1. Introduction
Aerosol particles play important role in the ambient air related to air quality, urban and regional air pollution, visibility, global climate, biogeochemical cycles, human health, etc. [
1,
2]. Aerosols can offset regional greenhouse warming effects directly by scattering sunlight back into space and indirectly by enhancing cloud albedo, thereby cooling the climate [
3]. However, aerosol components such as soot heat the atmosphere by absorbing sunlight and thereby augmenting the warming produced by the greenhouse effect [
3,
4]. Over polluted regions, long-range transport of mineral dust mixed with anthropogenic species could induce substantial changes in the optical and chemical properties of aerosols [
3,
5].
Research on the concentration, size, and chemical composition at the receptors is essential to elucidate the sources of aerosols and the processes associated with their formation [
6]. Both natural and anthropogenic sources, such as road dust, vehicular emissions, secondary aerosols, sea-salt, and black carbon from oil burning, influence aerosol characteristics [
7,
8]. Source apportionment is an important approach for identifying and quantifying various sources of air pollutants [
9,
10]. The most common method is receptor modeling [
11], which can provide a theoretical and mathematical framework for quantifying contributions from potential sources. The purpose of a receptor source apportionment model is to estimate the contributions of specific source types to pollutant levels in the atmosphere at a sampling (or receptor) site [
12]. The contributions of each source are distinguished through differences in their physical and chemical properties [
12]. Computer-generated source apportionment results must be interpreted by experts with knowledge about the site and the associated potential sources [
12]. Principal component analysis is one of the most widely used multivariate statistical techniques in the atmospheric sciences [
12,
13].
In this study, data on the optical and chemical properties of atmospheric aerosols were collected and analyzed in real time at the Seoul Meteorological Station, South Korea during a consecutive high concentration episode from 30 December 2013 to 2 January 2014. This period was selected as a case study of dust and sand storm (DSS) events by working group-1 (WG1) of the Tripartite Environment Ministers Meeting among China, Japan, and Korea (TEMM) [
14]. More specifically, the solar radiation properties and concentrations of the water-soluble ionic components of the ambient aerosols were analyzed to identify the possible sources of the main aerosol constituents.
2. Method
Seoul Meteorological Station (37.57° N, 126.97° E) is situated in the Songwol-dong area located in the middle of Seoul, South Korea at a height of 85.5 m. An intensive observation study of atmospheric aerosols was carried out during the high aerosol concentration episode from 30 December 2013 to 2 January 2014. Surface weather map charts and meteorological parameters, including wind speed, wind direction, humidity, and visibility, were continuously measured at this station by the Korean Meteorological Administration. The HYSPLIT 4.0 model developed at National Oceanic and Atmospheric Administration (NOAA) was used to estimate the upstream path of air flow during this period. The HYSPLIT simulations were run using the Unified Model-Global Data Assimilation and Prediction System (UM-GDAPS) with weather data from Korea Meteorological Administration (KMA) for 72 h before each episode at altitude of 500 m above ground level [
15].
Direct and diffuse solar radiations were measured using a collocated sky radiometer (POM-02L, Prede Co. Ltd, Japan) at Seoul Meteorological Station.
The aerosol optical depth (AOD) and single scattering albedo (SSA) at five wavelengths (400, 500, 675, 870, and 1020 nm) were retrieved using the SKYRAD.pack software (version 5.0) package [
16]. The Ångström exponent (AE) was calculated as a log-linear fitting for the five wavelengths.
The PM10 (particle matter with an aerodynamic diameter of less than or equal to 10 μm), PM2.5, and PM1.0 concentrations were measured using the Environmental Dust Monitoring (EDM, #180, GRIMM, Germany) system with 31 channels of different sizes at a flow rate of 1.2 L min−1 at Seoul Meteorological Station. The instrument is designed to measure the particle size distribution and particulate mass based on light scattering by individual aerosol particles in the ambient air.
Semi-continuous measurements of the ionic concentrations in PM10 aerosols were taken at 20 min intervals using a particle-into-liquid sampler (PILS, model ADI-2081, Metrohm, Switzerland) coupled to two ion chromatography systems (IC, model ICS-5000, Thermo Fisher Scientific, United States). The PILS nucleates aerosol particles to form water droplets by mixing a denuded aerosol stream with a supersaturated steam. The nucleated droplets are collected in a flowing liquid stream by inertial impaction. The liquid stream, which contains an internal Lithium bromide (LiBr) standard to determine dilution by condensed water vapor, is split into two streams. These are then injected into two ion chromatography systems every 20 min to analyze the ionic concentrations for the major water-soluble inorganic species (Na+, NH4+, K+, Mg2+, Ca2+, SO42−, NO2−, NO3−, Cl−). Eluent for the cationic analysis was 20 mM methane sulfonic acid with a flow rate of 0.5 mL min−1 using CG12A/CS12A column and CSRS-ULTRA II suppressor. The analysis of anionic species was performed with an ion chromatograph equipped using AG19/AS19 column (13 mM KOH eluent) and ASRS-ULTRA II suppressor. The standard calibration curve for the analysis of cations and anions showed linearity with R2 = 0.9999 or better. During the analysis period and depending on the species, the limit of detection (LOD) for IC analyses of the ionic species ranged from 0.05 to 0.1 μg m−3.
3. Results and Discussion
3.1. Meteorological Conditions and Mass Concentrations
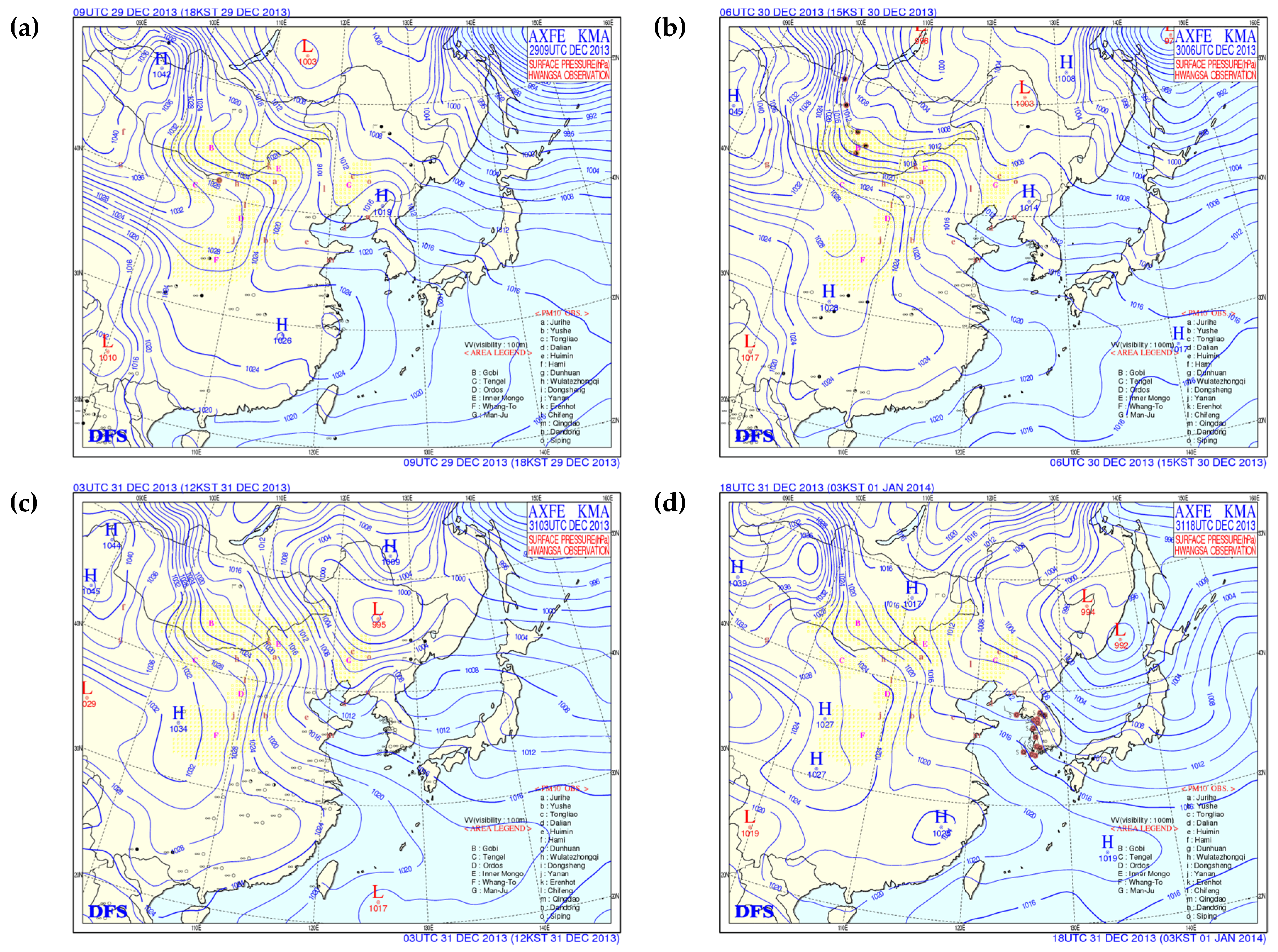
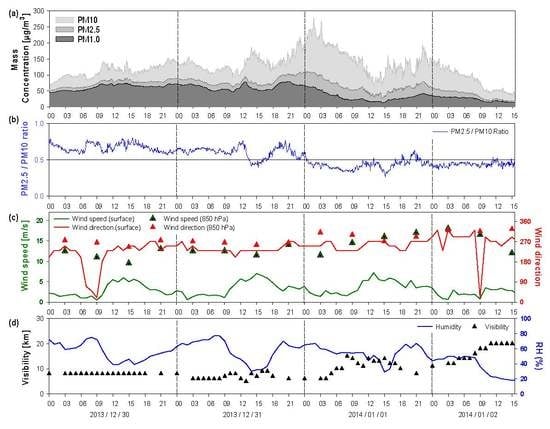
Surface weather maps for 29 December 2013 to 1 January 2014 are shown in
Figure 1. The atmosphere around the Korean peninsula was stable due to the effect of high pressure from 29 December to the morning of 31 December (
Figure 1a,
Figure 2b). Haze was observed in Seoul on 30 and 31 December. On the afternoon of 31 December the Korean peninsula was affected by a low-pressure system over northeastern China. The 850 hPa wind speed increased, and the wind direction changed from southwest to northwest after January 1, 2014. Asian dust was observed from 02:30 KST 1 January 2014, and the ratios of PM
2.5 to PM
10 were mainly below 0.5 after this time.
Figure 2 presents the temporal variations of PM
10, PM
2.5, and PM
1.0 mass concentrations; the ratio of PM
2.5 to PM
10; and the meteorological conditions (wind speed, wind direction, humidity, and visibility) from 30 December 2013 to 2 January 2014. From December 30 to the morning of 31 December, the ratio of PM
2.5 to PM
10 exceeded 0.6, thus indicating that fine particles were predominant. The maximum concentration of PM
10 during this period was 156 µg m
–3. After 12:00 KST on 31 December, the concentration began to increase again and reached the highest value of 279.7 µg m
−3 between 00:00 - 03:00 KST 1 January (
Figure 2a).
Figure 2c,d shows that the wind speed increased while humidity decreased from 09:00 KST on 31 December 2013. In addition, the mass concentration both PM
10 and PM
2.5 were increased, and the concentration ratio of PM
2.5/PM
10 showed a tendency to increase after 15:00 KST on 31 December Therefore, it was determined that the mass concentration of PM
10 was increased relative to PM
2.5 and PM
1.0 after 09:00 KST on 31 December 2013.
3.2. Aerosol Optical Properties
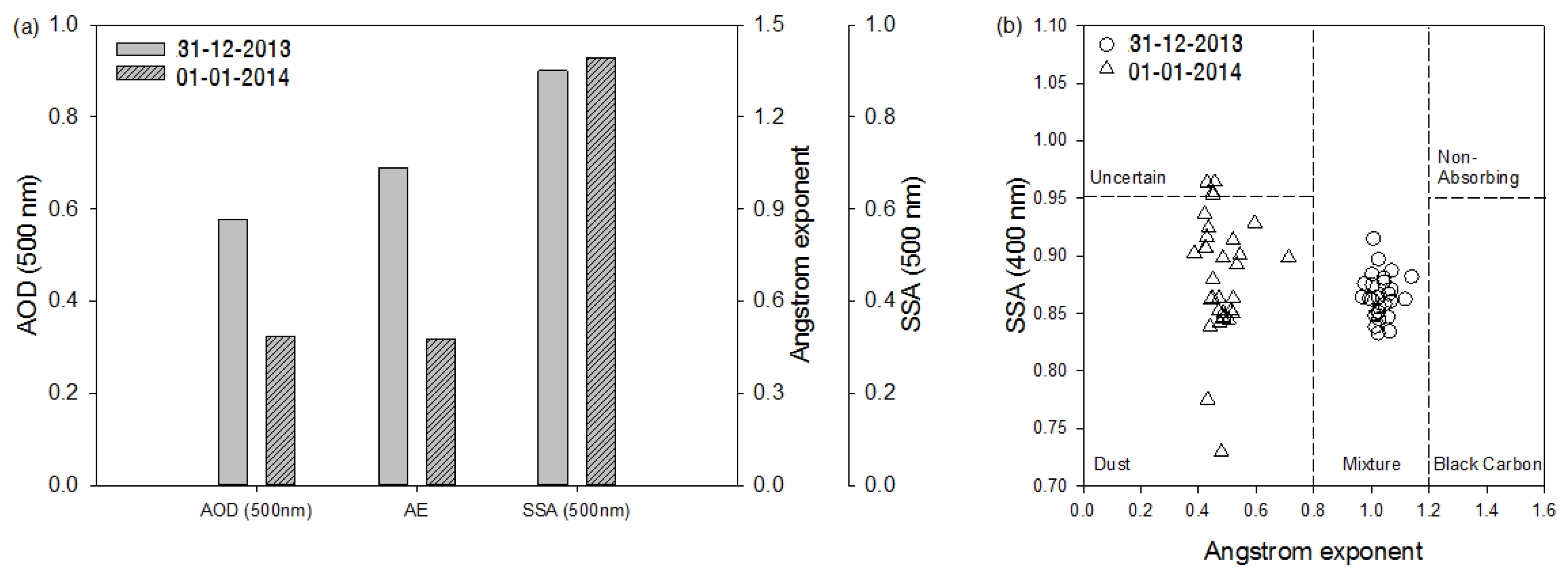
Figure 3a shows the means of AOD (500 nm), AE, and SSA (500 nm) retrieved from the sky radiometer data for 31 December and 1 January. There was no sky radiometer observation on 30 December due to clouds. The daily mean AOD was 0.57 on 31 December 2013, which was higher by 80% as compared to that on 1 January. The AE values were 1.03 and 0.48 on 31 December and 1 January, respectively, indicating that coarse particles dominated on 1 January. The average values for SSA were 0.90 and 0.93 for 31 December and 1 January, respectively. Aerosol types can be classified using AE and SSA, which determine the size of aerosols and distinguish absorbing/non-absorbing aerosols, respectively. Finer particles have a higher AE, and more absorbing aerosols have a lower SSA. Black carbon is a well-known absorbing aerosol, but dust particles also appeared to be solar absorbing aerosols [
17]. Aerosol type classification for columnar aerosols was suggested by previous studies [
18,
19,
20]. This study adopted one of these aerosol type classification criteria, suggested in Mielonen et al. [
20]. Fine-mode aerosols are defined as having an AE greater than 1.2, while coarse-mode aerosols are defined as having an AE less than 0.8. Aerosols between these threshold values are classified as a “Mixture” mode. The SSA threshold of 0.95 is used to distinguish absorbing from non-absorbing aerosols. Values below 0.95 in the coarse mode indicate “Dust” and values above 0.95 indicate “Uncertain” SSA values below 0.95 in the fine mode indicate “black carbon (BC),” and values above 0.95 indicate “non-absorption (NA)” The dominant aerosol types on two days are shown in
Figure 3b. The means of SSA for both days were similar, but SSAs on 1 January were more scattered (0.73–0.95) than on 31 December. All data on 31 December were in the “Mixture” category indicating that both coarse and fine particles exist, whereas “Dust” was most frequently detected on 1 January.
3.3. Ionic Concentrations of Aerosols
The concentrations of the major water-soluble ionic species in PM
10 were analyzed semi-continuously using the PILS-IC system to examine the chemical characteristics of the aerosol particles. Time series variations in the concentrations of ionic species (Na
+, NH
4+, K
+, Mg
2+, Ca
2+, SO
42−, NO
2−, NO
3−, Cl
−) are shown in
Figure 4a. Here, the concentrations of calcium carbonate and magnesium carbonates were calculated as the soluble (sol) fractions of Ca and Mg and were added to the calculated CO
32− concentrations [
21]. The CO
32− was calculated using Equation (1):
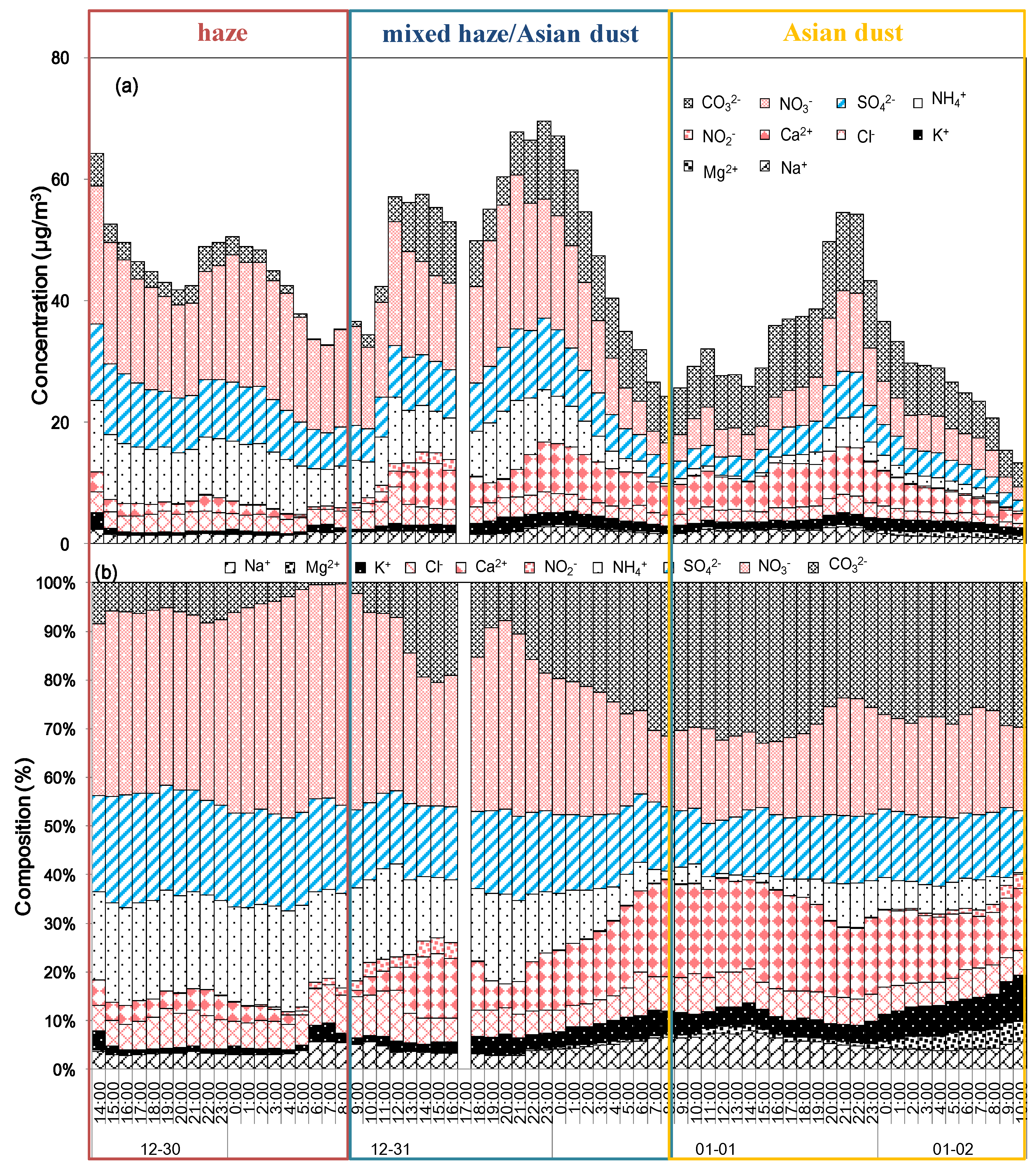
The concentrations of the ionic species were ordered as NO3− (12.43 µg m−3) > CO32− (6.89 µg m−3) > SO42− (6.82 µg m−3) > NH4+ (5.35 µg m−3) > Ca2+ (4.29 µg m−3) > Cl- (2.49 µg m−3) > Na+ (1.78 µg m−3) > K+(1.29 µg m−3) > NO2− (0.26 µg m−3) > Mg2+ (0.26 µg m−3). The concentrations of NO3−, SO42-, Ca2+, and NH4+ were 2.28–25.37, 1.71–12.65, 0.03–8.26, and 0.03–11.67 µg m–3, respectively. In addition, the concentrations of NO3−, SO42−, and NH4+ were relatively high from 02:00 KST on 30 December to 08:00 KST on 31 December. The concentrations of NO3− and CO32− were high from 09:00 KST on 31 December to 08:00 KST on 1 January. The concentrations of Ca2+ and CO32− were high from 09:00 KST on 1 January to 10:00 KST on 2 January. Based on this analysis, the study period was divided into three episodes: the haze period (02:00 KST on 30 December to 08:00 KST on 31 December), the mixed haze/Asian dust period (09:00 KST on 31 December to 08:00 KST on 1 January), and the Asian dust period (09:00 KST on 1 January to 10:00 KST on 2 January). This chemical characteristic was similar to the PM10 mass concentration, the ratio of the PM2.5/PM10, and optical characteristics. Using these results, we investigated the chemical compositions, neutralization factors, and pollution sources of the three episodes.
The composition ratios of the ionic species in PM
10 during the study period are shown in
Figure 4b. The composition ratios of secondary inorganic aerosols (NH
4+, NO
3−, and SO
42−), soil (Ca
2+ and CO
32−), sea-salt (Na
+, Cl
−, and Mg
2+), and other species in PM
10 during the study periods were 55.7%, 28.8%, 11.5%, and 4.10%, respectively. Their species ranges were 28.3–85.8%, 0.3–53.5%, 7.9–16.3%, and 0.9–12.6%, respectively. The composition ratios of the secondary inorganic aerosol species were 80.2%, 57.0%, and 36.5%, and those of the soil species were 7.9%, 28.1%, and 44.6% of the total ionic species for the haze, mixed haze/Asian dust, and Asian dust episodes, respectively. Sea-salt species accounted for 9.9%, 10.8%, and 13.2%, while the others accounted for 1.9%, 4.2%, and 5.6% of the haze, mixed haze/Asian dust, and Asian dust episodes, respectively. The composition ratios of secondary inorganic aerosol species were higher during the haze episode, whereas those of the soil and other species were higher during the Asian dust episode. In particular, CO
32− was the most dominant anion, and Ca
2+ and Mg
2+ were major cations during the mixed haze/Asian dust and Asian dust episodes.
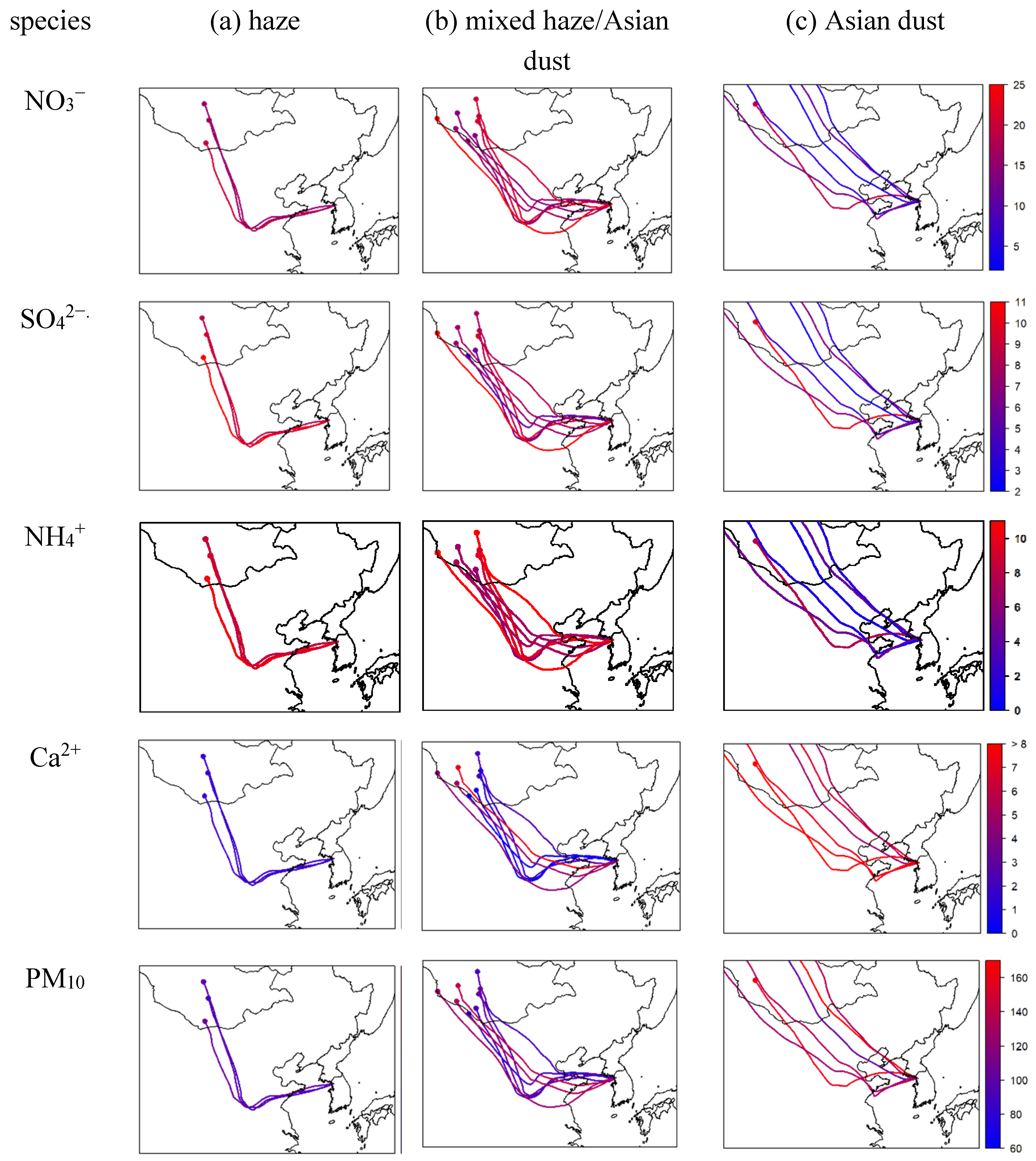
Figure 5 shows the 72 h air mass backward trajectories at a height of 500 m colored according to the concentration of the ionic component pollutants. The backward trajectories were similar for the haze and mixed haze/Asian dust episodes, while air masses for the Asian dust case seemed to move faster than those for the other cases. NO
3−, SO
42−, NH
4+, i.e., the anthropogenic pollutants, showed elevated concentrations when the trajectories passed over the industrialized area in East China. During the Asian dust episode, the air masses passed over Mongolia and Inner Mongolia, the major Asian Dust source regions, and Ca
2+ mainly originating from soil dust showed higher concentrations.
3.4. Ammonium Salt Formation and Neutralization
The secondary inorganic aerosol species, including SO
42−, NO
3−, and NH
4+, often exist in various forms, such as (NH
4)
2SO
4, NH
4HSO
4, and NH
4NO
3 [
22]. The ratios for (NH
4)
2SO
4 and NH
4NO
3 that existed mainly in the ambient air could be calculated using Equations (2) and (3) [
23].
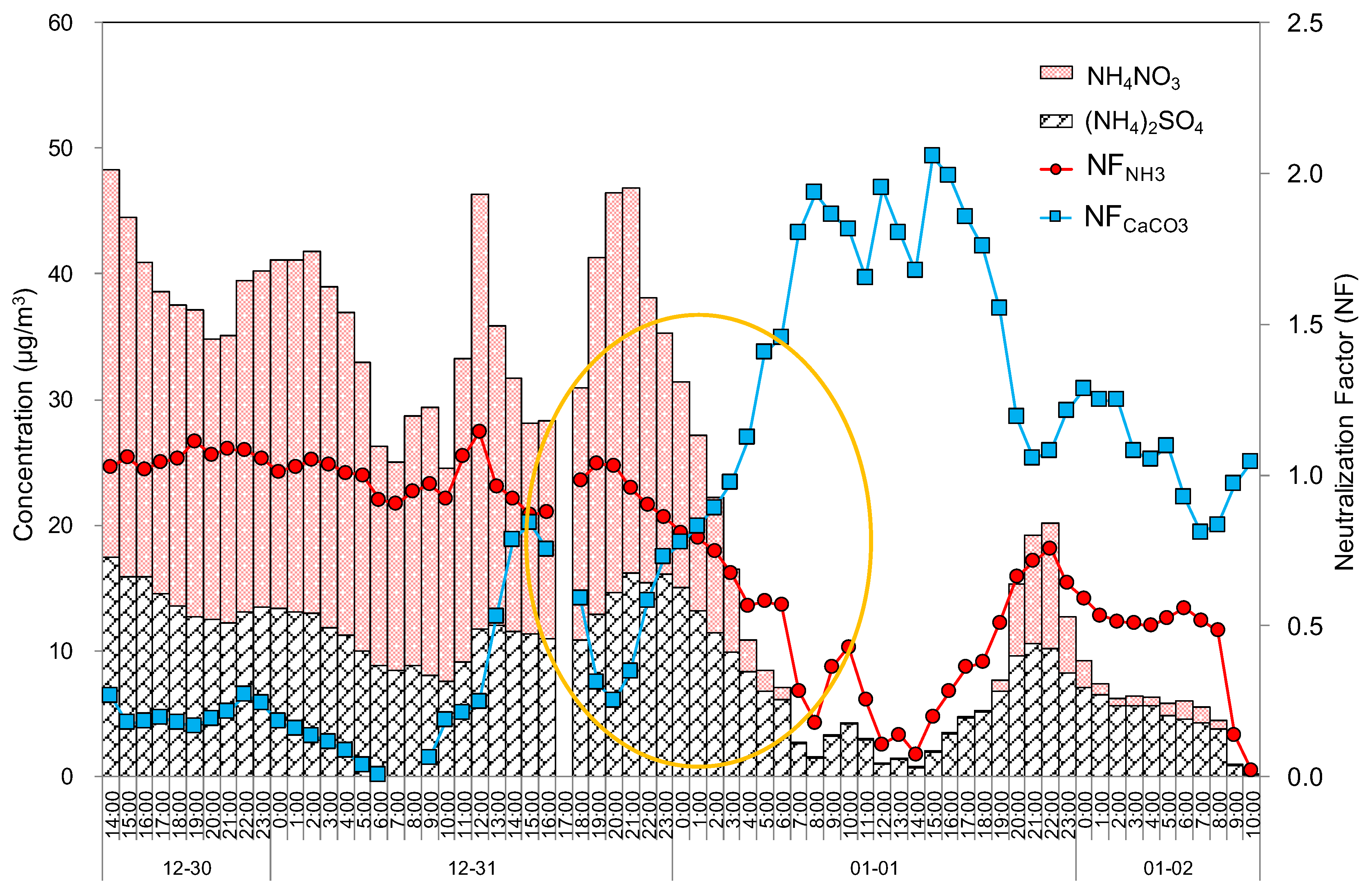
Figure 6 shows the concentrations of (NH
4)
2SO
4 and NH
4NO
3 estimated from the ionic analysis and the neutralization factors caused by the reaction between inorganic acids and bases, such as ammonia and calcium carbonate in PM
10 aerosols. The estimated concentrations of (NH
4)
2SO
4 in Seoul were 12.6, 10.6, and 4.7 µg m
–3, and those of NH
4NO
3 were 24.7, 16.6, and 1.5 µg m
−3 for the haze, mixed haze/Asian dust, and Asian dust episodes, respectively, indicating relatively high concentrations in the haze period but lower concentrations in the Asian dust period. The estimated concentrations of (NH
4)
2SO
4 in PM
10 at the Gosan, South Korea site were 22.9, 10.5, and 8.0 µg m
−3, and those of NH
4NO
3 were 24.3, 0.1, and 0.09 µg m
–3 for the haze, mixed haze/Asian dust, and Asian dust, respectively [
24].
Generally, the acid compounds in the air are neutralized by basic ammonia and carbonate compounds through the reactions between inorganic acids and base species, which are mostly ammonia or soil components, such as calcium carbonate and magnesium carbonate [
25,
26]. The neutralization factors are presented in
Figure 6. The neutralization factors by ammonia were 1.03, 0.81, and 0.41 for the haze, mixed haze/Asian dust, and Asian dust episodes, respectively. Meanwhile, the neutralization factors by calcium carbonate were 0.16, 0.77, and 1.39, for haze, mixed haze/Asian dust, and Asian dust episodes, respectively.
It has been shown that the neutralization by ammonia was noticeably higher during the haze episode but decreased as the Asian dust episode began to occur. Conversely, the neutralization by calcium carbonate increased during the Asian dust episode. Interestingly, the neutralization by ammonia and calcium carbonate were reversed during the haze and mixed haze/Asian dust episodes. Therefore, it is thought that the concentrations of ammonium sulfate and ammonium nitrate would increase during the haze episode, while the soil-originated CaCO3 would increase during the Asian dust episode.
3.5. Emission Sources of Ambient Aerosols
Table 1 shows the correlation coefficients between the water-soluble ions in PM
10 aerosols. In the results, NH
4+ was strongly correlated with SO
42− in all haze (r = 0.880), mixed haze/Asian dust (r = 0.848), and Asian dust (r = 0.911) episodes. Strong correlations were also found between NH
4+ and NO
3− in haze (r = 0.783), mixed haze/Asian dust (r = 0.971), and Asian dust (r = 0.969). Ca
2+ was strongly correlated with CO
32− for all three episodes. These results suggest that (NH
4)
2SO
4, NH
4HSO
4, NH
4NO
3, and CaCO
3 were the major chemical fractions in all three episodes.
Principal component analysis (PCA) was applied to estimate the major emission sources of aerosol components [
27]. PCA is the most common multivariate statistical method applied in environmental studies. The Statistical Package for the Social Sciences (SPSS) software (version 18.0) was used for the multivariate analysis. We applied PCA for the water-soluble ions analyzed during the episodes for source attribution. The varimax rotated factors of the loading matrix are presented in
Table 2.
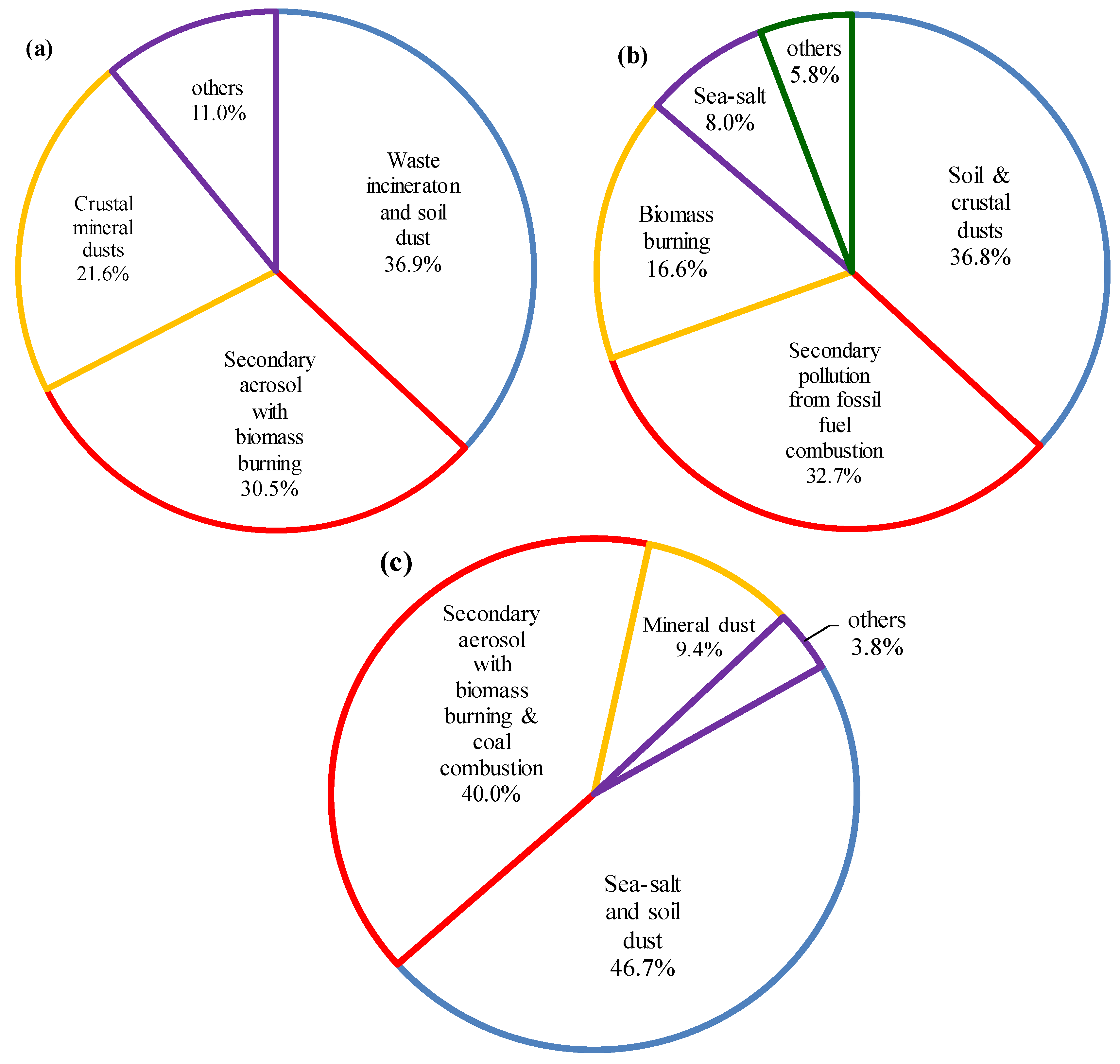
In the haze episode, the first three principal components with eigenvalues greater than one were extracted; they accounted for 89.0% of the total variance. The first principal component (PC1), which explained 36.9% of the total variance with an eigenvalue of 3.7 was mostly dependent on Ca
2+, Cl
−, SO
42−, and CO
32−. The Ca
2+ and CO
32− species are considered to be soil dust sources [
28], while SO
42− and Cl
− are typically produced by waste incinerators [
29]. This indicates that PC1 is associated with soil dust and emissions from waste incinerators. The second principal component, PC2 accounted for 30.5% of the total variance and showed high loadings for NH
4+, SO
42−, NO
3−, and K
+. Because K
+ is regarded as a tracer for biomass burning [
30], PC2 can be associated with secondary aerosols mixed with biomass-burning aerosols [
31]. SO
42− showed a significant loading on both PC1 and PC2 indicating composite sources. Finally, PC3 explained 21.6% of the total variance, which was dominated by Na
+ and Mg
2+. A strong correlation between Na
+ and Mg
2+ indicates that Na
+ and Mg
2+ had the same origin
Table 1a. Thus, the major sources of PC3 were natural minerals characteristics (
Figure 7a) [
32].
In the mixed haze/Asian dust episode, four principal components were identified and accounted for 94.2% of the total variance. PC1, accounting for 36.8% of the total variance was heavily loaded by Na
+, Mg
2+, Ca
2+, and CO
32−, with loadings of 0.702, 0.866, 0.985, and 0.987, respectively. Mg
2+, Ca
2+, and CO
32− are regarded as tracers for soil dust sources from natural and a variety of crustal minerals [
33]. The high correlation between Na
+ and Mg
2+ (r = 0.777) indicates that Na
+ and Mg
2+ share the same origin
Table 1b. PC2 showed high loadings of the three secondary ions as well as Cl
- indicating that the secondary pollutants originated from fossil fuel combustion and other anthropogenic sources [
31]. The loading of K
+ on PC3 (16.6% of the total variance) mainly originated from biomass burning. PC4, which explained another 8.0% of the total variance grouped Na
+ and Cl
− and appears to represent the sea-salt source (
Figure 7b) [
34].
Three principal components that explained 96.2% of the total variance were identified in the Asian dust episode. PC1, with 46.7% of the total variance, was loaded with Na
+, Ca
2+, Cl
−, and CO
32−, with values of 0.830, 0.888, 0.682, and 0.840, respectively, indicating the presence of a mixture of sea-salt aerosols and soil dust. PC2, accounting for 40.0% of the variance was mainly affected by NH
4+ (0.963), K
+ (0.649), Cl
− (0.669), NO
3− (0.929), and SO
42− (0.824). NO
3−, SO
42−, and NH
4+ are the typical tracers of secondary origins, and Cl
- and K
+ are emitted mainly from coal combustion and biomass burning [
33]. PC3 accounted for 9.4% of the total variance and was strongly loaded with K
+ and Mg
2+. These are regarded as tracers for crustal and construction dusts (
Figure 7c).
4. Conclusions
Through optical analyses using the sky radiometer, aerosol types were classified as “Mixture” and “Dust” particles for 31 December 2013 and 1 January 2014, respectively. The secondary inorganic aerosol species (NH4+, NO3−, and SO42−) during the haze episode had relatively higher concentrations (80.2%) as compared to the other episodes. In addition, the composition ratios of soil (Ca2+ and CO32−) were 3.6 and 5.6 times increased in both the mixed haze/Asian dust and Asian dust episodes, respectively. The NH4NO3 content was higher than (NH4)2SO4 during the haze, but it was relatively low during the Asian dust episode. Comparing the high aerosol concentration episodes, the neutralization by ammonia was noticeably higher during the haze episode, and calcium carbonate was more prominent during the Asian dust episode. The backward trajectories analysis indicated that the concentrations of the secondary inorganic aerosol species (NH4+, NO3−, and SO42−) were higher when the air masses moved over the industrialized area in East China. However, when the air mass was transported over Mongolia and Inner Mongolia during the Asian dust episode, the concentration of Ca2+ species was relatively higher. The neutralization factors of ammonia were 1.03, 0.81, 0.41, respectively, for the haze, mixed haze/Asian dust, and Asian dust episodes. Moreover, the neutralization factors of calcium carbonate were 0.16, 0.77, 1.39, respectively. Based on PCA, the haze episode compositions might be influenced by a mixture of soil dust and emissions from waste incinerators, a mixture of secondary aerosols and biomass burning, and crustal mineral dust. The compositions of the mixed haze/Asian dust period were most affected by a mixture of soil and crustal dusts, followed by secondary pollutants from fuel combustion, biomass burning, and sea-salt emissions. Moreover, a mixture of sea-salts and soil dust, a mixture of secondary aerosols with biomass burning and coal combustion, and mineral dust were the potential sources in the Asian dust episode.
Author Contributions
Conceptualization, H.-J.K. and J.-M.S.; methodology, H.-J.K., S.J.S., and J.E.K.; software, S.J.S.; validation, H.-J.K. and J.-M.S.; formal analysis, H.-J.K. and S.J.S.; investigation, J.W.C.; writing—original draft preparation, H.-J.K. and J.-M.S.; writing—review and editing, H.-J.K., S.J.S., J.E.K., and J.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Korea Meteorological Administration Research and Development Program “Development of Asian Dust and Haze Monitoring and Prediction Technology” under Grant (1365003013).
Acknowledgments
The authors would like to thank technical and scientific staff members of the Seoul Meteorological Station and collocated Seoul Hwangsa Monitoring Centre and for their efforts to collect meteorological and aerosol data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heal, M.R.; Kumar, P.; Harrison, R.M. Particles, air quality, policy and health. Chem. Soc. Rev. 2012, 41, 6606–6630. [Google Scholar] [CrossRef]
- Jiang, J.; Zhou, W.; Cheng, Z.; Wang, S.; He, K.; Hao, J. Particulate matter distributions in China during a winter period with frequent pollution episodes (January 2013). Aerosol Air Qual. Res. 2015, 15, 494–503. [Google Scholar] [CrossRef]
- Gautam, R.; Hsu, N.C.; Lau, K.M. Premonsoon aerosol characterization and radiative effects over the Indo-Gangetic plains: Implications for regional climate warming. J. Geophys. Res. 2010, 115, D17208. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Strong radiative heating due to mixing state of black carbon on atmospheric aerosols. Nature. 2001, 409, 695–697. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Carmichael, G.R.; Arimoto, R.; Conant, W.C.; Brechtel, F.J.; Bates, T.S.; Cahill, T.A.; Clarke, A.D.; Doherty, S.J.; Flatau, P.J.; et al. ACE-ASIA—Regional climatic and atmospheric chemical effects of Asian dust and pollution. Bull. Am. Meteorol. Soc. 2004, 85, 367–380. [Google Scholar] [CrossRef]
- Aldabe, J.; Elustondo, D.; Santamaría, C.; Lasheras, E.; Pandolfi, M.; Alastuey, A.; Querol, X.; Santamaría, J.M. Chemical characterization and source apportionment of PM2.5 and PM10 at rural, urban and traffic sites in Navarra (North of Spain). Atmos. Res. 2011, 102, 191–205. [Google Scholar] [CrossRef]
- Guo, H.; Ding, A.J.; So, K.L.; Ayoko, G.; Li, Y.S.; Hung, W.T. Receptor modeling of source apportionment of Hong Kong aerosols and implication of urban and regional contribution. Atmos. Environ. 2009, 43, 1159–1169. [Google Scholar] [CrossRef]
- Xu, L.; Chen, X.; Chen, J.; Zhang, F.; He, C.; Du, K.; Wang, Y. Characterization of PM10 atmospheric aerosol at urban and urban background sites in Fuzhou city, China. Environ. Sci. Pollut. Res. 2012, 19, 1443–1453. [Google Scholar] [CrossRef]
- Watson, J.G.; Zhu, T.; Chow, J.C.; Engelbrecht, J.; Fujita, E.M.; Wilson, W.E. Receptor modeling application framework for particle source apportionment. Chemosphere 2002, 49, 1093–1136. [Google Scholar] [CrossRef]
- Wagstrom, K.M.; Pandis, S.N. Source–receptor relationships for fine particulate matter concentrations in the Eastern United States. Atmos. Environ. 2011, 45, 347–356. [Google Scholar] [CrossRef]
- Amil, N.; Latif, M.T.; Khan, M.F.; Mohamad, M. Seasonal variability of PM2.5 composition and sources in the Klang Valley urban-industrial environment. Atmos. Chem. Phys. 2016, 16, 5357–5381. [Google Scholar] [CrossRef]
- Ho, K.F.; Cao, J.J.; Lee, S.C.; Chan, C.K. Source apportionment of PM2.5 in urban area of Hong Kong. J. Hazard. Mater. 2006, 138, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Hopke, P.K.; Gladnet, E.S.; Gordon, G.E.; Zoller, W.H.; Jones, A.G. The use of multivariate analysis to identify sources of selected elements in the Boston aerosol. Atmos. Environ. 1976, 10, 1015–1025. [Google Scholar] [CrossRef]
- Uno, I.; Yuminoto, K.; Osada, K.; Wang, Z.; Pan, X. Dust acid uptake analysis during long-lasting dust and pollution episodes over east Asia based on synergetic observation and chemical transport model. SOLA 2017, 13, 109–113. [Google Scholar] [CrossRef]
- Lim, Y.K.; Kim, J.; Lee, H.C.; Lee, S.S.; Cha, J.W.; Ryoo, S.B. Aerosol Physical Characteristics over the Yellow Sea During the KORUS-AQ Field Campaign: Observation and Air Quality Model Simulations. Asia-Pacific J. Atmos. Sci. 2019, 55, 629–640. [Google Scholar] [CrossRef]
- Nakajima, T.; Tonna, G.; Rao, R.; Kaufman, Y.; Holben, B. Use of sky-brightness measurements from ground for remote sensing of particulate poly dispersions. Appl. Opt. 1996, 35, 2672–2686. [Google Scholar] [CrossRef]
- Sohn, B.J.; Nakajima, T.; Chun, H.W.; Aoki, K. More Absorbing Dust Aerosol Inferred from Sky Radiometer Measurements at Anmyeon, Korea. J. Meteorol. Soc. Jpn. 2007, 85, 815–823. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Song, C.H.; Kim, S.B.; Chun, Y.; Sohn, B.J.; Holben, B.N. Characteristics of aerosol types from AERONET sunphotometer measurements. Atmos. Environ. 2010, 44, 3110–3117. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.H.; Lee, H.C.; Higurashi, A.; Takemura, T.; Song, C.H. Consistency of the aerosol type classification from satellite remote sensing during the ABC EAREX campaign. J. Geophys. Res. 2007, 112, D22S33. [Google Scholar] [CrossRef]
- Mielonen, T.; Arola, A.; Komppula, M.; Kukkonen, J.; Koskinen, J.; De Leeuw, G.; Lehtinen, K.E.J. Comparison of CALIOP level 2 aerosol subtypes to aerosol types derived from AERONET inversion data. Geophys. Res. Lett. 2009, 36, L18804. [Google Scholar] [CrossRef]
- Kchih, H.; Perrino, C.; Cherif, S. Investigation of desert dust contribution to source apportionment of PM10 and PM2.5 from a Southern Mediterranean Coast. Aerosol Air Qual. Res. 2015, 15, 454–464. [Google Scholar] [CrossRef]
- Xu, H.; Bi, X.H.; Zheng, W.W.; Wu, J.H.; Feng, Y.C. Particulate matter mass and chemical component concentrations over four Chinese cities along the western Pacific coast. Environ. Sci. Pollut. Res. 2015, 22, 1940–1953. [Google Scholar] [CrossRef] [PubMed]
- Rogula-Kozłowska, W.; Klejnowski, K.; Rogula-Kopiec, P.; Ośródka, L.; Krajny, E.; Błaszczak, B.; Mathews, B. Spatial and seasonal variability of the mass concentration and chemical composition of PM2.5 in Poland. Air Qual. Atmos. Health. 2014, 7, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.J.; Song, J.M.; Cha, J.W.; Kim, J.; Ryoo, S.B.; Kang, C.H. Chemical composition characteristics of atmospheric aerosols in relation to haze, Asian dust and mixed haze-Asian dust episodes at Gosan Site in 2013. J. Kor. Soc. Atmos. Environ. 2016, 32, 289–304. [Google Scholar] [CrossRef][Green Version]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Satsangi, A.; Pachauri, T.; Singla, V.; Lakhani, A.; Maharaj Kumari, K. Water soluble ionic species in atmospheric aerosols: Concentrations and Sources at Agra in the Indo-Gangetic Plain (IGP). Aerosol Air Qual. Res. 2013, 13, 1877–1889. [Google Scholar] [CrossRef]
- Harrison, R.M.; Smith, D.T.J.; Luhana, L. Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK. Environ. Sci. Technol. 1996, 30, 825–832. [Google Scholar] [CrossRef]
- Koçak, M.; Theodosi, C.; Zarmapas, P.; Im, U.; Bougiatioti, A.; Yenigun, O.; Mihalopoulos, N. Particulate matter(PM10) in Istanbul: Origin, source areas and potential impact on surrounding regions. Atmos. Environ. 2011, 45, 6891–6900. [Google Scholar] [CrossRef]
- Mustaffa, N.I.H.; Latif, M.T.; Ali, M.M.; Khan, M.F. Source apportionment of surfactants in marine aerosols at different locations along the Malacca Straits. Environ. Sci. Pollut. Res. 2014, 21, 6590–6602. [Google Scholar] [CrossRef]
- Deshmukh, D.K. Characterization of dicarboxylates inorganic ions in urban PM10 aerosols in the Eastern Central India. Aerosol Air Qual. Res. 2012, 12, 592–607. [Google Scholar] [CrossRef]
- Yin, L.; Niu, Z.; Chen, X.; Chen, J.; Zhang, F.; Xu, L. Characteristics of water-soluble inorganic ions in PM2.5 and PM2.5-10 in the coastal urban agglomeration along the western Taiwan Strait Region, China. Environ. Sci. Pollut. Res. 2014, 21, 5141–5156. [Google Scholar] [CrossRef]
- Khamkaew, C.; Chantara, S.; Janta, R.; Pani, S.K.; Prapamontol, T.; Kawichai, S.; Wiriya, W.; Lin, N.H. Investigation of Biomass Burning Chemical Components over Northern Southeast Asia during 7-SEAS/BASELInE 2014 Campaign. Aerosol Air Qual. Res. 2016, 16, 2655–2670. [Google Scholar] [CrossRef]
- Meng, C.C.; Wang, L.T.; Zhang, F.F.; Wei, Z.; Ma, S.M.; Ma, X.; Yang, J. Characteristics of concentrations and water-soluble inorganic ions in PM2.5 in Handan City, Hebei province, China. Atmos. Res. 2016, 171, 133–146. [Google Scholar] [CrossRef]
- Gao, Y.; Lee, S.C.; Huang, Y.; Chow, J.C.; Watson, J.G. Chemical characterization and source apportionment of size-resolved particles in Hong Kong sub-urban area. Atmos. Res. 2016, 170, 112–122. [Google Scholar] [CrossRef]
Figure 1.
Surface weather chart for (a) 29 December 2013 at 18:00 KST, (b) 30 December 2013 at 15:00 KST, (c) 31 December 2013 at 12:00 KST, and (d) 1 January 2014 at 03:00 KST.
Figure 1.
Surface weather chart for (a) 29 December 2013 at 18:00 KST, (b) 30 December 2013 at 15:00 KST, (c) 31 December 2013 at 12:00 KST, and (d) 1 January 2014 at 03:00 KST.
Figure 2.
Time series of (a) PM10, PM2.5, and PM1.0 mass concentrations; (b) PM2.5/PM10 ratio; (c) wind direction and wind speed (─: surface (Seoul), △: 850 hPa (Osan)); and (d) humidity and visibility from 30 December 2013 to 2 January 2014.
Figure 2.
Time series of (a) PM10, PM2.5, and PM1.0 mass concentrations; (b) PM2.5/PM10 ratio; (c) wind direction and wind speed (─: surface (Seoul), △: 850 hPa (Osan)); and (d) humidity and visibility from 30 December 2013 to 2 January 2014.
Figure 3.
(a) Aerosol optical depth (500 nm), Ångström Exponent, and Single Scattering Albedo (500 nm) and (b) the frequency distribution of aerosol types from 31 December 2013 and 1 January 2014.
Figure 3.
(a) Aerosol optical depth (500 nm), Ångström Exponent, and Single Scattering Albedo (500 nm) and (b) the frequency distribution of aerosol types from 31 December 2013 and 1 January 2014.
Figure 4.
Time series of 1 h averaged (a) ionic concentrations and (b) their ionic compositions during the study period.
Figure 4.
Time series of 1 h averaged (a) ionic concentrations and (b) their ionic compositions during the study period.
Figure 5.
72 h HYSPLIT backward trajectories at a height of 500 m arriving at the Seoul, colored according to the concentration of pollutants (in µg m−3) for the (a) haze, (b) mixed haze/Asian dust, and (c) Asian dust episodes.
Figure 5.
72 h HYSPLIT backward trajectories at a height of 500 m arriving at the Seoul, colored according to the concentration of pollutants (in µg m−3) for the (a) haze, (b) mixed haze/Asian dust, and (c) Asian dust episodes.
Figure 6.
Time series variations of 1 h averaged estimated concentrations of (NH4)2SO4 and NH4NO3 and neutralization factors by NH3 and CaCO3 during the high concentration episode.
Figure 6.
Time series variations of 1 h averaged estimated concentrations of (NH4)2SO4 and NH4NO3 and neutralization factors by NH3 and CaCO3 during the high concentration episode.
Figure 7.
Source apportionments of water-soluble species in each episode: (a) haze, (b) mixed haze/Asian dust, and (c) Asian dust.
Figure 7.
Source apportionments of water-soluble species in each episode: (a) haze, (b) mixed haze/Asian dust, and (c) Asian dust.
Table 1.
Correlation between the ionic species during the (a) haze, (b) mixed haze/Asian dust, and (c) Asian dust episodes.
Table 1.
Correlation between the ionic species during the (a) haze, (b) mixed haze/Asian dust, and (c) Asian dust episodes.
| (a) Haze |
|---|
| Species | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | Cl− | NO2− | NO3− | SO42− | CO32− |
|---|
| Na+ | 1.000 | | | | | | | | | |
| NH4+ | 0.268 | 1.000 | | | | | | | | |
| K+ | 0.471 | 0.737 | 1.000 | | | | | | | |
| Mg2+ | 0.863 | 0.441 | 0.530 | 1.000 | | | | | | |
| Ca2+ | 0.450 | 0.645 | 0.404 | 0.631 | 1.000 | | | | | |
| Cl− | 0.276 | 0.200 | 0.062 | 0.158 | 0.543 | 1.000 | | | | |
| NO2− | −0.232 | −0.514 | −0.302 | −0.334 | −0.827 | −0.683 | 1.000 | | | |
| NO3− | 0.142 | 0.783 | 0.632 | 0.351 | 0.231 | −0.352 | 0.056 | 1.000 | | |
| SO42− | 0.342 | 0.880 | 0.650 | 0.486 | 0.773 | 0.358 | −0.773 | 0.470 | 1.000 | |
| CO32− | 0.473 | 0.647 | 0.415 | 0.654 | 1.000 | 0.536 | −0.819 | 0.239 | 0.773 | 1.000 |
| (b) Mixed haze/Asian dust |
| Species | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | Cl− | NO2− | NO3− | SO42− | CO32− |
| Na+ | 1.000 | | | | | | | | | |
| NH4+ | 0.071 | 1.000 | | | | | | | | |
| K+ | 0.439 | 0.107 | 1.000 | | | | | | | |
| Mg2+ | 0.777 | −0.314 | 0.663 | 1.000 | | | | | | |
| Ca2+ | 0.609 | −0.223 | 0.526 | 0.838 | 1.000 | | | | | |
| Cl− | 0.117 | 0.700 | −0.077 | −0.368 | −0.321 | 1.000 | | | | |
| NO2− | −0.158 | 0.188 | −0.536 | −0.440 | −0.069 | 0.324 | 1.000 | | | |
| NO3− | 0.212 | 0.971 | 0.260 | −0.144 | −0.131 | 0.605 | 0.053 | 1.000 | | |
| SO42− | 0.415 | 0.848 | 0.436 | 0.190 | 0.254 | 0.427 | −0.069 | 0.904 | 1.000 | |
| CO32− | 0.626 | −0.231 | 0.540 | 0.857 | 0.999 | −0.327 | −0.094 | −0.133 | 0.252 | 1.000 |
| (c) Asian dust |
| Species | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | Cl− | NO2− | NO3− | SO42− | CO32− |
| Na+ | 1.000 | | | | | | | | | |
| NH4+ | 0.538 | 1.000 | | | | | | | | |
| K+ | −0.063 | 0.702 | 1.000 | | | | | | | |
| Mg2+ | −0.785 | −0.098 | 0.419 | 1.000 | | | | | | |
| Ca2+ | 0.947 | 0.573 | 0.100 | −0.744 | 1.000 | | | | | |
| Cl- | 0.901 | 0.743 | 0.203 | −0.644 | 0.888 | 1.000 | | | | |
| NO2− | −0.844 | −0.284 | 0.116 | 0.764 | −0.871 | −0.753 | 1.000 | | | |
| NO3- | 0.695 | 0.969 | 0.579 | −0.258 | 0.709 | 0.848 | −0.438 | 1.000 | | |
| SO42- | 0.760 | 0.911 | 0.564 | -0.347 | 0.824 | 0.849 | -0.556 | 0.955 | 1.000 | |
| CO32- | 0.912 | 0.649 | 0.226 | -0.618 | 0.985 | 0.879 | -0.828 | 0.768 | 0.880 | 1.000 |
Table 2.
Principal component analysis results for water-soluble species during the haze, mixed haze/Asian dust, and Asian dust episodes, with loading > 0.5 in bold. The analysis used a varimax rotation with Kaiser normalization.
Table 2.
Principal component analysis results for water-soluble species during the haze, mixed haze/Asian dust, and Asian dust episodes, with loading > 0.5 in bold. The analysis used a varimax rotation with Kaiser normalization.
| Species | Haze | Mixed haze/Asian Dust | Asian Dust |
|---|
| PC 1 | PC 2 | PC 3 | PC 1 | PC 2 | PC 3 | PC 4 | PC 1 | PC 2 | PC 3 |
|---|
| Na+ | 0.171 | | 0.951 | 0.702 | 0.155 | 0.166 | 0.624 | 0.830 | 0.450 | |
| NH4+ | 0.429 | 0.875 | 0.100 | | 0.973 | | | 0.186 | 0.963 | 0.154 |
| K+ | 0.145 | 0.752 | 0.380 | 0.531 | 0.302 | 0.651 | | | 0.649 | 0.712 |
| Mg2+ | 0.238 | 0.301 | 0.889 | 0.866 | | 0.421 | 0.178 | | | 0.511 |
| Ca2+ | 0.825 | 0.323 | 0.346 | 0.985 | | | | 0.888 | 0.437 | |
| Cl− | 0.823 | | 0.113 | | 0.602 | | 0.586 | 0.682 | 0.669 | |
| NO2− | | | | | | | | | | |
| NO3− | | 0.941 | 0.105 | | 0.978 | | 0.105 | 0.350 | 0.929 | |
| SO42− | 0.672 | 0.659 | 0.148 | 0.295 | 0.928 | 0.120 | | 0.519 | 0.824 | 0.176 |
| CO32− | 0.813 | 0.326 | 0.372 | 0.987 | | | | 0.840 | 0.507 | |
| Eigenvalue | 3.7 | 3.1 | 2.2 | 3.7 | 3.3 | 1.7 | 0.8 | 4.7 | 4.0 | 0.9 |
| Variance (%) | 36.9 | 30.5 | 21.6 | 36.8 | 32.7 | 16.6 | 8.0 | 46.7 | 40.0 | 9.4 |
| Cumulative (%) | 36.9 | 67.5 | 89.0 | 36.8 | 69.6 | 86.2 | 94.2 | 46.7 | 86.7 | 96.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).