, H.S.; Park, K. Comparison of the physical and chemical characteristics of fine road dust at different urban sites. J. Air Waste Manag. Assoc. 2018, 68, 812–823. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Pilot, E.; Yu, J.; Nie, C.; Holdaway, J.; Yang, L.; Li, Y.; Wang, W.; Vardoulakis, S.; et al. Taking Action on Air Pollution Control in the Beijing-Tianjin-Hebei (BTH) Region: Progress, Challenges and Opportunities. Int. J. Environ. Res. Public Health 2018, 15, 306. [Google Scholar] [CrossRef]
- Kaul, D.S.; Gupta, T.; Tripathi, S.N.; Tare, V.; Collett, J.L. Secondary Organic Aerosol: A Comparison between Foggy and Nonfoggy Days. Environ. Sci. Technol. 2011, 45, 7307–7313. [Google Scholar] [CrossRef]
- Hennigan, C.J.; Bergin, M.H.; Russell, A.G.; Nenes, A.; Weber, R.J. Gas/particle partitioning of water-soluble organic aerosol in Atlanta. Atmos. Chem. Phys. 2009, 9, 3613–3628. [Google Scholar] [CrossRef]
- Rastogi, N.; Patel, A.; Singh, A.; Singh, D. Diurnal Variability in Secondary Organic Aerosol Formation over the Indo-Gangetic Plain during Winter Using Online Measurement of Water-Soluble Organic Carbon. Aerosol Air Qual. Res. 2015, 15, 2225–2231. [Google Scholar] [CrossRef]

| Average | EP1 | EP2 | CD | |||||
|---|---|---|---|---|---|---|---|---|
| Average ± Standard Deviation (Median) | Sample Number (N) | Average ± Standard Deviation (Median) | Sample Number (N) | Average ± Standard Deviation (Median) | Sample Number (N) | Average ± Standard Deviation (Median) | Sample Number (N) | |
| Temperature (°C) | 10 ± 4 (10) | 292 | 12 ± 3 (12) | 90 | 8 ± 3 (7) | 88 | 10 ± 3 (10) | 51 |
| Pressure (hPa) | 1013 ± 2 (1012) | 292 | 1013 ± 3 (1013) | 90 | 1014 ± 3 (1015) | 88 | 1012 ± 1 (1012) | 51 |
| Wind speed (m/s) | 2 ± 1 (2) | 292 | 2 ± 1 (2) | 90 | 2 ± 1 (2) | 88 | 4 ± 2 (4) | 51 |
| Wind direction (°) | 201 ± 70 (199) | 292 | 162 ± 61 (165) | 90 | 200 ± 71 (207) | 88 | 263 ± 55 (286) | 51 |
| Relative humidity (%) | 51 ± 22 (46) | 292 | 48 ± 13 (47) | 90 | 67 ± 18 (75) | 88 | 31 ± 10 (32) | 51 |
| PM2.5 (μg/m3) | 98 ± 68 (91) | 283 | 164 ± 56 (163) | 90 | 109 ± 37 (102) | 88 | 13 ± 3 (13) | 51 |
| WSOC (μg/m3) | 5.14 ± 3.25 (4.89) | 281 | 8.14 ± 2.17 (8.39) | 90 | 6.05 ± 2.16 (5.33) | 77 | 0.87 ± 0.21 (0.86) | 51 |
| OC (μg/m3) | 9.54 ± 4.98 (9.01) | 287 | 14.01 ± 3.43 (14.59) | 88 | 10.47 ± 3.39 (9.32) | 87 | 2.80 ± 0.50 (2.67) | 51 |
| EC (μg/m3) | 2.63 ± 1.71 (2.35) | 276 | 4.14 ± 1.40 (4.24) | 88 | 2.70 ± 1.27 (2.18) | 87 | 0.46 ± 0.13 (0.45) | 40 |
| WSOC/OC | 0.49 ± 0.12 (0.52) | 276 | 0.58 ± 0.08 (0.59) | 88 | 0.56 ± 0.05 (0.56) | 76 | 0.31 ± 0.06 (0.31) | 51 |
| OC/EC | 4.38 ± 1.31 (4.12) | 276 | 3.56 ± 0.72 (3.35) | 88 | 4.20 ± 0.88 (4.07) | 87 | 6.20 ± 1.31 (6.12) | 40 |
| City | Seasons | Sampling Method | EC | OC | WSOC | WSOC/OC | References |
|---|---|---|---|---|---|---|---|
| Beijing | Spring 2017 | Online PILS-TOC | 2.63 ± 1.71 | 9.54 ± 4.98 | 5.14 ± 3.25 | 0.49 ± 0.12 | This study |
| Beijing | Spring 2009 Summer 2009 Autumn 2009 Winter 2009 | Offline TOC | 2.8 ± 1.1 | 13.7 ± 4.4 | 6.7 ± 1.8 | [17] | |
| 4.2 ± 1.2 | 11.1 ± 1.8 | 3.2 ± 1.1 | |||||
| 5.3 ± 2.8 | 17.8 ± 5.6 | 7.7 ± 5.0 | |||||
| 7.5 ± 7.4 | 24.9 ± 15.6 | 7.7 ± 3.6 | |||||
| Beijing | Summer 2011 Autumn 2011 Winter 2011 Spring 2012 Summer 2012 | Offline TOC | 3.08 | 13.55 | 4.48 | 0.33 | [6] |
| 5.4 | 25.42 | 5.82 | 0.25 | ||||
| 4.7 | 28.16 | 5.53 | 0.2 | ||||
| 2.98 | 16.57 | 3.9 | 0.27 | ||||
| 3.15 | 16.54 | 5.81 | 0.34 | ||||
| Beijing | Summer 2013 Winter 2012 | Offline TOC | 1.90 ± 0.63 | 9.65 ± 2.87 | 6.42 ± 2.17 | 0.66 ± 0.06 | [27] |
| 5.58 ± 1.44 | 32.9 ± 16.8 | 10.8 ± 3.13 | 0.39 ± 0.16 | ||||
| Tianjin | Winter 2011 | Online PILS-TOC | 6.0 ± 4.8 | 21.5 ± 19.2 | 14.3 ± 11.8 | [49] | |
| Beijing Shanghai Lanzhou Guangzhou | Summer 2005 Spring 2005 Summer 2006 Spring 2004 | Offline TOC | 4.9 ± 2.9 | 8.2 ± 3.7 | 4.5 ± 2.3 | 0.55 ± 0.1 | [54] |
| 10.0 ± 5.5 | 16.9 ± 12.2 | 5.8 ± 4.2 | 0.35 ± 0.1 | ||||
| 2.9 ± 1.4 | 6.6 ± 2.9 | 2.4 ± 0.8 | 0.4 ± 0.1 | ||||
| 10.5 ± 3.7 | 14.9 ± 5.8 | 4.3 ± 1.2 | 0.32 ± 0.1 | ||||
| Chengdu | Spring 2009 | Offline TOC | 5.7 ± 1.8 | 20.7 ± 6.0 | 10.4 ± 3.4 | 0.5 | [50] |
| Atlanta | Summer 2004 | Online PILS-TOC | 3.22 ± 1.12 | 1.98 ± 1.00 | 0.60 ± 0.13 | [37] | |
| Kanpur | Autumn 2008 | Offline TOC | 7.7 ± 2.9 | 47 ± 21 | 31.6 ± 15.3 | 0.66 ± 0.10 (day) 0.47 ± 0.07 (night) | [34] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Yan, C.; Sullivan, A.P.; Liu, Y.; Wang, X.; Dong, H.; Chen, S.; Zeng, L.; Collett, Jr., J.L.; Zheng, M. Significant Contribution of Primary Sources to Water-Soluble Organic Carbon During Spring in Beijing, China. Atmosphere 2020, 11, 395. https://doi.org/10.3390/atmos11040395
Jin Y, Yan C, Sullivan AP, Liu Y, Wang X, Dong H, Chen S, Zeng L, Collett, Jr. JL, Zheng M. Significant Contribution of Primary Sources to Water-Soluble Organic Carbon During Spring in Beijing, China. Atmosphere. 2020; 11(4):395. https://doi.org/10.3390/atmos11040395
Chicago/Turabian StyleJin, Yali, Caiqing Yan, Amy P. Sullivan, Yue Liu, Xinming Wang, Huabin Dong, Shiyi Chen, Limin Zeng, Jeffrey L. Collett, Jr., and Mei Zheng. 2020. "Significant Contribution of Primary Sources to Water-Soluble Organic Carbon During Spring in Beijing, China" Atmosphere 11, no. 4: 395. https://doi.org/10.3390/atmos11040395
APA StyleJin, Y., Yan, C., Sullivan, A. P., Liu, Y., Wang, X., Dong, H., Chen, S., Zeng, L., Collett, Jr., J. L., & Zheng, M. (2020). Significant Contribution of Primary Sources to Water-Soluble Organic Carbon During Spring in Beijing, China. Atmosphere, 11(4), 395. https://doi.org/10.3390/atmos11040395







