Physico-Chemical Characterization of an Urban Rainwater (Zagreb, Croatia)
Abstract
1. Introduction
2. Experiments
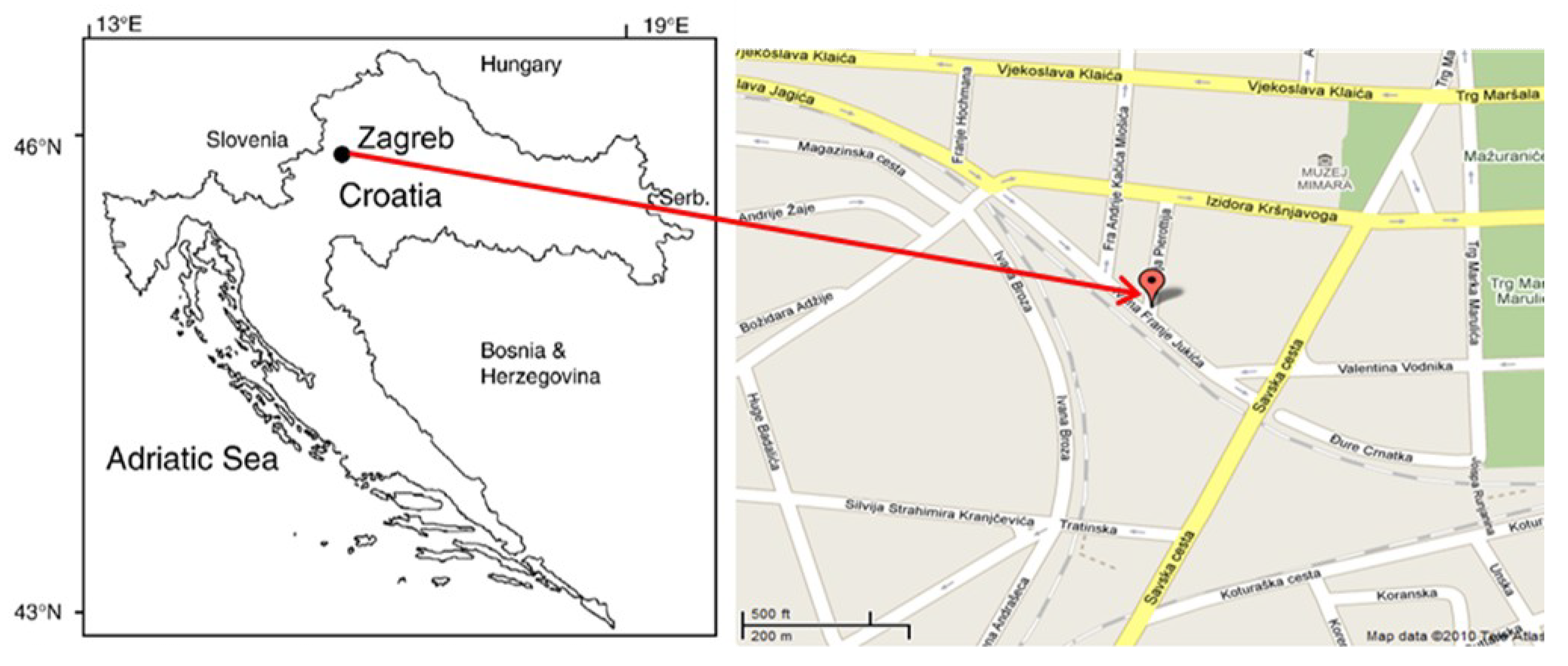
2.1. Area Description
2.2. Sampling Method and Sample Treatment Prior Analyses
2.3. Chemical Analyses
2.3.1. Organic Matter (DOC, POC Measurements)
2.3.2. Surface Activity of Rainwater DOC
2.3.3. CuCC Measurements
2.3.4. Reduced Sulfur Species
2.3.5. Trace Elements
2.3.6. pH Measurements
2.3.7. Correlation Analysis
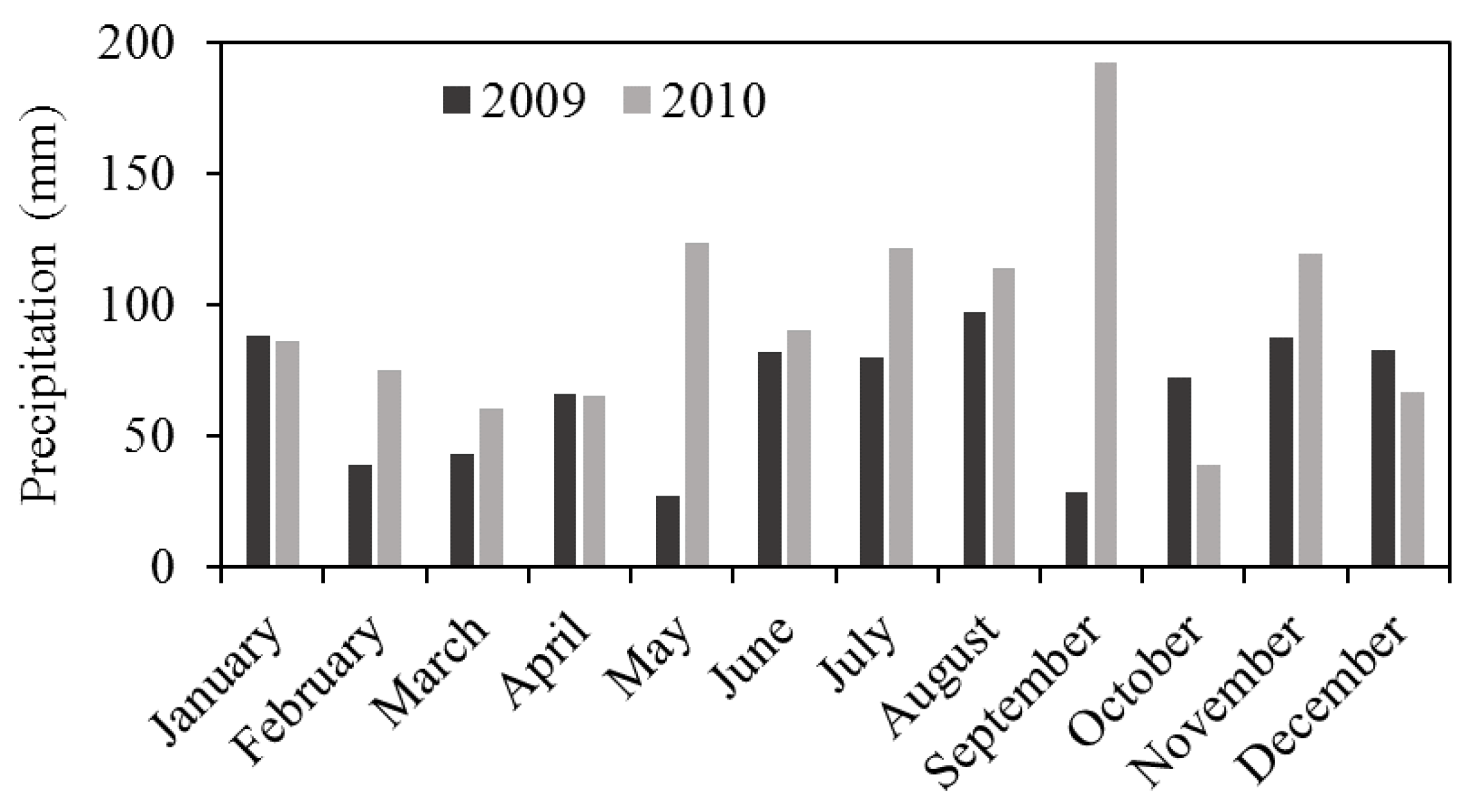
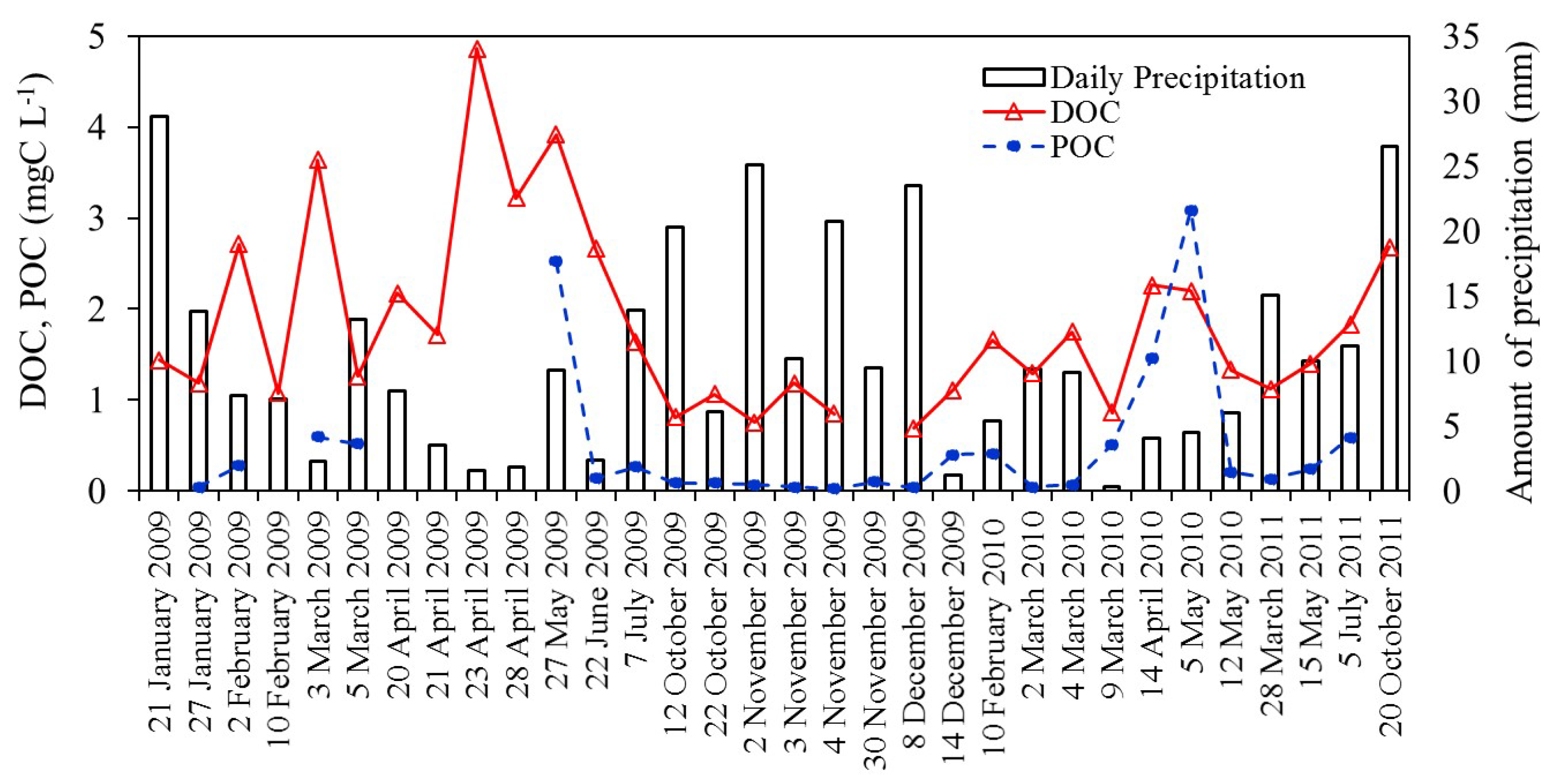
3. Results and Discussion
3.1. Organic Carbon
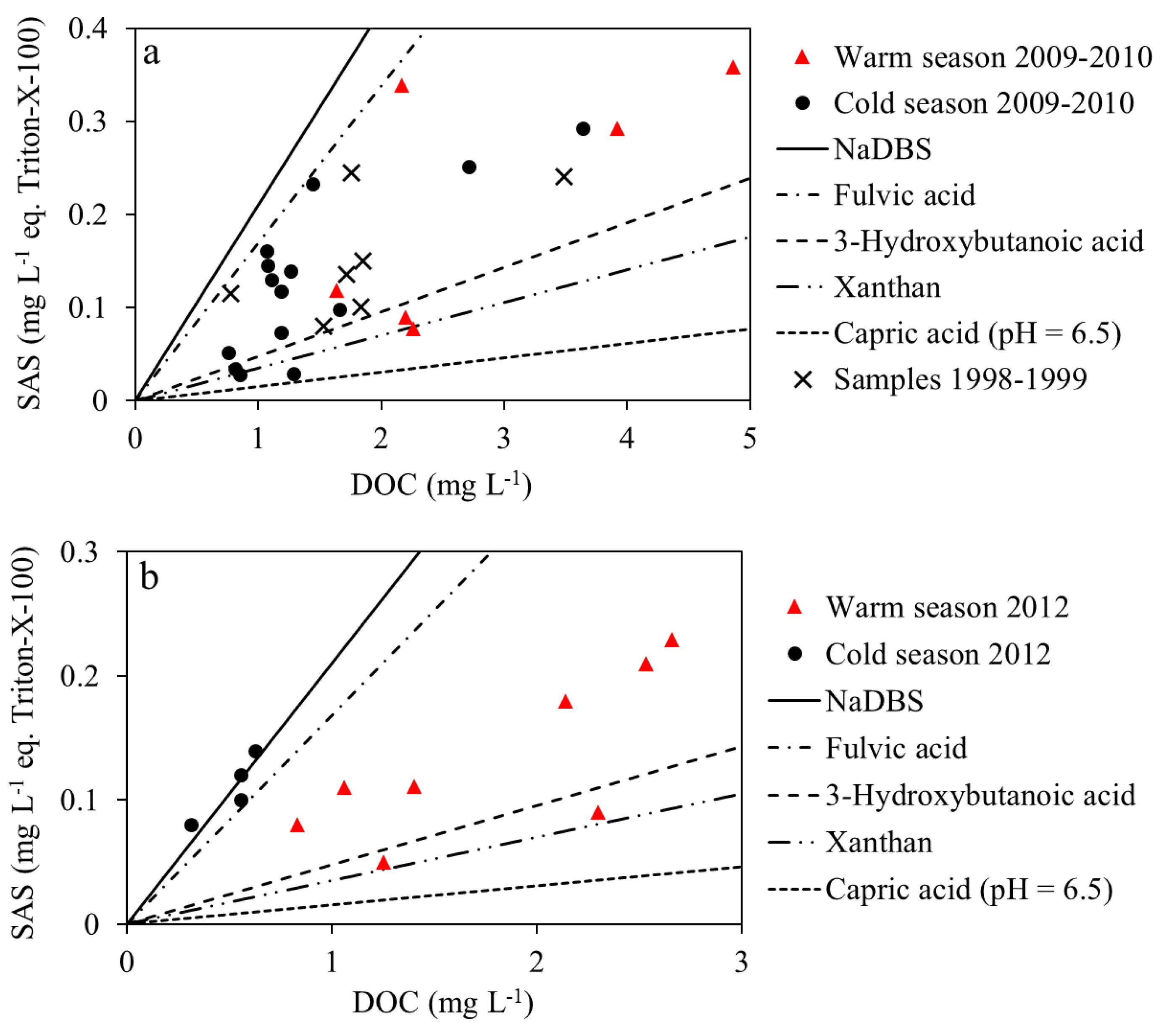
3.2. Surface Active Reactivity Characterization of DOC in Rainwater
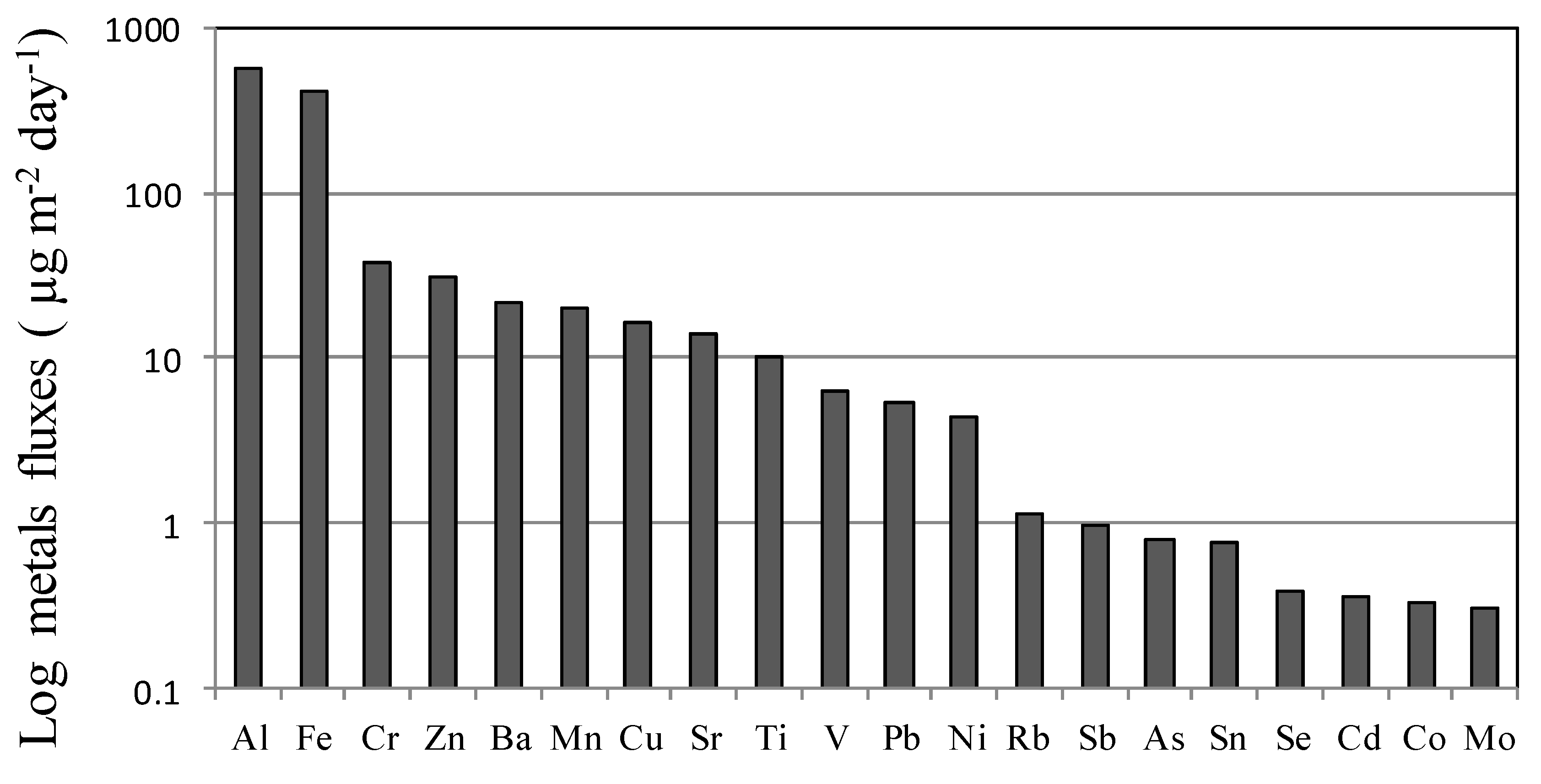
3.3. Metal Content in Rainwater
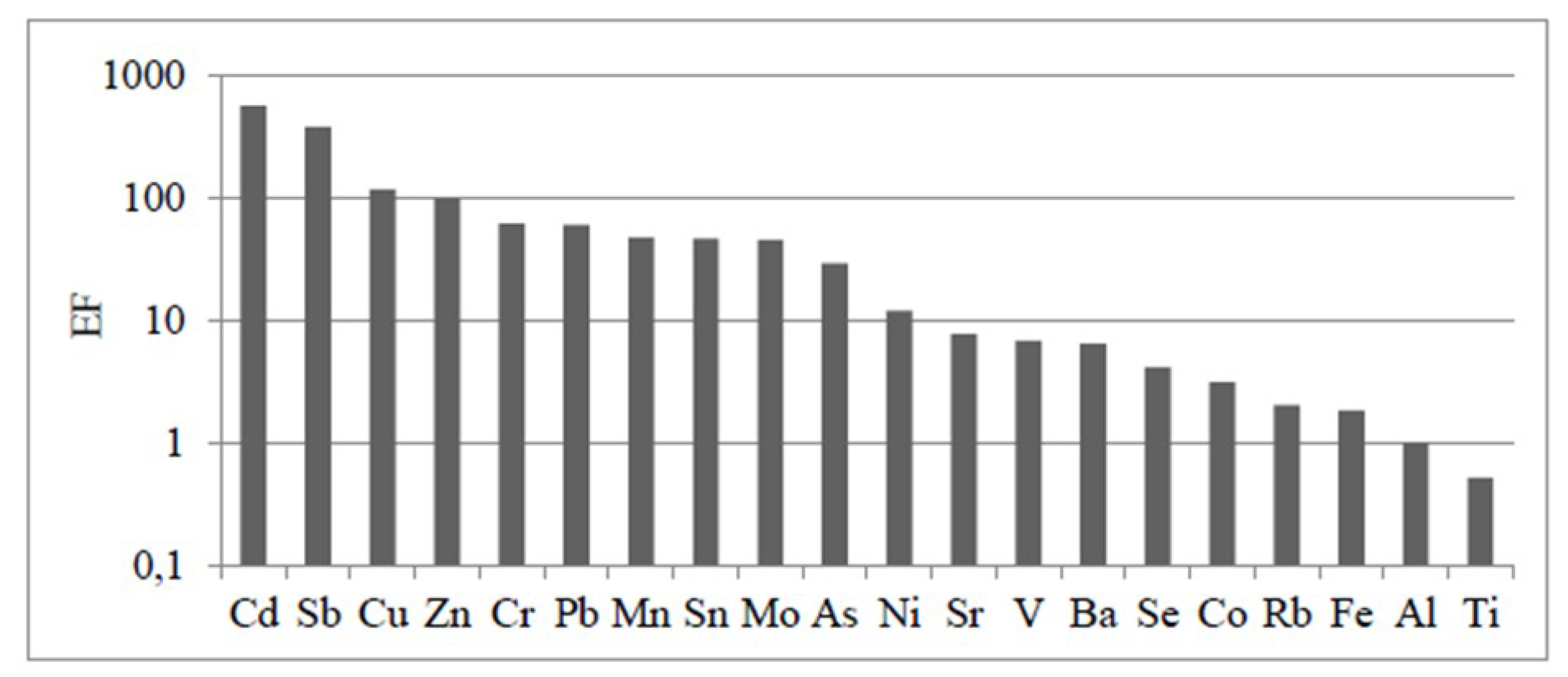
3.3.1. Sources of TEs
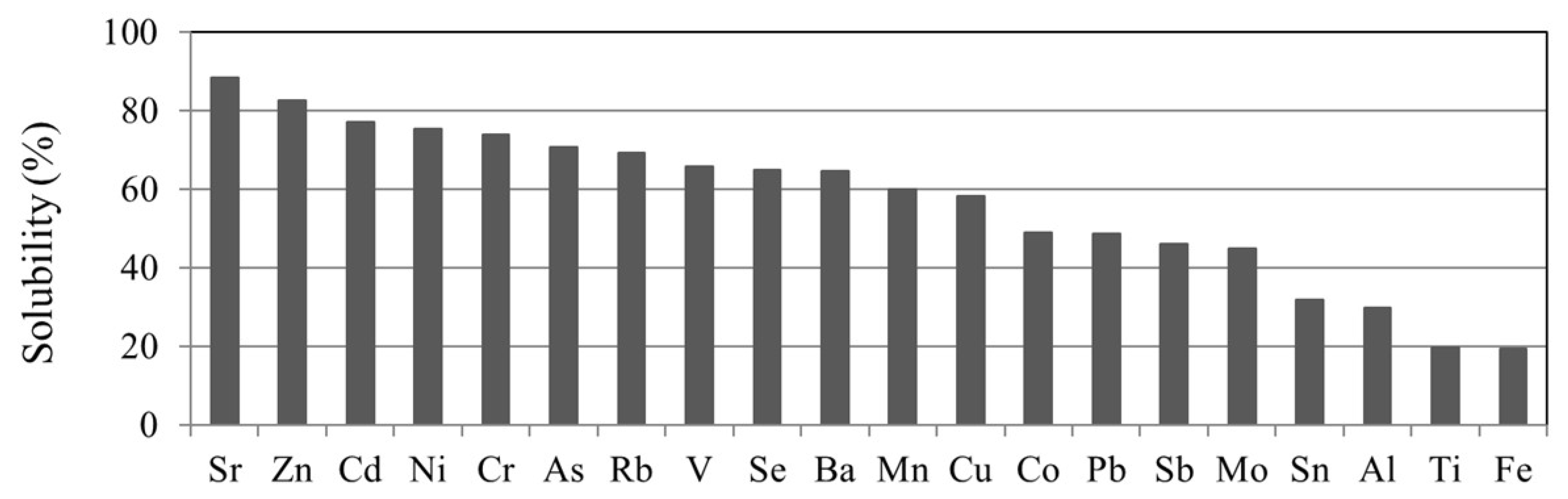
3.3.2. Solubility of TEs in Rainwater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brimblecombe, P. Air Composition and Chemistry; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Willey, J.D.; Glinski, D.A.; Southwell, M.; Long, M.S.; Brooks Avery, G.; Kieber, R.J. Decadal variations of rainwater formic and acetic acid concentrations in Wilmington, NC, USA. Atmos. Environ. 2011, 45, 1010–1014. [Google Scholar] [CrossRef]
- Jeong, G.Y.; Kim, J.Y.; Seo, J.; Kim, G.M.; Jin, H.C.; Chun, Y. Long-range transport of giant particles in Asian dust identified by physical, mineralogical, and meteorological analysis. Atmos. Chem. Phys. 2014, 14, 505–521. [Google Scholar] [CrossRef]
- Richon, C.; Dutay, J.C.; Dulac, F.; Wang, R.; Balkanski, Y. Modeling the biogeochemical impact of atmospheric phosphate deposition from desert dust and combustion sources to the Mediterranean Sea. Biogeosciences 2018, 15, 2499–2524. [Google Scholar] [CrossRef]
- Willey, J.D.; Kieber, R.J.; Eyman, M.S.; Avery, G.B., Jr. Rainwater dissolved organic carbon: Concentrations and global flux. Glob. Biogeochem. Cycles 2000, 14, 139–148. [Google Scholar] [CrossRef]
- Canic, K.S.; Vidic, S.; Klaic, Z.B. Precipitation chemistry in Croatia during the period 1981–2006. J. Environ. Monit. 2009, 11, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Špoler Čanić, K.K., I; Bencetić Klaić, Z. Impact of Saharan Dust on Precipitation Chemistry in Croatia. Available online: https://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.680.5060 (accessed on 13 October 2019).
- Iavorivska, L.; Boyer, E.W.; DeWalle, D.R. Atmospheric deposition of organic carbon via precipitation. Atmos. Environ. 2016, 146, 153–163. [Google Scholar] [CrossRef]
- Iavorivska, L.; Boyer, E.W.; Grimm, J.W. Wet atmospheric deposition of organic carbon: An underreported source of carbon to watersheds in the northeastern United States. J. Geophys. Res. Atmospheres 2017, 122, 3104–3115. [Google Scholar] [CrossRef]
- Bao, H.Y.; Yi, Y.Y.; Wang, C.; Spencer, R.G.M.; Deng, X.; Guo, W.D. Dissolved organic matter in coastal rainwater: Concentration, bioavailability and depositional flux to seawater in southeastern China. Mar. Chem. 2018, 205, 48–55. [Google Scholar] [CrossRef]
- Lawlor, A.; Tipping, E. Metals in bulk deposition and surface waters at two upland locations in northern England. Environ. Pollut. 2003, 121, 153–167. [Google Scholar] [CrossRef]
- Tuncel, G.W.a.d.d.f.o.m.i.i.t.E.M. Black Sea and Central Anatolia. Map Tech. Rep. Series 2001, 133, 1–34. [Google Scholar]
- Rossini, P.; Guerzoni, S.; Molinaroli, E.; Rampazzo, G.; De Lazzari, A.; Zancanaro, A. Atmospheric bulk deposition to the lagoon of Venice - Part I. Fluxes of metals, nutrients and organic contaminants. Environ. Int. 2005, 31, 959–974. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chow, M.F.; Lai, C.C.; Kuo, H.Y.; Lin, C.H.; Chen, T.Y.; Shiah, F.K. Long Term Trends and Dynamics of Dissolved Organic Carbon (DOC) in a Subtropical Reservoir Basin. Water 2017, 9, 14. [Google Scholar] [CrossRef]
- Kieber, R.J.; Peake, B.; Willey, J.D.; Avery, G.B. Dissolved organic carbon and organic acids in coastal New Zealand rainwater. Atmos. Environ. 2002, 36, 3557–3563. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Xin, J.; Tang, G.; Song, T.; Wang, Y.; Li, X.; Wu, F. Study on dissolved organic carbon in precipitation in Northern China. Atmos. Environ. 2010, 44, 2350–2357. [Google Scholar] [CrossRef]
- Siudek, P.; Frankowski, M.; Siepak, J. Seasonal variations of dissolved organic carbon in precipitation over urban and forest sites in central Poland. Environ. Sci. Pollut. Res. 2015, 22, 11087–11096. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Kim, G. Dissolved organic carbon in the precipitation of Seoul, Korea: Implications for global wet depositional flux of fossil-fuel derived organic carbon. Atmos. Environ. 2012, 59, 117–124. [Google Scholar] [CrossRef]
- Montero-Martínez, G.; Rivera-Arellano, J.; Roy, P.D.; Rosado-Abón, A.; Hernández-Nagay, D.P.; Mendoza-Trejo, A.; Andraca-Ayala, G.L. Content and composition of dissolved organic carbon in precipitation at the southern part of Mexico City. Atmosfera 2018, 31, 331–346. [Google Scholar] [CrossRef]
- Niu, H.; Kang, S.; Shi, X.; Zhang, G.; Wang, S.; Pu, T. Dissolved organic carbon in summer precipitation and its wet deposition flux in the Mt. Yulong region, southeastern Tibetan Plateau. J. Atmos. Chem. 2019, 76, 1–20. [Google Scholar] [CrossRef]
- Leko, P.O.; Kozarac, Z.; Ćosović, B. Surface active substances (SAS) and dissolved organic matter (DOC) in atmospheric precipitation of urban area of Croatia (Zagreb). Water Air Soil Pollut. 2004, 158, 295–310. [Google Scholar] [CrossRef]
- Orlović-Leko, P.; Plavšić, M.; Bura-Nakić, E.; Kozarac, Z.; Ćosović, B. Organic matter in the bulk precipitations in Zagreb and Šibenik, Croatia. Atmos. Environ. 2009, 43, 805–811. [Google Scholar] [CrossRef]
- Orlović-Leko, P.; Kozarac, Z.; Ćosović, B.; Strmečki, S.; Plavšić, M. Characterization of atmospheric surfactants in the bulk precipitation by electrochemical tools. J. Atmos. Chem. 2010, 66, 11–26. [Google Scholar] [CrossRef]
- Renard, P.; Canet, I.; Sancelme, M.; Wirgot, N.; Deguillaume, L.; Delort, A.M. Screening of cloud microorganisms isolated at the Puy de Dome (France) station for the production of biosurfactants. Atmos. Chem. Phys. 2016, 16, 12347–12358. [Google Scholar] [CrossRef]
- Ćosović, B. Adsorption kinetics of the complex mixture of organic solutes at model and natural phase boundaries. In Aquatic Chemical Kinetics, Environmental Science and Technology Series; Weily: New York, NY, USA, 1990; pp. 291–311. [Google Scholar]
- Seidl, W. Model for a surface film of fatty acids on rain water and aerosol particles. Atmos. Environ. 2000, 34, 4917–4932. [Google Scholar] [CrossRef]
- Graber, E.; Rudich, Y. Atmospheric HULIS: How humic-like are they? A comprehensive and critical review. Atmos. Chem. Phys. 2006, 6, 729–753. [Google Scholar] [CrossRef]
- Kristensen, T.B.; Du, L.; Nguyen, Q.T.; Nojgaard, J.K.; Koch, C.B.; Nielsen, O.F.; Hallar, A.G.; Lowenthal, D.H.; Nekat, B.; van Pinxteren, D.; et al. Chemical properties of HULIS from three different environments. J. Atmos. Chem. 2015, 72, 65–80. [Google Scholar] [CrossRef]
- Santos, P.S.; Santos, E.B.; Duarte, A.C. First spectroscopic study on the structural features of dissolved organic matter isolated from rainwater in different seasons. Sci. Total Environ. 2012, 426, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.D.; Kieber, R.J.; Seaton, P.J.; Miller, C. Rainwater as a source of Fe(II)-stabilizing ligands to seawater. Limnol. Oceanogr. 2008, 53, 1678–1684. [Google Scholar] [CrossRef]
- Okochi, H.; Brimblecombe, P. Potential trace metal–organic complexation in the atmosphere. The Sci. World J. 2002, 2, 767–786. [Google Scholar] [CrossRef]
- Witt, M.; Jickells, T. Copper complexation in marine and terrestrial rain water. Atmos. Environ. 2005, 39, 7657–7666. [Google Scholar] [CrossRef]
- Witt, M.; Skrabal, S.; Kieber, R.; Willey, J. Copper complexation in coastal rainwater, southeastern USA. Atmos. Environ. 2007, 41, 3619–3630. [Google Scholar] [CrossRef]
- Plavšić, M.; Orlović-Leko, P.; Kozarac, Z.; Bura-Nakić, E.; Strmečki, S.; Ćosović, B. Complexation of copper ions in atmospheric precipitation in Croatia. Atmos. Res. 2008, 87, 80–87. [Google Scholar] [CrossRef]
- Louis, Y.; Garnier, C.; Lenoble, V.; Mounier, S.; Cukrov, N.; Omanovic, D.; Pizeta, I. Kinetic and equilibrium studies of copper-dissolved organic matter complexation in water column of the stratified Krka River estuary (Croatia). Mar. Chem. 2009, 114, 110–119. [Google Scholar] [CrossRef]
- Baker, A.R.; Croot, P.L. Atmospheric and marine controls on aerosol iron solubility in seawater. Mar. Chem. 2010, 120, 4–13. [Google Scholar] [CrossRef]
- Allen, A.; Nemitz, E.; Shi, J.; Harrison, R.; Greenwood, J. Size distributions of trace metals in atmospheric aerosols in the United Kingdom. Atmos. Environ. 2001, 35, 4581–4591. [Google Scholar] [CrossRef]
- Johansson, C.; Norman, M.; Burman, L. Road traffic emission factors for heavy metals. Atmos. Environ. 2009, 43, 4681–4688. [Google Scholar] [CrossRef]
- Faloona, I. Sulfur processing in the marine atmospheric boundary layer: A review and critical assessment of modeling uncertainties. Atmos. Environ. 2009, 43, 2841–2854. [Google Scholar] [CrossRef]
- Watts, S.F. The mass budgets of carbonyl sulfide, dimethyl sulfide, carbon disulfide and hydrogen sulfide. Atmos. Environ. 2000, 34, 761–779. [Google Scholar] [CrossRef]
- Orlović-Leko, P.; Omanović, D.; Ciglenečki, I.; Vidović, K.; Brenko, T. Application of electrochemical methods in the physicochemical characterization of atmospheric precipitation. Bulg. Chem. Commun. 2017, 49, 211–217. [Google Scholar]
- Rozan, T.F.; Lassman, M.E.; Ridge, D.P.; Luther, G.W., III. Evidence for iron, copper and zinc complexation as multinuclear sulphide clusters in oxic rivers. Nature 2000, 406, 879. [Google Scholar] [CrossRef]
- Wong, C.S.C.; Li, X.; Thornton, I. Urban environmental geochemistry of trace metals. Environ. Pollut. 2006, 142, 1–16. [Google Scholar] [CrossRef]
- Al-Momani, I.F. Trace elements in atmospheric precipitation at Northern Jordan measured by ICP-MS: Acidity and possible sources. Atmos. Environ. 2003, 37, 4507–4515. [Google Scholar] [CrossRef]
- Hou, H.; Takamatsu, T.; Koshikawa, M.; Hosomi, M. Trace metals in bulk precipitation and throughfall in a suburban area of Japan. Atmos. Environ. 2005, 39, 3583–3595. [Google Scholar] [CrossRef]
- Okubo, A.; Takeda, S.; Obata, H. Atmospheric deposition of trace metals to the western North Pacific Ocean observed at coastal station in Japan. Atmos. Res. 2013, 129, 20–32. [Google Scholar] [CrossRef]
- Fernández-Olmo, I.; Puente, M.; Montecalvo, L.; Irabien, A. Source contribution to the bulk atmospheric deposition of minor and trace elements in a Northern Spanish coastal urban area. Atmos. Res. 2014, 145, 80–91. [Google Scholar] [CrossRef]
- Başak, B.; Alagha, O. Trace metals solubility in rainwater: Evaluation of rainwater quality at a watershed area, Istanbul. Environ. Monit. Assess. 2010, 167, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Heimburger, A.; Losno, R.; Triquet, S. Solubility of iron and other trace elements in rainwater collected on the Kerguelen Islands (South Indian Ocean). Biogeosciences 2013, 10, 6617–6628. [Google Scholar] [CrossRef]
- Statistics Cost-Based Optimizer. Statistical Yearbook of the Republic of Croatia. Available online: http://www.dzs.hr/Hrv_Eng/ljetopis/2010/SLJH2010.pdf (accessed on 13 October 2019).
- Statistics Cost-Based Optimizer. Statistical Yearbook of the Republic of Croatia. Available online: http://www.dzs.hr/Hrv_Eng/ljetopis/2011/SLJH2011.pdf (accessed on 12 September 2019).
- Statistical Yearbook of the Republic of Croatia. Available online: https://www.dzs.hr/Hrv_Eng/ljetopis/2012/sljh2012.pdf (accessed on 12 September 2019).
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J. Organic aerosol and global climate modelling: A review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar] [CrossRef]
- Cosovic, B.; Vojvodic, V. Voltammetric analysis of surface active substances in natural seawater. Electroanalysis 1998, 10, 429–434. [Google Scholar] [CrossRef]
- Orlović-Leko, P.; Vidović, K.; Plavšić, M.; Ciglenečki, I.; Šimunić, I.; Minkina, T. Voltammetry as a tool for rough and rapid characterization of dissolved organic matter in the drainage water of hydroameliorated agricultural areas in Croatia. J. Solid State Electrochem. 2016, 20, 3097–3105. [Google Scholar] [CrossRef]
- Balla, D.; Papageorgiou, A.; Voutsa, D. Carbonyl compounds and dissolved organic carbon in rainwater of an urban atmosphere. Environ. Sci. Pollut. Res. 2014, 21, 12062–12073. [Google Scholar] [CrossRef]
- Strmecki, S.; Ciglenecki, I.; Udovic, M.G.; Margus, M.; Bura-Nakic, E.; Dautovic, J.; Plavsic, M. Voltammetric Study of Organic Matter Components in the Upper Reach of the Krka River, Croatia. Croat. Chem. Acta 2018, 91, 447–454. [Google Scholar] [CrossRef]
- Šimunić, I.; Orlović-Leko, P.; Marić, A.S.; Ciglenečki, I.; Tatiana, M.; Filipović, V. Quality of Surface Water in the Agricultural District Lonja Field (Croatia). Agric. For. 2017, 63, 5–14. [Google Scholar]
- Orlović-Leko, L.Š.I.; DautovićOrlović-Leko, J.; Ciglenečki, I. Dissolved Organic Carbon (DOC) in the surface waters in the agricultural area (Lonja field, Croatia). In 7th Croatian Water Conference, Croatian Waters in Environmetal and Nature Protection; Biondić, D., Holjević, D., Vizner, M., Eds.; Opatija, Croatia, 2019; pp. 287–291. [Google Scholar]
- Cvrković-Karloci, Ž.; Krznarić, D.; Šeruga, M.; Ćosović, B. Simple electrochemical determination of surface-active substances in natural waters. Int. J. Electrochem. 2011, 2011. [Google Scholar] [CrossRef]
- Cukrov, N.; Cmuk, P.; Mlakar, M.; Omanovic, D. Spatial distribution of trace metals in the Krka River, Croatia: An example of the self-purification. Chemosphere 2008, 72, 1559–1566. [Google Scholar] [CrossRef]
- Capel, P.D.; Gunde, R.; Zuercher, F.; Giger, W. Carbon speciation and surface tension of fog. Environ. Sci. Technol. 1990, 24, 722–727. [Google Scholar] [CrossRef]
- Buchard, V.; Randles, C.A.; da Silva, A.M.; Darmenov, A.; Colarco, P.R.; Govindaraju, R.; Ferrare, R.; Hair, J.; Beyersdorf, A.J.; Ziemba, L.D. The MERRA-2 Aerosol Reanalysis, 1980 Onward. Part II: Evaluation and Case Studies. J. Clim. 2017, 30, 6851–6872. [Google Scholar] [CrossRef]
- Global Modeling and Assimilation Office. Available online: https://gmao.gsfc.nasa.gov/GMAO_products/reanalysis_products.php (accessed on 20 January 2020).
- Ćosović, B.; Orlović Leko, P.; Kozarac, Z. Rainwater dissolved organic carbon: Characterization of surface active substances by electrochemical method. Electroanal. Int. J. Devoted Fundam. Pract. Aspects Electroanal. 2007, 19, 2077–2084. [Google Scholar]
- Simcik, M.F. The importance of surface adsorption on the washout of semivolatile organic compounds by rain. Atmos. Environ. 2004, 38, 491–501. [Google Scholar] [CrossRef]
- Wang, M.; Perroux, H.; Fleuret, J.; Bianco, A.; Bouvier, L.; Colomb, A.; Borbon, A.; Deguillaume, L. Anthropogenic and biogenic hydrophobic VOCs detected in clouds at the puy de Dôme station using Stir Bar Sorptive Extraction: Deviation from the Henry’s law prediction. Atmos. Res. 2020, 104844. [Google Scholar] [CrossRef]
- Orlović-Leko, P.F.S.; Penezić, A.; Vidović, K.; Plećaš, S. Surface activity of precipitation and aerosol on the Zagreb area. In Proceedings of the 6th Croatian Water Conference, Opatija, Croatia, 20–23 May 2015. [Google Scholar]
- Avery, G.B.; Biswas, K.F.; Mead, R.; Southwell, M.; Willey, J.D.; Kieber, R.J.; Mullaugh, K.M. Carbon isotopic characterization of hydrophobic dissolved organic carbon in rainwater. Atmos. Environ. 2013, 68, 230–234. [Google Scholar] [CrossRef]
- Vidović, K. Seasonal Variation of Properties of Organic Matter in Aerosol in the Atmosphere over Zagreb. Master’s Thesis, University of Zagreb, Zagreb, Croatian, 2014. [Google Scholar]
- Tang, X.; Zhang, X.S.; Wang, Z.W.; Ci, Z.J. Water-soluble organic carbon (WSOC) and its temperature-resolved carbon fractions in atmospheric aerosols in Beijing. Atmos. Res. 2016, 181, 200–210. [Google Scholar] [CrossRef]
- Bura-Nakić, E.; Helz, G.R.; Ciglenečki, I.; Ćosović, B. Seasonal variations in reduced sulfur species in a stratified seawater lake (Rogoznica Lake, Croatia); evidence for organic carriers of reactive sulfur. Geochim. Cosmochim. Acta 2009, 73, 3738. [Google Scholar] [CrossRef]
- Cvitešić Kušan, A.F.S.; Ciglenečki, I. Electrochemical Evidence of non-Volatile Reduced Sulfur Species in Water-Soluble Fraction of Fine Marine Aerosols. Atmosphere 2019, 10, 674. [Google Scholar] [CrossRef]
- Feng, J.; Möller, D. Characterization of water-soluble macromolecular substances in cloud water. J. Atmos. Chem. 2004, 48, 217–233. [Google Scholar] [CrossRef]
- Gašparović, B.; Ćosović, B. Electrochemical estimation of the dominant type of surface active substances in seawater samples using o-nitrophenol as a probe. Mar. Chem. 1994, 46, 179–188. [Google Scholar] [CrossRef]
- Jang, K.-S.; Choi, A.Y.; Choi, M.; Kang, H.; Kim, T.-W.; Park, K.-T. Size-Segregated Chemical Compositions of HULISs in Ambient Aerosols Collected during the Winter Season in Songdo, South Korea. Atmosphere 2019, 10, 226. [Google Scholar] [CrossRef]
- Strmečki, S.; Plavšić, M.; Steigenberger, S.; Passow, U. Characterization of phytoplankton exudates and carbohydrates in relation to their complexation of copper, cadmium and iron. Mar. Ecol. Prog. Ser. 2010, 408, 33–46. [Google Scholar] [CrossRef]
- Strmečki, S.; Paleček, E. Adsorption/desorption of biomacromolecules involved in catalytic hydrogen evolution. Bioelectrochemistry 2018, 120, 87–93. [Google Scholar] [CrossRef]
- Al-Khashman, O.A. Study of chemical composition in wet atmospheric precipitation in Eshidiya area, Jordan. Atmos. Environ. 2005, 39, 6175–6183. [Google Scholar] [CrossRef]
- Potential for Human Exposure. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp7-c6.pdf (accessed on 7 October 2019).
- Gunawardena, J.; Egodawatta, P.; Ayoko, G.A.; Goonetilleke, A. Atmospheric deposition as a source of heavy metals in urban stormwater. Atmos. Environ. 2013, 68, 235–242. [Google Scholar] [CrossRef]
- Mijic, Z.; Stojic, A.; Perisic, M.; Rajsic, S.; Tasic, M.; Radenkovic, M.; Joksic, J. Seasonal variability and source apportionment of metals in the atmospheric deposition in Belgrade. Atmos. Environ. 2010, 44, 3630–3637. [Google Scholar] [CrossRef]
- Fabretti, J.F.; Sauret, N.; Gal, J.F.; Maria, P.C.; Scharer, U. Elemental characterization and source identification of PM2.5 using Positive Matrix Factorization: The Malraux road tunnel, Nice, France. Atmos. Res. 2009, 94, 320–329. [Google Scholar] [CrossRef]
- Sternbeck, J.; Sjodin, A.; Andreasson, K. Metal emissions from road traffic and the influence of resuspension—Results from two tunnel studies. Atmos. Environ. 2002, 36, 4735–4744. [Google Scholar] [CrossRef]
- Duce, R.A.; Hoffman, G.L.; Zoller, W.H. Atmospheric Trace-Metals at Remote Northern and Southern-Hemisphere Sites—Pollution or Natural. Science 1975, 187, 59–61. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. The Crust, 3.01—The Composition of the Continental Crust. In Treatise on Geochemistry; Elsevier-Pergamon: Oxford, UK; pp. 1–64.
- Giusti, L.; Yang, Y.L.; Hewitt, C.N.; Hamiltontaylor, J.; Davison, W. The Solubility and Partitioning of Atmospherically Derived Trace-Metals in Artificial and Natural-Waters—A review. Atmos. Environ. Part A Gen. Top. 1993, 27, 1567–1578. [Google Scholar] [CrossRef]
- Motelay-Massei, A.; Ollivon, D.; Tiphagne, K.; Garban, B. Atmospheric bulk deposition of trace metals to the Seine River Basin, France: Concentrations, sources and evolution from 1988 to 2001 in Paris. Water Air Soil Pollut. 2005, 164, 119–135. [Google Scholar] [CrossRef]
| Aquatic System (Croatia) | DOC (mgC L−1) | References |
|---|---|---|
| Lake Brljan (Krka River) | 0.505–1.530 | [57] |
| River Sava (Zagreb) | 1.14–2.31 a | |
| River Lonja | 4.62–7.75 | [58,59] |
| River Drava (Osijek) | 4.91 | [60] |
| River Dunav (Batina) | 4.21 | [60] |
| Rainwater (Zagreb) | 0.69–4.86 b |
| Aquatic system (Croatia) | SAS | References |
|---|---|---|
| Rainwater (Zagreb) | 0.090–0.37 | [21] |
| Rainwater (Zagreb) | 0.055–0.450 (0.140 *) | [22] |
| Lake Brljan (Krka River) | 0.018–0.150 | [57] |
| River Sava | 0.153–0.255 | [58] |
| River Lonja | 0.209–0.258 a | |
| Lake Maksimir (Zagreb) | 0.263 b | |
| Rainwater (Zagreb) | 0.030–0.358 (0.156 *) c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlović-Leko, P.; Vidović, K.; Ciglenečki, I.; Omanović, D.; Sikirić, M.D.; Šimunić, I. Physico-Chemical Characterization of an Urban Rainwater (Zagreb, Croatia). Atmosphere 2020, 11, 144. https://doi.org/10.3390/atmos11020144
Orlović-Leko P, Vidović K, Ciglenečki I, Omanović D, Sikirić MD, Šimunić I. Physico-Chemical Characterization of an Urban Rainwater (Zagreb, Croatia). Atmosphere. 2020; 11(2):144. https://doi.org/10.3390/atmos11020144
Chicago/Turabian StyleOrlović-Leko, Palma, Kristijan Vidović, Irena Ciglenečki, Dario Omanović, Mathieu Dutour Sikirić, and Ivan Šimunić. 2020. "Physico-Chemical Characterization of an Urban Rainwater (Zagreb, Croatia)" Atmosphere 11, no. 2: 144. https://doi.org/10.3390/atmos11020144
APA StyleOrlović-Leko, P., Vidović, K., Ciglenečki, I., Omanović, D., Sikirić, M. D., & Šimunić, I. (2020). Physico-Chemical Characterization of an Urban Rainwater (Zagreb, Croatia). Atmosphere, 11(2), 144. https://doi.org/10.3390/atmos11020144