Contribution of Satellite-Derived Aerosol Optical Depth PM2.5 Bayesian Concentration Surfaces to Respiratory-Cardiovascular Chronic Disease Hospitalizations in Baltimore, Maryland
Abstract
1. Introduction
2. Methods
2.1. Description of Study Participants
2.2. HBM Inputs
2.2.1. Monitor PM2.5
2.2.2. CMAQ PM2.5
2.2.3. AOD-PM2.5
2.3. AOD-PM2.5 Fused Surfaces
2.4. ED/IP Chronic Diseases
2.4.1. Subjects
2.4.2. Cases–Controls
2.5. Confounders
2.5.1. Co-Morbid Conditions (Diabetes, Hypertension, Atherosclerosis)
2.5.2. Apparent Temperature (AT and AT2)
2.5.3. Pollen
2.5.4. Holidays
2.5.5. Snowstorms
2.6. Effect Modifiers
2.6.1. Poverty and Population Density
2.6.2. Season
2.7. File Linkage
2.7.1. Case-Crossover Analyses
2.7.2. Statistical Analyses
2.8. Conditional Logistic Regression Analyses
2.8.1. Variable Selection
2.8.2. Variable Evaluation of Effect Modifiers
2.8.3. Final Models
2.8.4. Season and Monitor
3. Results
3.1. Cases and Controls
3.2. AOD-PM2.5 Concentration Surfaces
3.2.1. PMB and AOD-PM2.5
3.2.2. Correlations between AOD-PM2.5 and PMB
3.2.3. PM2.5 Concentration Values
3.3. Conditional Logistic Regression
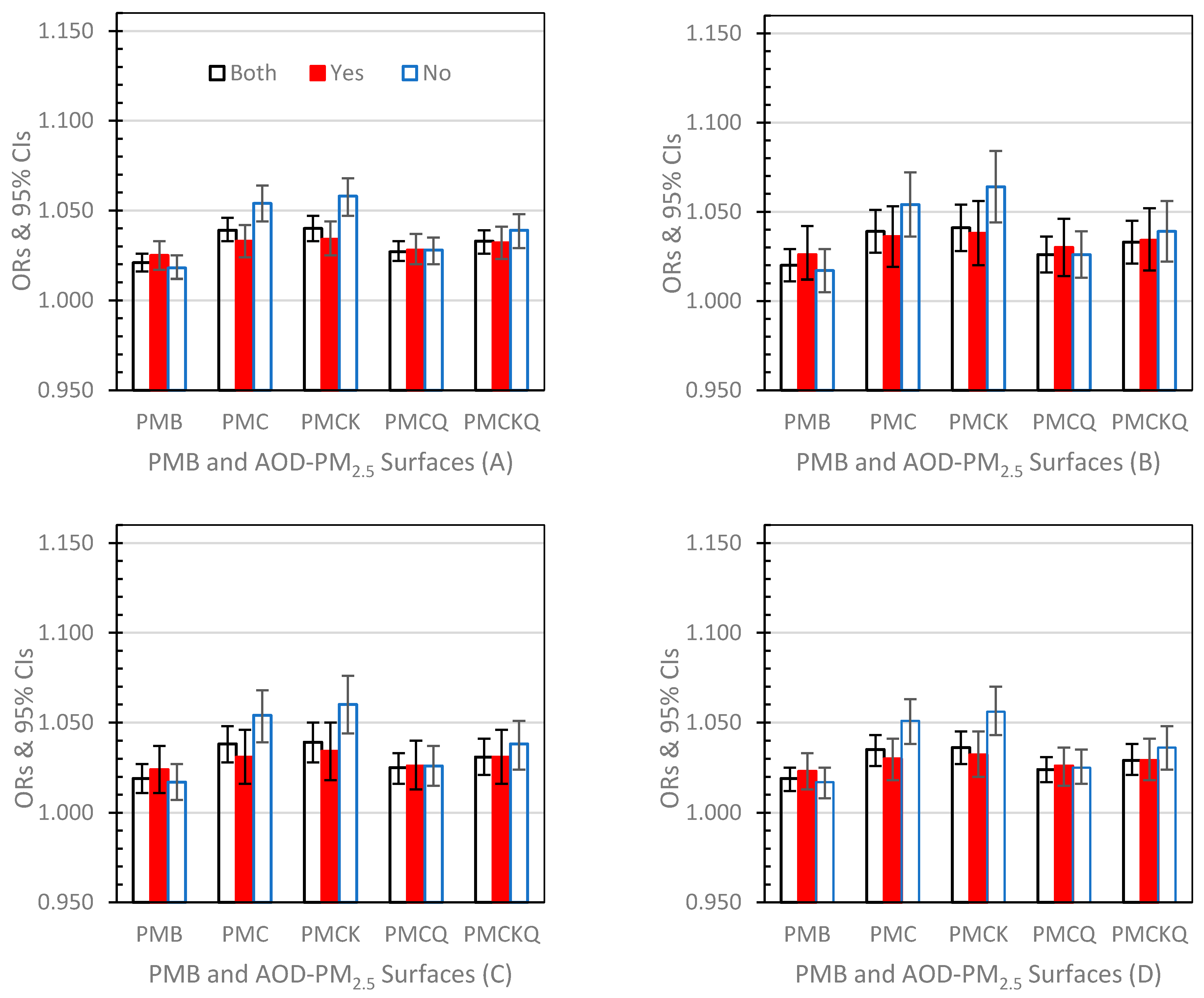
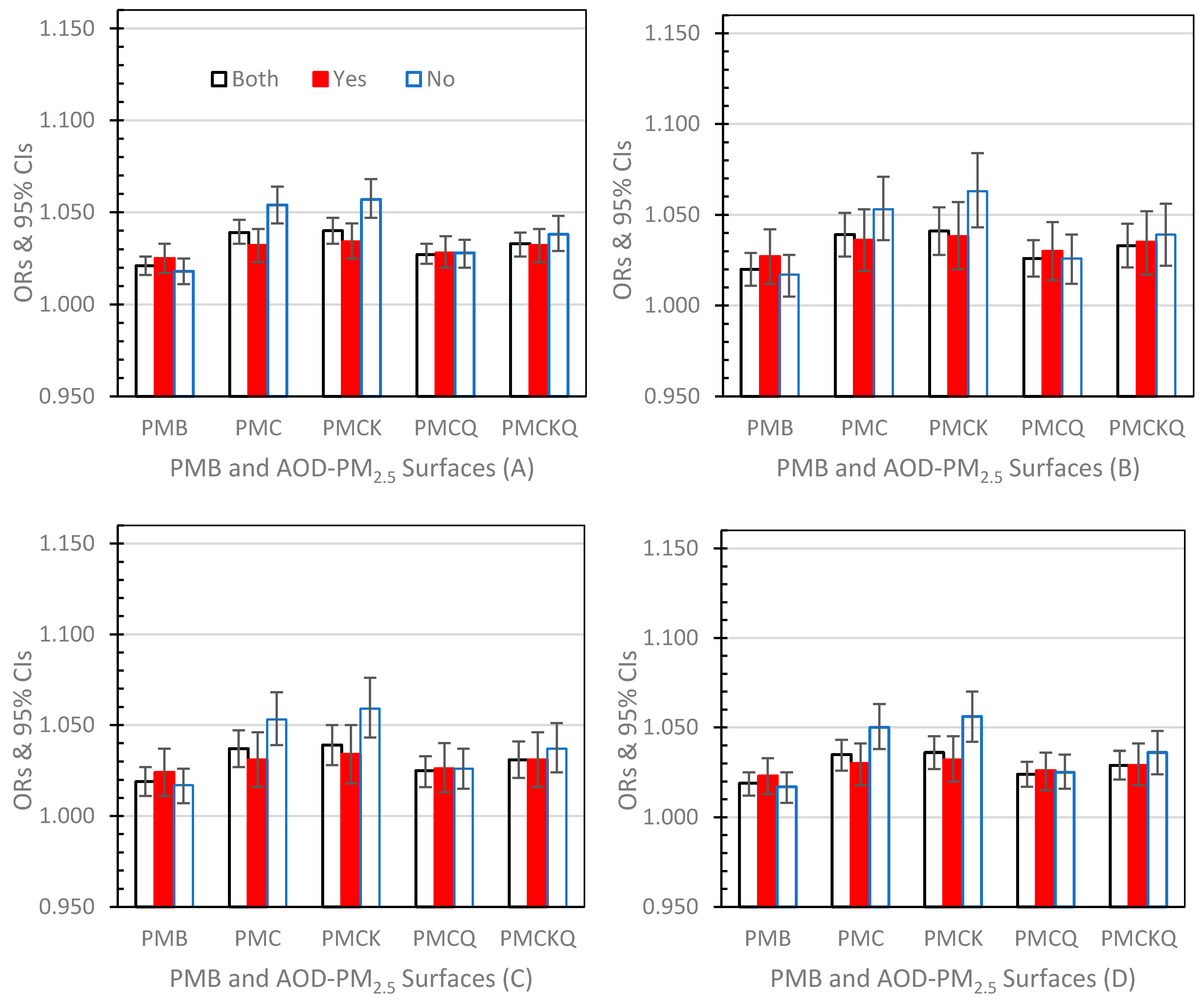
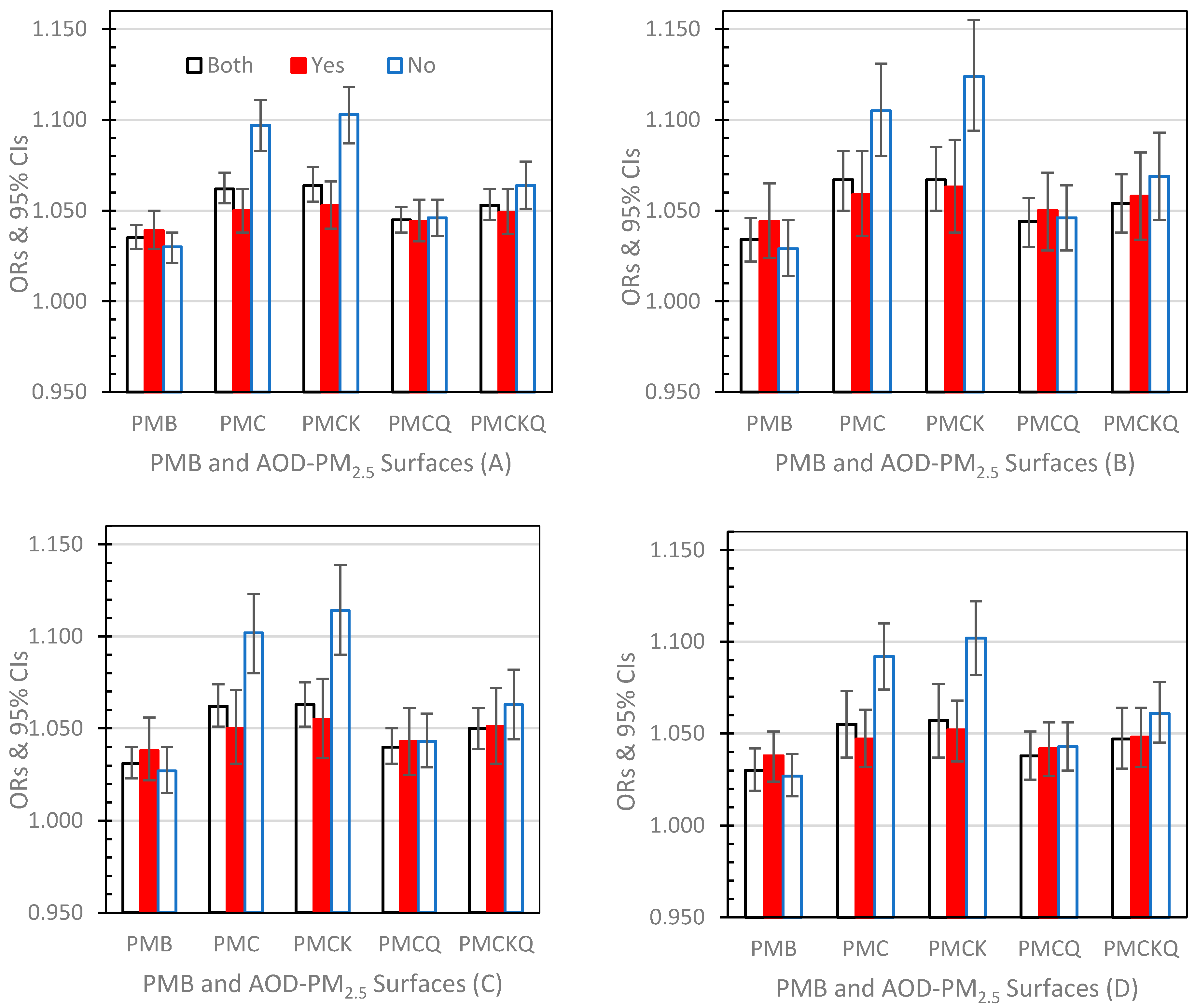
3.3.1. Lag Day 0 (Figure 2A–D)
3.3.2. Lag Day 1 (Figure 3A–D)
3.3.3. Lag Day 01 (Figure 4A–D)
3.4. Lag Day and Monitor
3.5. Lag Day and Season
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
Abbreviations
| AIC | Akaike Information Criterion |
| AOD | Aerosol Optical Depth |
| AQS | Air Quality System |
| AT | Apparent Temperature |
| AT2 | Product of AT, AT*AT |
| CDC | U.S. Centers for Disease Control and Prevention |
| CI | 95% Confidence Interval |
| CMAQ | Community Multiscale Air Quality Model |
| ED | Emergency Department |
| EPA | U.S. Environmental Protection Agency |
| EPHT | Environmental Public Health Tracking |
| FRM | Federal Reference Method |
| GIS | Geographic Information System |
| HBM | Hierarchical Bayesian Model |
| HF | Heart Failure |
| HSCRC | Maryland Health Services Cost Review Commission |
| ICD-9-CM | International Classification of Diseases, Ninth Revision, Clinical Modification |
| IP | Inpatient Hospitalization |
| MDH | Maryland Department of Health |
| MDP | Maryland Department of Planning |
| MI | Myocardial Infarction |
| MODIS | MODerate resolution Imaging Spectroradiometer |
| LAADS | Level-1 and Atmosphere Archive and Distribution System |
| NASA | National Aeronautics and Space Administration |
| NCEI | National Centers for Environmental Information |
| NOAA | National Oceanic and Atmospheric Administration |
| OPM | U.S. Office of Personnel Management |
| OR | Odds Ratio |
| PHASE | Public Health Air Surveillance Evaluation |
| PHREG | Proportional Hazards Regression |
| PM2.5 | Fine Particulate Matter |
| PMB | PM2.5 Baseline Model (monitor PM2.5 and CMAQ PM2.5) |
| PMC | AOD PM2.5 Model (monitor PM2.5 and AOD PM2.5) |
| PMCK | AOD PM2.5 Kriged Model (monitor PM2.5 and AOD PM2.5 Kriged) |
| PMCKQ | AOD PM2.5 Kriged and CMAQ PM2.5 Model (monitor PM2.5 and AOD PM2.5 Kriged and CMAQ PM2.5) |
| PMCQ | AOD PM2.5 and CMAQ PM2.5 Model (monitor PM2.5 and AOD PM2.5 and CMAQ PM2.5) |
| SAS | Statistical Analysis System |
| USCB | U.S. Census Bureau/Department of Commerce |
| ZCTA | ZIP Code Tabulation Area (US Census) |
| ZIP Code | Zone Improvement Plan (US Postal Service) |
References
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xu, X.; Chu, M.; Guo, Y.; Wang, J. Air particulate matter and cardiovascular disease: The epidemiological, biomedical, and clinical evidence. J. Thorac. Dis. 2016, 8, E8–E19. [Google Scholar] [CrossRef]
- Egondi, T.; Ettarh, R.; Kyobutungi, C.; Ng, N.; Rocklöv, J. Exposure to outdoor particles (PM2.5) and associated child morbidity and mortality in socially deprived neighborhoods of Nairobi, Kenya. Atmosphere 2018, 9, 351. [Google Scholar] [CrossRef]
- Garcia, E.; Berhane, K.T.; Islam, T.; McConnel, R.; Urman, R.; Chen, Z.; Gilliland, F.D. Association of changes in air quality with incident asthma in children in California, 1993–2014. JAMA 2019, 321, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Kirrane, E.; Svendsgaard, D.; Ross, M.; Buckley, B.; Davis, A.; Johns, D.; Kotchmar, D.; Long, T.C.; Luben, T.J.; Smith, G.; et al. A comparison of risk estimates for the effect of short-term exposure to PM, NO2 and CO on cardiovascular hospitalizations and emergency department visits: Effect size modeling of study findings. Atmosphere 2011, 2, 688–701. [Google Scholar] [CrossRef]
- Kloog, I.; Nordio, F.; Zanobetti, A.; Coull, B.A.; Koutrakis, P.; Schwartz, J.D. Short term effects of particle exposure on hospital admissions in the Mid-Atlantic states: A population estimate. PLoS ONE 2014, 9, e88578. [Google Scholar] [CrossRef]
- Liu, H.Y.; Dunea, D.; Iordache, S.; Pohoata, A. A review of airborne matter effects on young children’s respiratory systems and diseases. Atmosphere 2018, 9, 150. [Google Scholar] [CrossRef]
- Miller, L.; Xu, X. Ambient PM2.5 human health effects—Findings in China and research directions. Atmosphere 2018, 9, 424. [Google Scholar] [CrossRef]
- Peters, A.; Dockery, D.W.; Muller, J.E.; Mittleman, M.A. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 2001, 103, 2810–2815. [Google Scholar] [CrossRef]
- Strickland, M.J.; Hao, H.; Hu, X.; Chang, H.H.; Darrow, L.A.; Liu, Y. Pediatric emergency visits and short-term changes in PM2.5 concentrations in the U.S. State of Georgia. Environ. Health Perspect. 2016, 124, 690–696. [Google Scholar] [CrossRef]
- Tétreault, L.F.; Doucet, M.; Gamache, P.; Fornier, M.; Brand, A.; Kosatsky, T.; Smargiassi, A. Childhood exposure to the ambient air pollutants and the onset of asthma: An administrative cohort study in Québec. Environ. Health Perspect. 2016, 124, 1276–1282. [Google Scholar] [CrossRef]
- Wu, Y.; Li, M.; Tian, Y.; Cao, Y.; Song, J.; Huang, Z.; Wang, X.; Hu, Y. Short-term effects of ambient fine particulate air pollution on inpatient visits for myocardial infarction in Beijing, China. Environ. Sci. Pollut. Res. Int. 2019, 26, 14178–14183. [Google Scholar] [CrossRef] [PubMed]
- Haley, V.B.; Talbot, T.O.; Felton, H.D. Surveillance of the short-term impact of fine particle air pollution on cardiovascular disease hospitalizations in New York State. Environ. Health 2009, 8, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Hirshon, J.M.; Shardell, M.; Alles, S.; Powell, J.L.; Squibb, K.; Ondov, J.; Blaisdell, C.J. Elevated ambient air zinc increases pediatric asthma morbidity. Environ. Health Perspect. 2008, 116, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Symons, J.M.; Wang, L.; Guallar, E.; Howell, E.; Dominici, F.; Schwab, M.; Ange, B.A.; Samet, J.; Ondov, J.; Harrison, D.; et al. A case-crossover study of fine particulate matter air pollution and onset of congestive heart failure symptom exacerbation leading to hospitalization. Am. J. Epidemiol. 2006, 164, 421–433. [Google Scholar] [CrossRef]
- Weber, S.A.; Insaf, T.Z.; Hall, E.S.; Talbot, T.O.; Huff, A.K. Assessing the impact of fine particulate matter (PM2.5) on respiratory-cardiovascular chronic diseases in the New York City Metropolitan area using hierarchical Bayesian Model estimates. Environ. Res. 2016, 151, 399–409. [Google Scholar] [CrossRef] [PubMed]
- EPA (U.S. Environmental Protections Agency). Air Quality System (AQS). Available online: https://www.epa.gov/aqs (accessed on 10 November 2019).
- Vaidyanathan, A.; Dimmick, W.F.; Kegler, S.R.; Qualters, J.R. Statistical air quality predictions for public health surveillance: Evaluation and generation of county level metrics of PM2.5 for the environmental public health tracking network. Int. J. Health Geogr. 2013, 12, 1–13. [Google Scholar] [CrossRef]
- Goldman, G.T.; Mulholland, J.A.; Russell, A.G.; Srivastava, A.; Strickland, M.J.; Klein, M.; Waller, L.A.; Tolbert, P.E.; Edgerton, E.S. Ambient air pollutant measurement error: Characterization and impacts in a time-series epidemiologic study in Atlanta. Environ. Sci. Technol. 2010, 44, 7692–7698. [Google Scholar] [CrossRef]
- Boothe, V.; Dimmick, F.; Haley, V.; Paulu, C.; Bekkedal, M.; Holland, D.; Talbot, T.; Smith, A.; Warner, M.; Baldridge, E.; et al. A review of public health air surveillance evaluation project. Epidemiology 2006, 17, S450–S451. [Google Scholar] [CrossRef]
- Boothe, V.; Dimmick, W.F.; Talbot, T.O. Relating air quality and environmental public health tracking data. WIT Trans. Ecol. Environ. 2005, 85, 43–52. [Google Scholar] [CrossRef]
- Talbot, T.O.; Haley, V.B.; Dimmick, W.F.; Paulu, C.; Talbott, E.O.; Rager, J. Developing consistent data and methods to measure the public health impacts of ambient air quality for Environmental Public Health Tracking: Progress to date and future directions. Air Q. Atmos. Health 2009, 2, 199–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Appel, K.W.; Napelenok, S.L.; Foley, K.M.; Pye, H.O.T.; Hogrefe, C.; Luecken, D.J.; Bash, J.O.; Roselle, S.J.; Pleim, J.E.; Foroutan, H.; et al. Description and evaluation of the Community Multiscale Air Quality (CMAQ) modeling system version 5.1. GeoSci. Model Dev. 2017, 10, 1703–1732. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.M.; Roselle, S.J.; Appel, K.W.; Bhave, P.V.; Pleim, J.E.; Otte, T.L.; Mathur, R.; Sarwar, G.; Young, J.O.; Gilliam, R.C.; et al. Incremental testing of the Community Multiscale Air Quality (CMAQ) modeling system version 4.7. GeoSci. Model Dev. 2010, 3, 205–226. [Google Scholar] [CrossRef]
- EPA. Community Modeling and Analysis System (CMAS), CMAQ. Available online: https://www.cmascenter.org/ (accessed on 10 November 2019).
- Kearney, G.D.; Namulanda, G.; Qualters, J.R.; Talbott, E.O. A decade of environmental public health tracking (2002–2012): Progress and challenges. J. Public Health Manag. Pract. 2015, 2, S23–S35. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Liu, Y.; Li, X.; Liu, Z.; Lu, H.; Lu, Y.; Mao, Z.; Chen, X.; Li, N.; Ren, M.; et al. A Review on predicting ground PM2.5 concentration using satellite aerosol optical depth. Atmosphere 2016, 7, 129. [Google Scholar] [CrossRef]
- Lee, H.J.; Coull, B.A.; Bell, M.L.; Koutrakis, P. Use of satellite-based aerosol optical depth and spatial clustering to predict ambient PM2.5 concentrations. Environ. Res. 2012, 118, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sarnat, J.A.; Kilaru, V.; Jacob, D.J.; Koutrakis, P. Estimating ground-level PM2.5 in the eastern United States using satellite remote sensing. Environ. Sci. Technol. 2005, 39, 269–3278. [Google Scholar] [CrossRef]
- McGuinn, L.A.; Ward-Caviness, C.K.; Neas, L.M.; Schneider, A.; Diaz-Sanchez, D.; Cascio, W.E.; Kraus, W.E.; Hauser, E.; Dowdy, E.; Haynes, C.; et al. Association between satellite-based estimates of long-term PM2.5 exposure and coronary artery disease. Environ. Res. 2016, 145, 9–17. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Hu, M.; Pan, X.; Shi, J.; Chen, F.; He, K.; Koutrakis, P.; Christiani, D.C. Acute health impacts of airborne particles estimated from satellite remote sensing. Environ. Int. 2013, 51, 150–159. [Google Scholar] [CrossRef]
- Huang, J.; Kondragunta, S.; Laszlo, I.; Liu, H.; Remer, L.A.; Zhang, H.; Superczynski, S.; Ciren, P.; Holben, B.N.; Petrenko, M. Validation and expected error estimation of Suomi-NPP VIIRS aerosol optical thickness and Ångström exponent with AERONET. J. Geophys. Res. Atmos. 2016, 121, 7139–7160. [Google Scholar] [CrossRef]
- Kumar, N.; Chu, A.D.; Foster, A.D.; Peters, T.; Willis, R. Satellite remote sensing for developing time and space resolved estimates of ambient particulate in Cleveland, OH. Aerosol. Sci. Technol. 2011, 45, 1090–1108. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Liang, D.; Comellas, A.; Chu, A.D.; Abrams, T. Satellite-based PM concentrations and their application to COPD in Cleveland, OH. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, Z. High-resolution satellite-based analysis of ground-level PM2.5 for the City of Montreal. Sci. Total Environ. 2016, 541, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, Y.; Bilal, M.; Dong, W. Mapping daily PM2.5 at 500 m resolution over Beijing with improved hazy day performance. Sci. Total Environ. 2019, 659, 410–418. [Google Scholar] [CrossRef]
- Zhang, H.; Kondragunta, S.; Laszlo, I.; Liu, H.; Remer, L.A.; Huang, J.; Superczynski, S.; Ciren, P. An enhanced VIIRS aerosol optical thickness (AOT) retrieval algorithm over land using a global surface reflectance ratio database. J. Geophys. Res. Atmos. 2016, 121, 10717–10738. [Google Scholar] [CrossRef]
- Duncan, B.N.; Prados, A.I.; Lamsal, L.N.; Liu, Y.; Streets, D.G.; Gupta, P.; Hilsenrath, E.; Kahn, R.A.; Nielsen, J.E.; Beyersdorf, A.J.; et al. Satellite data of atmospheric pollution for U.S. air quality applications: Examples of applications, summary of data end-user resources, and answers to FAQs, and common mistakes to avoid. Atmos. Environ. 2014, 94, 647–662. [Google Scholar] [CrossRef]
- Hoff, R.M.; Christopher, S.A. Remote sensing of particulate pollution from space: Have we reached the promised land? J. Air Waste Manag. Assoc. 2009, 59, 645–675. [Google Scholar] [CrossRef] [PubMed]
- Hidy, G.M.; Brook, J.R.; Chow, J.C.; Green, M.; Husar, R.B.; Lee, C.; Scheffe, R.D.; Swanson, A.; Watson, J.G. Remote sensing of particulate pollution from space: Have we reached the promised land? J. Air Waste Manag. Assoc. 2009, 59, 1130–1139. [Google Scholar] [CrossRef]
- Engel-Cox, J.A.; Holloman, C.H.; Coutant, B.W.; Hoff, R.M. Qualitative and quantitative evaluation of MODIS. Satellite sensor data for regional and urban scale quality. Atmos. Environ. 2004, 38, 2495–2509. [Google Scholar] [CrossRef]
- Lee, S.J.; Serre, M.L.; van Donkelaar, A.; Martin, R.V.; Burnett, R.T.; Jerrett, M. Comparison of geostatistical interpolation and remote sensing techniques for estimating long-term exposure to ambient PM2.5 concentrations across the continental United States. Environ. Health Perspect. 2012, 120, 1727–1732. [Google Scholar] [CrossRef]
- Van Donkelaar, A.; Martin, R.V.; Brauer, M.; Boys, B.L. Use of satellite observations for long-term exposure assessment of global concentrations of fine particulate matter. Environ. Health Perspect. 2015, 123, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hoff, R.M.; Engel-Cox, J.A. The relation between Moderate Resolution Imaging Spectroradiometer (MODIS) aerosol optical depth and PM2.5 over the United States: A geographical comparison by U.S. Environmental Protection Agency Regions. J. Air Waste Manag. Assoc. 2009, 59, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- NASA (National Aeronautics and Space Administration). Level-1 and Atmosphere Archive and Distribution System (LAADS). Available online: https://ladsweb.modaps.eosdis.nasa.gov (accessed on 10 November 2019).
- McMillan, N.J.; Holland, D.M.; Morara, M.; Feng, J. Combining numerical model output and particulate data using Bayesian space-time modeling. Environmetrics 2009, 21, 48–65. [Google Scholar] [CrossRef]
- Weber, S.A.; Engel-Cox, J.A.; Hoff, R.M.; Prados, A.I.; Zhang, H. An improved method for estimating surface fine particle concentrations using seasonally adjusted satellite aerosol optical depth. J. Air Waste Manag. Assoc. 2010, 60, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Battelle Memorial Institute. User’s Guide: Temporal-Spatial Ambient Concentration Estimator (T-SpACE), Version 5_4h.0.1.21; Battelle Memorial Institute: Columbus, OH, USA, 2011. [Google Scholar]
- Hall, E.S. Temporal-Spatial Ambient Concentrator Estimator (T-SpACE): Hierarchical Bayesian Model Software Used to Estimate Ambient Concentrations of NAAQS Air Pollutants in Support of Health Studies; EPA/600/R-18/01; U.S. Environmental Protection Agency: Washington, DC, USA, 2018. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=339714 (accessed on 10 November 2019).
- HSCRC (Maryland Health Services Cost Review Commission). Available online: https://hscrc.maryland.gov (accessed on 10 November 2019).
- MDP (Maryland Department of Planning). Maryland State Data Center. Zip Code Boundary Area Files, 2004 and 2006. Available online: http://planning.maryland.gov/MSDC/Pages/s5_map_gis.aspx (accessed on 10 November 2019).
- CDC (U.S. Centers for Disease Control and Prevention). International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Available online: https://www.cdc.gov/nchs/icd/icd9cm.htm (accessed on 10 November 2019).
- Carracedo-Martinez, E.; Taracido, M.; Tobias, A.; Saez, M.; Figueiras, A. Case-crossover analysis of air pollution health effects: A systematic review of methodology and application. Environ. Health Perspect. 2010, 118, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, J.J.K. Case-crossover design in air pollution epidemiology. Eur. Respir. J. 2003, 40, 81s–85s. [Google Scholar] [CrossRef] [PubMed]
- Janes, H.; Sheppard, L.; Lumley, T. Case-crossover analyses of air pollution exposure data: Referent selection strategies and their implications for bias. Epidemiology 2005, 16, 717–726. [Google Scholar] [CrossRef]
- Maclure, M. The case-crossover design: A method for studying transient effects on the risk of acute events. Am. J. Epidemiol. 1991, 133, 144–153. [Google Scholar] [CrossRef]
- Stokes, M.E.; Davis, C.S.; Koch, G.G. Categorical Data Analysis Using the SAS System; SAS Institute, Inc.: Cary, NC, USA, 1995; ISBN 978-1-60764-664-8. [Google Scholar]
- Bai, Y.; Sun, Q. Fine particulate matter air pollution and atherosclerosis: Mechanistic insights. Biochim. Biophys. Acta 2016, 1860, 2863–2868. [Google Scholar] [CrossRef]
- Brook, R.D.; Kousha, T. Air pollution and emergency department visits for hypertension in Edmonton and Calgary, Canada: A case-crossover study. Am. J. Hypertens. 2015, 28, 1121–1126. [Google Scholar] [CrossRef]
- Chen, H.; Burnett, R.T.; Kwong, J.C.; Villeneuve, P.J.; Goldberg, M.S.; Brook, R.D.; van Donkelaar, A.; Jerrett, M.; Martin, R.V.; Brook, J.R.; et al. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ. Health Perspect. 2013, 121, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Dubowsky, S.D.; Suh, H.; Schwartz, J.; Coull, B.A.; Gold, D.R. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ. Health Perspect. 2006, 114, 992–998. [Google Scholar] [CrossRef]
- Hoshino, T.; Hoshino, A.; Nishino, J. Assessment of associations between ischaemic attacks in patients with type 2 diabetes mellitus and air concentrations of particulate matter <2.5 μm. J. Int. Med. Res. 2016, 44, 639–655. [Google Scholar] [CrossRef] [PubMed]
- NOAA (National Oceanic and Atmospheric Administration). The New Improved “Wind Chill Index. Available online: https://www.weather.gov/ffc/wci (accessed on 10 November 2019).
- Rothfusz, L.P. The Heat Index “Equation,” SR-90-23, 7/1/90. Available online: https://www.weather.gov/media/ffc/ta_htindx.PDF (accessed on 10 November 2019).
- DellaValle, C.T.; Triche, E.W.; Leaderer, B.P.; Bell, M.L. Effects of ambient pollen concentrations on frequency and severity of asthma symptoms among asthmatic children. Epidemiology 2012, 23, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Weichenthal, S.; Lavigne, E.; Villeneuve, P.J.; Reeves, F. Airborne pollen concentrations and emergency room visits for myocardial infarction: A multicity case-crossover study in Ontario, Canada. Am. J. Epidemiol. 2016, 183, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Sapkota, A.; Braggio, J.T. Pollen Effects on Asthma, Allergic Rhinitis, and Finger Wound Emergency Department Visits between 2000–2010 in Baltimore, Maryland. In Proceedings of the Council of State and Territorial Epidemiologists Annual Conference, Nashville, TN, USA, 25 June 2014; Available online: https://cste.confex.com/cste/2014/webprogram/Paper3178.html (accessed on 10 November 2019).
- Braggio, J.T.; Brunner, W.; Lutzker, L.; Mangan, A.; Simms, E.F.; Tomasallo, C.; Wahl, R.L.; Yip, F. Analysis of 2009 pollen readings. In Proceedings of the Council of State and Territorial Epidemiologists Annual Conference, Omaha, NE, USA, 4–7 June 2012. [Google Scholar]
- OPM (U.S. Office of Personnel Management). Federal Holidays, 2004–2006. Available online: https://archive.opm.gov/Operating_Status_Schedules/fedhol/2004.asp (accessed on 10 November 2019).
- NCEI (National Centers for Environmental Information). Climate Data Online. Available online: https://www.ncdc.noaa.gov/cdo-web/ (accessed on 10 November 2019).
- Brochu, P.J.; Yanosky, J.D.; Pacioreck, C.J.; Schwartz, J.; Chen, J.T.; Herrick, R.F.; Suh, H.H. Particulate air pollution and socioeconomic position in rural and urban areas of the northeastern United States. Am. J. Public Health 2011, 101, S224–S230. [Google Scholar] [CrossRef]
- Bell, M.L.; Ebisu, K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ. Health Perspect. 2012, 120, 1699–1704. [Google Scholar] [CrossRef]
- USCB (U.S. Census Bureau/Department of Commerce). 2000 Census of People and Housing, Summary File 3. Available online: https://www.census.gov/census2000/sumfile3.html (accessed on 10 November 2019).
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Walker, J.; Samet, J.M.; Zeger, S.L.; Dominici, F. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US counties, 1999–2005. Am. J. Epidemiol. 2008, 168, 1301–1310. [Google Scholar] [CrossRef]
- Chen, K.; Glonek, G.; Hansen, A.; Williams, S.; Tuke, J.; Salter, A.; Bi, P. The effects of air pollution on asthma hospital admissions in Adelaide, South Australia, 2003–2013: Time-series and case-crossover analyses. Clin. Exp. Allergy 2016, 46, 1416–1430. [Google Scholar] [CrossRef]
- Goldberg, M.S.; Burnett, R.T.; Stieb, D.M.; Brophy, J.M.; Daskalopoulou, S.S.; Valois, M.F.; Brook, J.R. Associations between ambient air pollution and daily mortality among elderly persons in Montreal, Quebec. Sci. Total Environ. 2013, 463–464, 931–942. [Google Scholar] [CrossRef]
- Hsu, W.H.; Hwang, S.A.; Kinney, P.L.; Lin, S. Seasonal and temperature modifications of the association between fine particulate air pollution and cardiovascular hospitalization in New York state. Sci. Total Environ. 2016, 578, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Pan, R.H.; Chan, C.K.; Wu, C.Y.; Phan, D.V.; Chan, C.L. Application of a time-stratified case-crossover design to explore the effects of air pollution and season on childhood asthma hospitalization in cities of differing urban patterns: Big data analytics of government open data. Int. J. Environ. Res. Public Health 2018, 15, 647. [Google Scholar] [CrossRef] [PubMed]
- Rodopoulou, S.; Samoli, E.; Chalbot, M.C.G.; Kavouras, I.G. Air pollution and cardiovascular and respiratory emergency visits in Central Arkansas: A time series analysis. Sci. Total Environ. 2015, 536, 872–879. [Google Scholar] [CrossRef] [PubMed]
- ESRI (Environmental Systems Research Institute). ArcGIS Desktop (ArcMap), Release 10.6.1; Environmental Systems Research Institute: Redlands, CA, USA, July 2018. [Google Scholar]
- SAS (Statistical Analysis System). Base SAS; SAS Institute Inc.: Cary, NC, USA, 2018. [Google Scholar]
- SAS (Statistical Analysis System). SAS/STAT 14.1 User’s Guide: High-Performance Procedures; SAS Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- Wang, S.V.; Coull, B.A.; Schwartz, J.; Mittleman, M.A.; Wellenius, G.A. Potential for bias in case-crossover studies with shared exposures analyzed using SAS. Am. J. Epidemiol. 2011, 174, 18–124. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-0-470-58247. [Google Scholar]
- Agresti, A. Categorical Data Analysis, 2nd ed.; John Wiley: Sons Hoboken, NJ, USA, 2002; ISBN 978-0-470-08289-8. [Google Scholar]
- Brook, R.D.; Bard, R.L.; Morishita, M.; Dvonch, T.; Wang, L.; Yang, H.Y.; Spino, C.; Mukherjee, B.; Kaplan, M.J.; Yalavarthi, S. Hemodynamic, autonomic, and vascular effects of exposure to course particulate matter air pollution from a rural location. Environ. Health Perspect. 2014, 122, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Chudnovsky, A.A.; Kostinski, A.; Lyapustin, A.; Koutrakis, P. Spatial scales of pollution from variable resolution satellite imaging. Environ. Pollut. 2013, 172, 131–138. [Google Scholar] [CrossRef]
- Gehring, U.; Wijga, A.H.; Hoek, G.; Bellander, T.; Berdel, D.; Brüske, I.; Fuertes, E.; Gruzieva, O.; Heinrich, J.; Hoffmann, B. Exposure to air pollution and development of Asthma and rhinoconjunctivitis throughout childhood and adolescence: A population-based birth cohort study. Lancet Respir. Med. 2015, 3, 933–942. [Google Scholar] [CrossRef]
- Hart, J.E.; Puett, R.C.; Rexrode, K.M.; Albert, C.M.; Laden, F. Effect modification of long-term air pollution exposures and the risk of incident cardiovascular disease in US women. J. Am. Heart Assoc. 2015, 4, e002301. [Google Scholar] [CrossRef]
- Lim, C.C.; Hayes, R.B.; Ahn, J.; Shao, Y.; Silverman, D.T.; Jones, R.R.; Garcia, C.; Thurston, G.D. Association between long-term exposure to ambient air pollution and diabetes mortality in the US. Environ. Res. 2018, 165, 330–336. [Google Scholar] [CrossRef]
- Yang, W.Y.; Zhang, Z.Y.; Thijs, L.; Bijnens, E.M.; Janssen, B.G.; Vanpoucke, C.; Lefebvre, W.; Cauwenberghs, N.; Wei, F.F.; Luttun, A.; et al. Left ventricular function in relation to chronic residential air pollution in a general population. Eur. J. Prev. Cardiol. 2017, 24, 1416–1428. [Google Scholar] [CrossRef]



| Variables 1–2 | ED Asthma Cases | ED Asthma Controls | IP Asthma Cases | IP Asthma Controls |
|---|---|---|---|---|
| Total | 11,723 (100) | 35,533 (100) | 3376 (100) | 10,139 (100) |
| Age Category 3–4 0–14 years | 5131 (43.8) † | 15,492 (43.6) † | 930 (27.6) | 2791 (27.5) |
| 15–34 years | 3223 (27.5) | 9765 (27.5) | 358 (10.6) | 1080 (10.6) |
| ≥35 years | 3369 (28.7) | 10,276 (28.9) | 2088 (61.8) | 6268 (61.8) |
| Gender—Female | 6093 (52.0) | 18,489 (52.0) | 2125 (62.9) | 6388 (63.0) |
| Male | 5628 (48.0) | 17,041 (48.0) | 1251 (37.1) | 3751 (37.0) |
| Race—Black | 5618 (48.1) | 17,078 (48.3) | 1130 (33.5) | 3380 (33.4) |
| Other | 749 (6.4) | 2311(6.5) | 164 (4.9) | 505 (5.0) |
| White | 5305 (45.4) | 15,989 (45.2) | 2076 (61.6) | 6236 (61.6) |
| Atherosclerosis—No | 11675 (99.6) | 35,387 (99.6) | 3055 (90.5) | 9176 (90.5) |
| Yes | 48 (0.4) | 146 (0.4) | 321 (9.5) | 963 (9.5) |
| Diabetes—No | 11458 (97.7) | 34,730 (97.7) | 2842 (84.2) | 8557 (84.4) |
| Yes | 265 (2.3) | 803 (2.3) | 534 (15.8) | 1582 (15.6) |
| Hypertension—No | 11,111 (94.8) | 33,650 (94.7) | 2316 (68.6) | 6950 (68.6) |
| Yes | 612 (5.2) | 1883 (5.3) | 1060 (31.4) | 3189 (31.4) |
| Insurance—No | 2099 (17.9) | 6409 (18.1) | 207 (6.1) | 623 (6.2) |
| Yes | 9606 (82.1) | 29,070 (81.9) | 3164 (93.9) | 9501 (93.8) |
| Poverty 5 | 9.6 (9.5–9.7) | 9.6 (9.5–9.7) | 9.4 (9.2–9.6) | 9.4 (9.2–9.5) |
| Monitor—No | 6.3 (6.3–6.4) * | 6.4 (6.3–6.4) * | 6.3 (6.2–6.5) * | 6.3 (6.3–6.4) * |
| Monitor—Yes | 13.7 (13.5–3.9) | 13.7 (13.6–13.8) | 13.6 (13.3–13.9) | 13.5 (13.4–13.7) |
| Population (Log10) 6 | 3.3 (3.3–3.3) | 3.3 (3.3–3.3) | 3.2 (3.2–3.2) | 3.2 (3.2–3.2) |
| Monitor—No | 3.1 (3.1–3.1) * | 3.1 (3.1–3.1) * | 2.9 (2.9–2.9) * | 2.9 (2.9–2.9) * |
| Monitor—Yes | 3.6 (3.6–3.6) | 3.6 (3.6–3.6) | 3.5 (3.5–3.6) | 3.5 (3.5–3.6) |
| Variables 1–2 | IP MI Cases | IP MI Controls | IP HF Cases | IP HF Controls |
|---|---|---|---|---|
| Total | 4745 (100) | 14276 (100) | 6919 (100) | 20,427 (100) |
| Age Category 3–4 35–59 years | 1477 (31.1) † | 4462 (31.3) † | 1279 (18.5) * | 3921 (19.2) |
| 60–75 years | 1638 (34.5) | 4907 (34.4) | 2531 (36.6) | 7119 (34.8) |
| ≥76 years | 1630 (34.4) | 4907 (34.4) | 3109 (44.9) | 9387 (46.0) |
| Gender—Female | 2041 (42.6) | 6155 (42.7) | 3559 (52.1) | 10,808 (52.2) |
| Male | 2749 (57.4) | 8256 (57.3) | 3267 (47.9) | 9884 (47.8) |
| Race-Black | 633 (13.2) | 1913 (13.3) | 1737 (25.5) | 5292 (25.6) |
| Other | 214 (4.5) | 634 (4.4) | 191 (2.8) | 602 (2.9) |
| White | 3937 (82.3) | 11,843 (82.3) | 4892 (71.7) | 14,780 (71.5) |
| Atherosclerosis—No | 1716 (35.8) | 5163 (35.8) | 3554 (52.1) | 10,777 (52.1) |
| Yes | 3074 (64.2) | 9248 (64.2) | 3272 (47.9) | 9915 (47.9) |
| Diabetes—No | 3363 (70.2) | 10,101 (70.1) | 3950 (57.9) | 11,948 (57.7) |
| Yes | 1427 (29.8) | 4310 (29.9) | 2876 (42.1) | 8744 (42.3) |
| Hypertension—No | 2511 (52.4) | 7499 (52.0) | 4022 (58.9) | 12197 (59.0) |
| Yes | 2279 (47.6) | 6912 (48.0) | 2804 (41.1) | 8495 (41.1) |
| Insurance—No | 149 (3.1) | 443 (3.1) | 111 (1.6) | 324 (1.6) |
| Yes | 4637 (96.9) | 13,956 (96.9) | 6710 (98.4) | 20,356 (98.4) |
| Poverty 5 | 8.3(8.2–8.4) | 8.4 (8.3–8.4) | 9.1 (9.0–9.2) | 9.2 (9.1–9.2) |
| Monitor—No | 6.0 (5.9–6.1) * | 6.0 (6.0–6.1) * | 6.4 (6.3–6.5) * | 6.4 (6.3–6.4) * |
| Monitor—Yes | 12.2 (11.9–12.4) | 12.3 (12.2–12.4) | 2.8 (12.6–13.0) | 12.8 (12.7–12.9) |
| Population (Log10) 6 | 3.1 (3.1–3.1) | 3.1 (3.1–3.1) | 3.2 (3.2–3.2) | 3.2 (3.2–3.2) |
| Monitor—No | 2.8 (2.8–2.8) * | 2.8 (2.8–2.8) * | 2.9 (2.9–2.9) * | 2.9 (2.9–2.9) * |
| Yes | 3.6 (3.5–3.6) | 3.6 (3.5–3.6) | 3.6 (3.6–3.6) | 3.6 (3.6–3.6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braggio, J.T.; Hall, E.S.; Weber, S.A.; Huff, A.K. Contribution of Satellite-Derived Aerosol Optical Depth PM2.5 Bayesian Concentration Surfaces to Respiratory-Cardiovascular Chronic Disease Hospitalizations in Baltimore, Maryland. Atmosphere 2020, 11, 209. https://doi.org/10.3390/atmos11020209
Braggio JT, Hall ES, Weber SA, Huff AK. Contribution of Satellite-Derived Aerosol Optical Depth PM2.5 Bayesian Concentration Surfaces to Respiratory-Cardiovascular Chronic Disease Hospitalizations in Baltimore, Maryland. Atmosphere. 2020; 11(2):209. https://doi.org/10.3390/atmos11020209
Chicago/Turabian StyleBraggio, John T., Eric S. Hall, Stephanie A. Weber, and Amy K. Huff. 2020. "Contribution of Satellite-Derived Aerosol Optical Depth PM2.5 Bayesian Concentration Surfaces to Respiratory-Cardiovascular Chronic Disease Hospitalizations in Baltimore, Maryland" Atmosphere 11, no. 2: 209. https://doi.org/10.3390/atmos11020209
APA StyleBraggio, J. T., Hall, E. S., Weber, S. A., & Huff, A. K. (2020). Contribution of Satellite-Derived Aerosol Optical Depth PM2.5 Bayesian Concentration Surfaces to Respiratory-Cardiovascular Chronic Disease Hospitalizations in Baltimore, Maryland. Atmosphere, 11(2), 209. https://doi.org/10.3390/atmos11020209





