Ambient Non-Methane Hydrocarbons (NMHCs) Measurements in Baoding, China: Sources and Roles in Ozone Formation
Abstract
:1. Introduction
2. Methodology
2.1. Ambient NMHCs Measurements
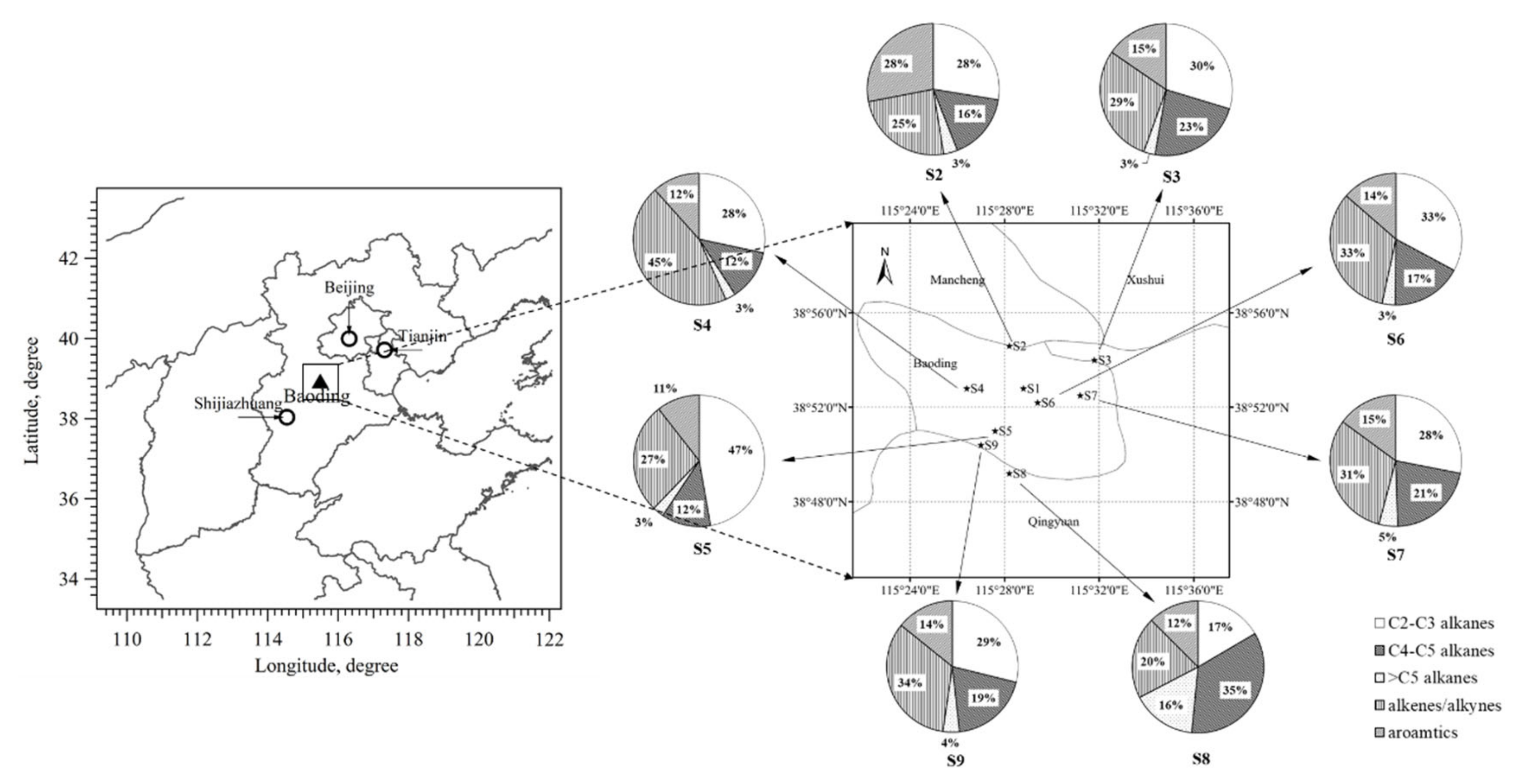
2.1.1. Sampling Sites
2.1.2. Online and Offline Measurements of NMHCs
2.2. Observation-Based Model (OBM)
2.3. Positive Matrix Factorization (PMF) Model
3. Results and Discussion
3.1. Characteristic of O3, NOX, and NMHCs
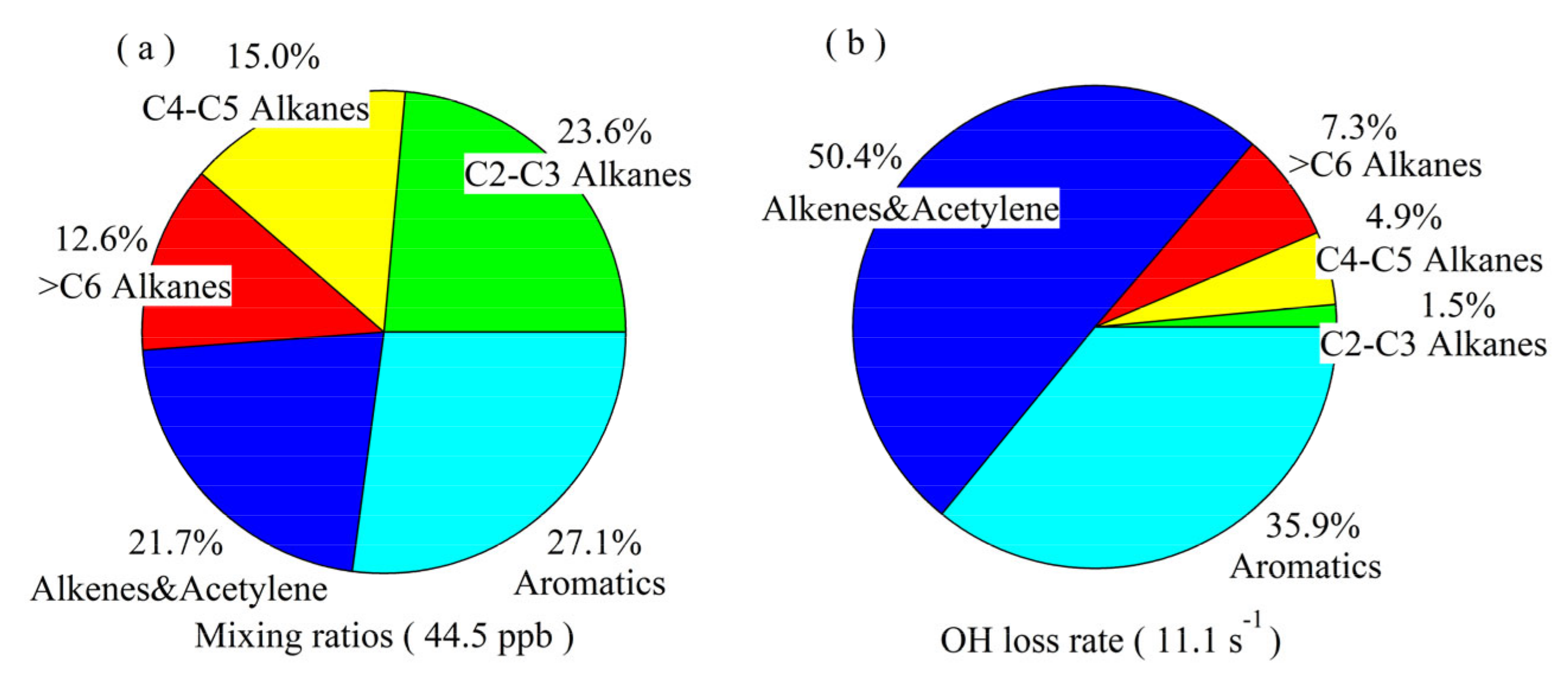
3.1.1. Mixing Ratios, Chemical Composition, and Reactivity of NMHCs
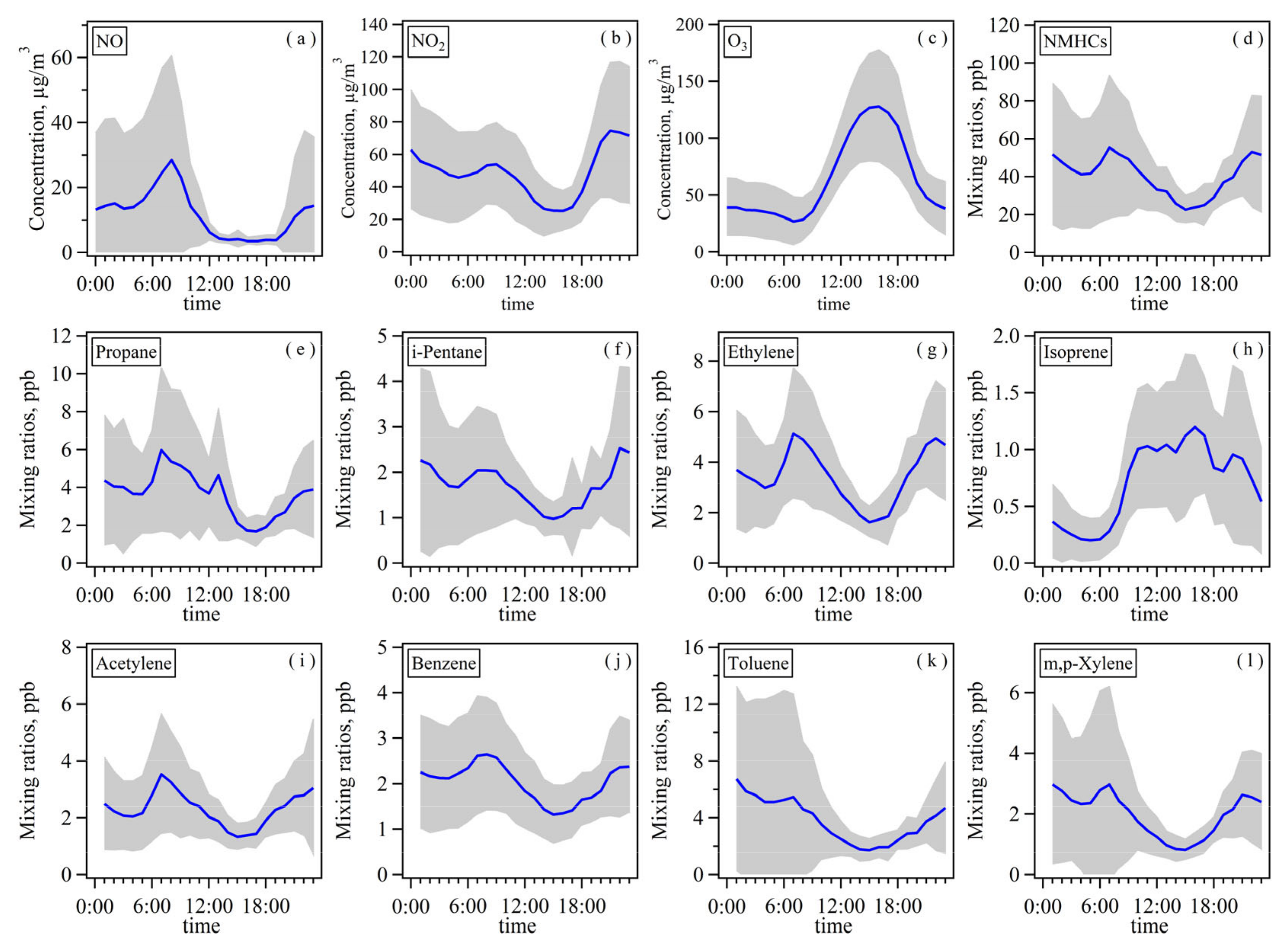
3.1.2. Diurnal Variations of NMHCs, NOX, and O3 Based on Online Measurements
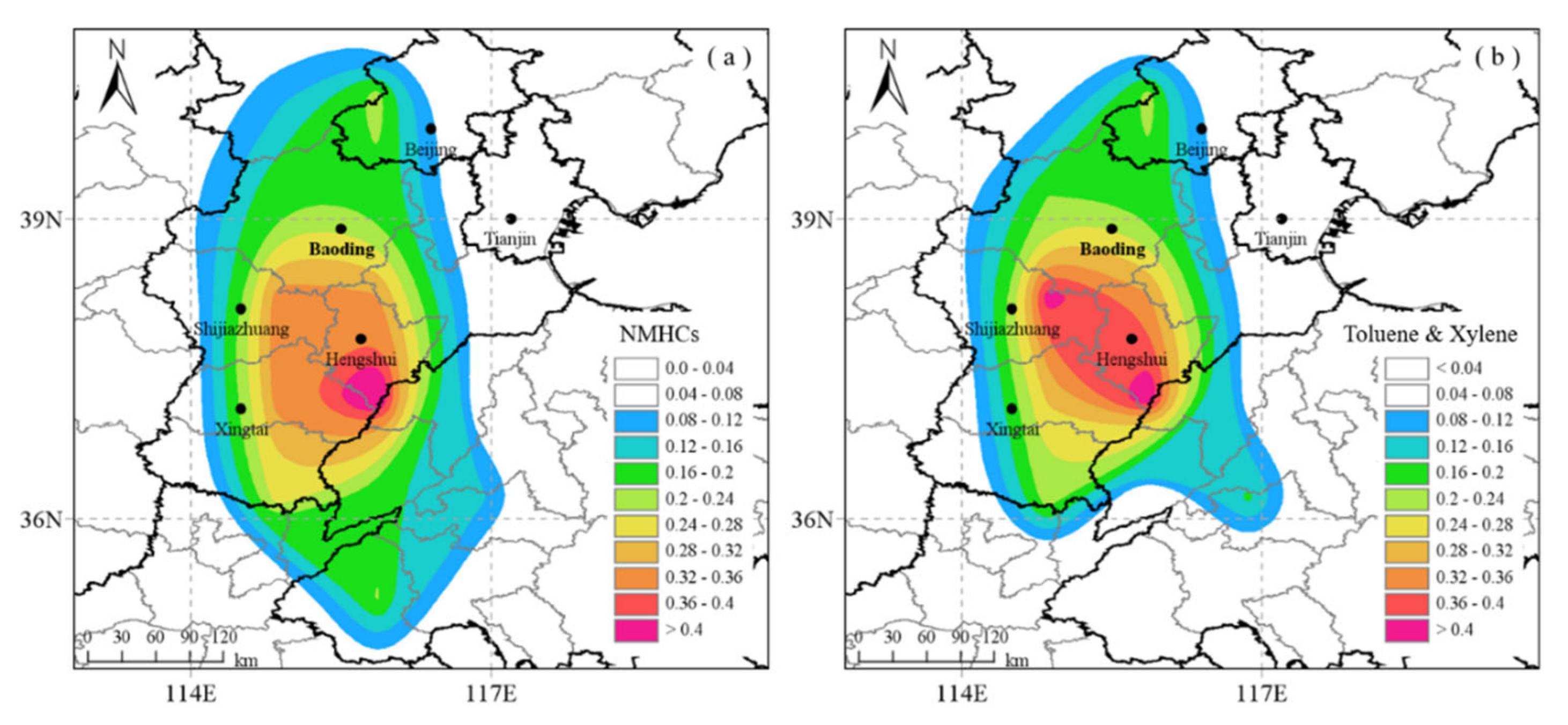
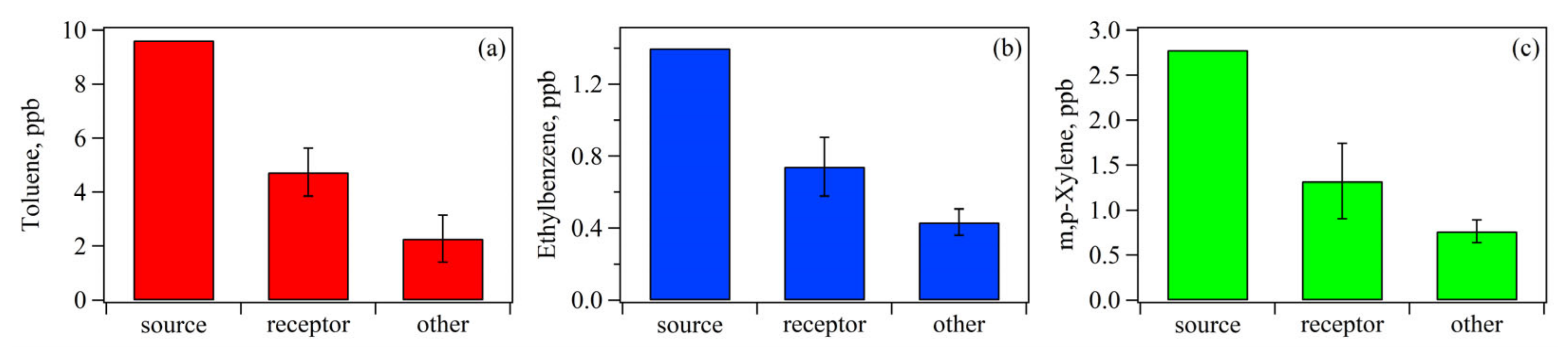
3.1.3. Influence of Transport: Potential Source Contributions and Spatial Distribution of Aromatics Levels
3.2. Source Apportionment of NMHCs
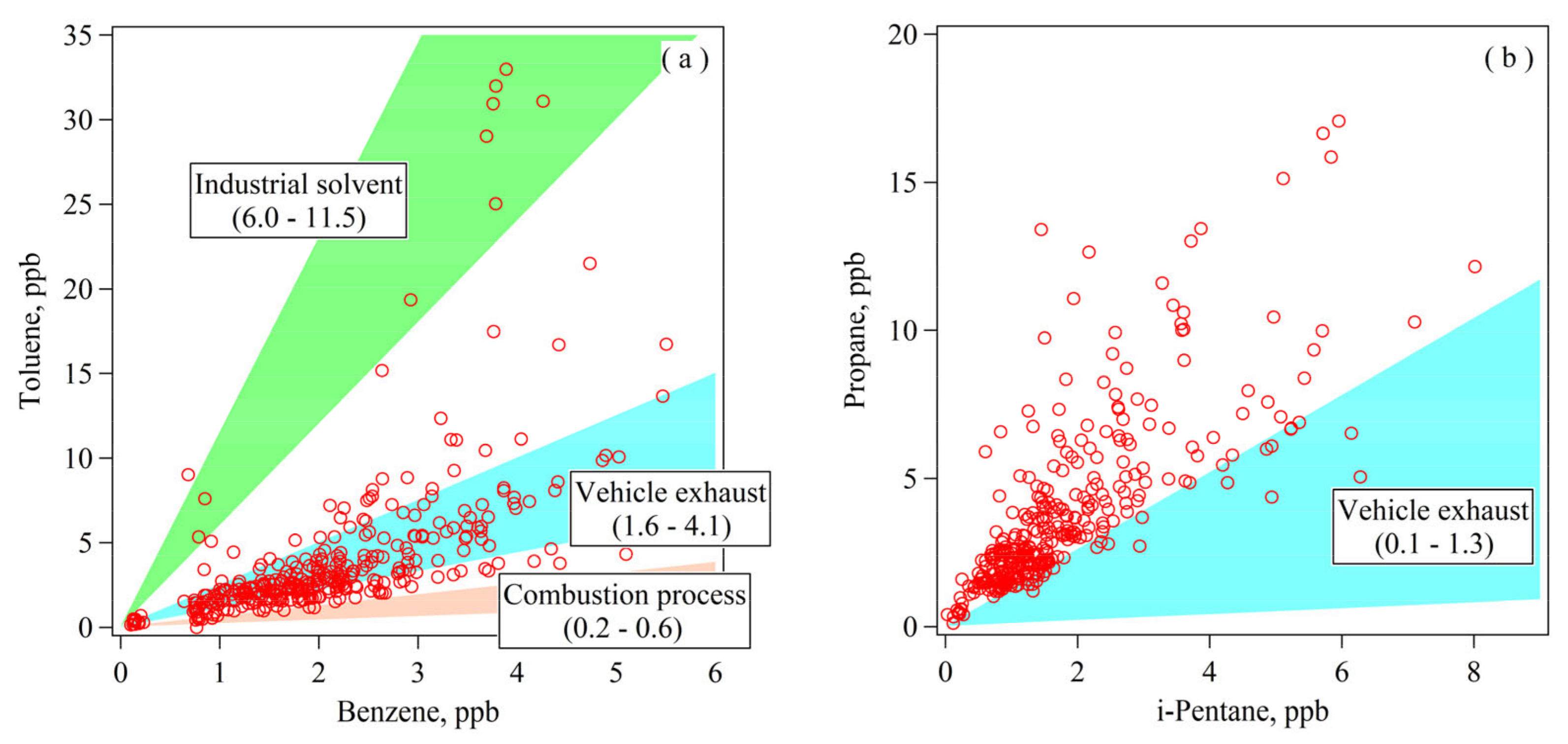
3.2.1. Preliminary Analysis of NMHC Sources Using Ratios
3.2.2. Identification of PMF-Resolved Factors
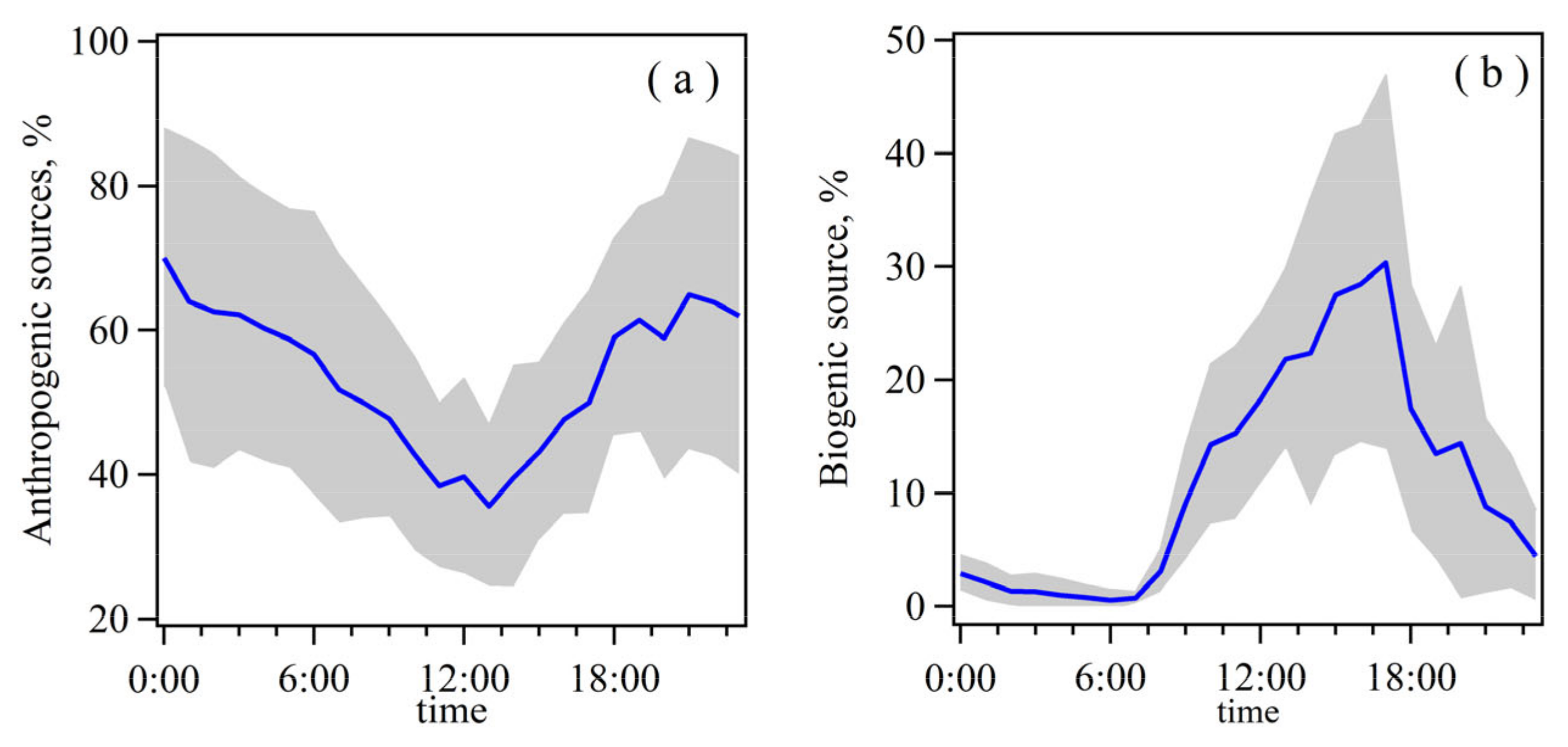
3.2.3. Relative Contributions of Individual Sources to Ambient NMHCs Concentrations
3.3. Roles of NMHCs in O3 Formation
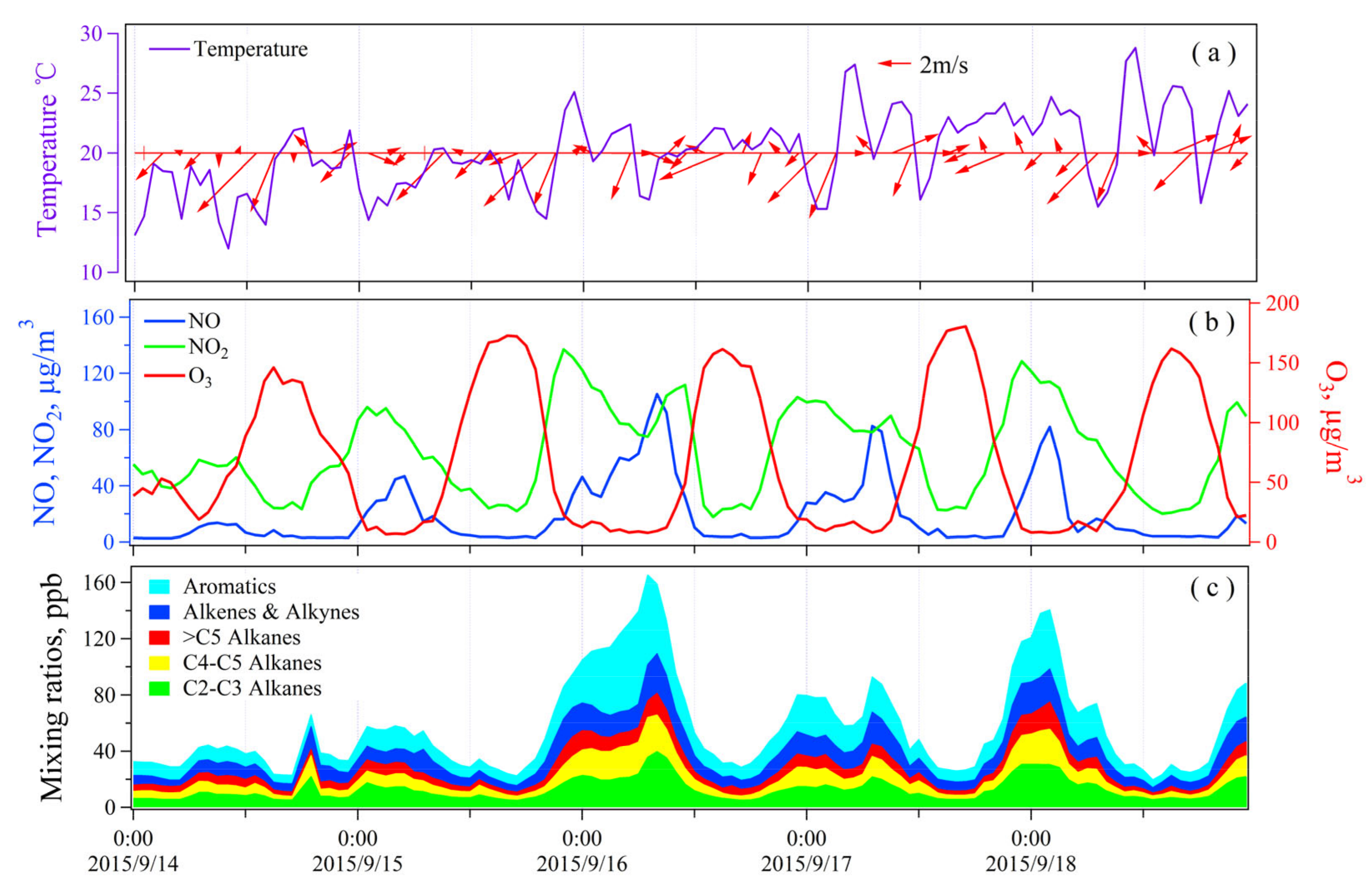
3.3.1. O3 Pollution Episodes
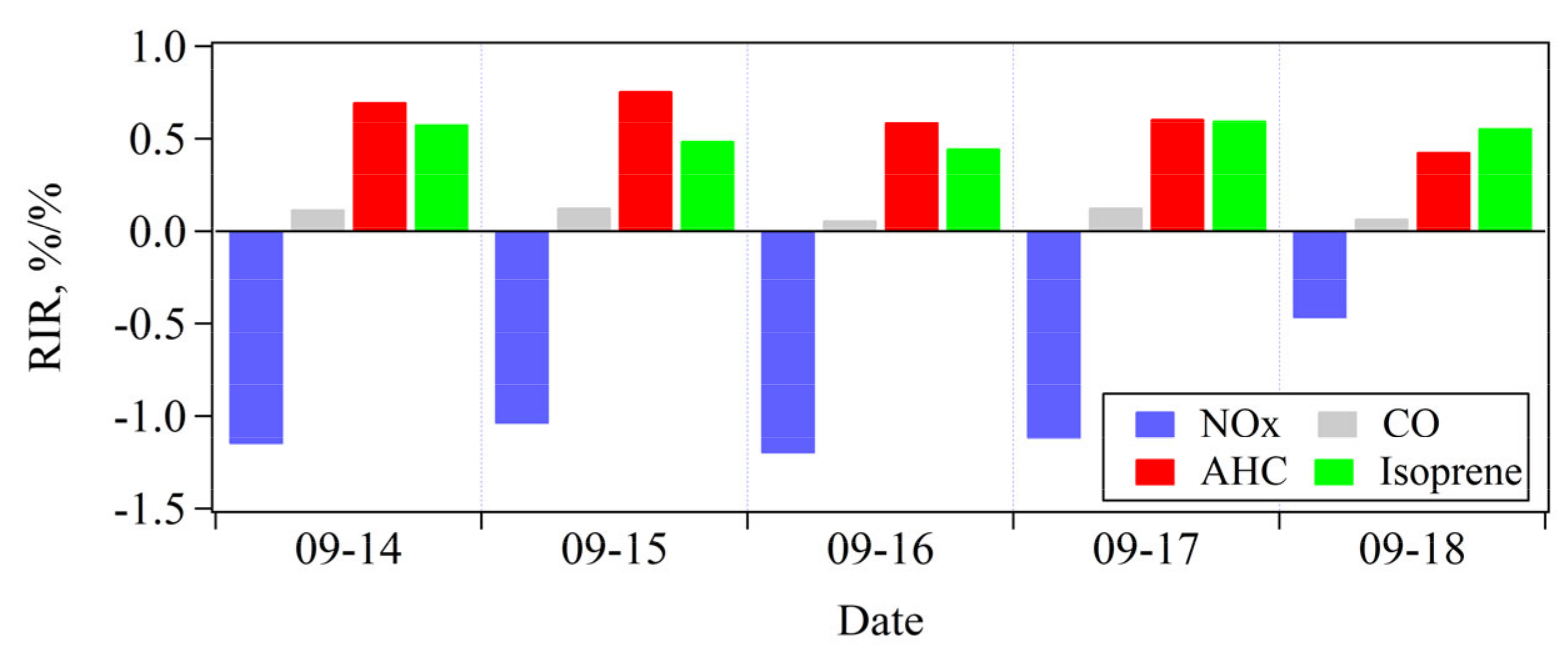
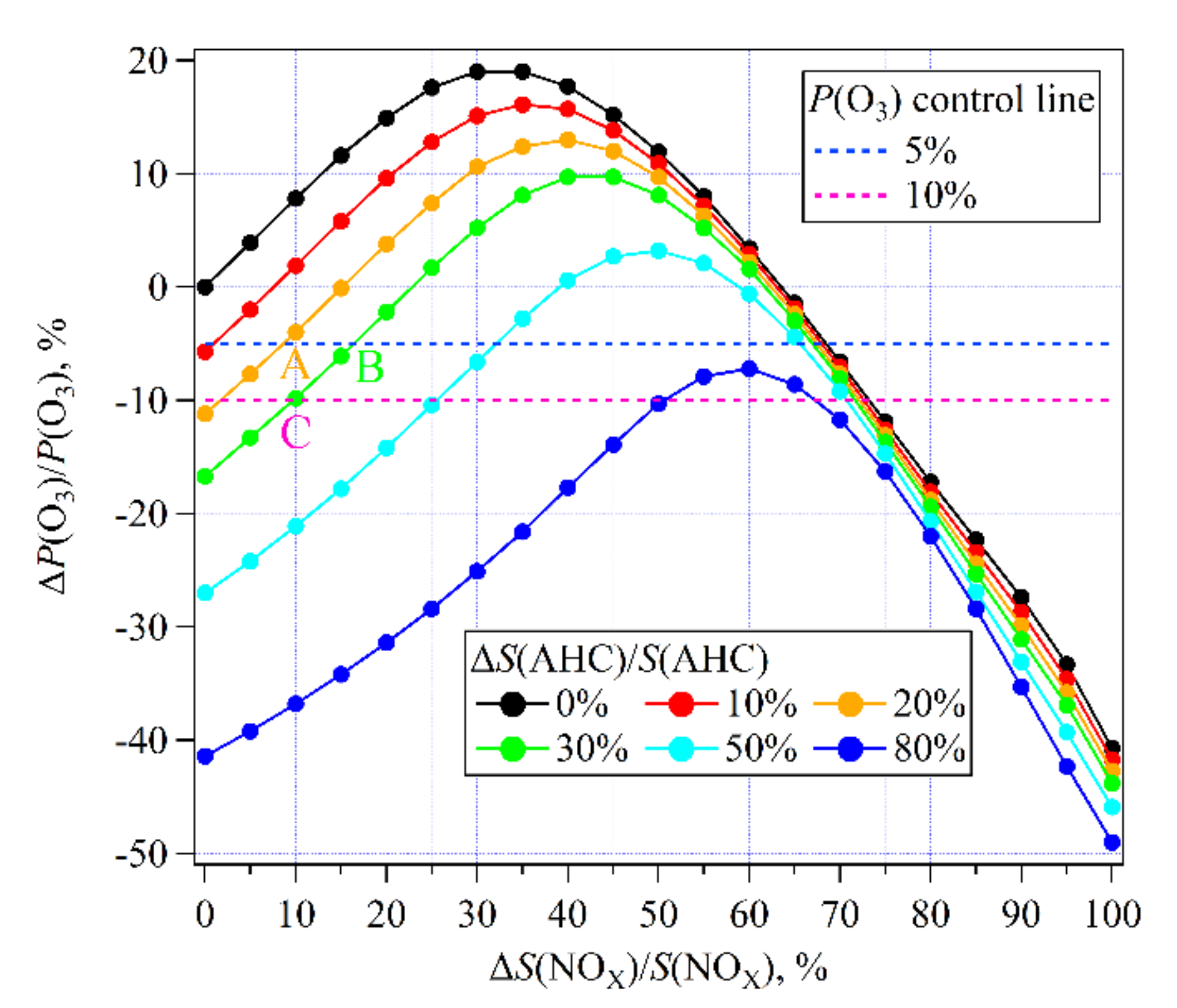
3.3.2. O3 Sensitivity to NMHCs and NOX
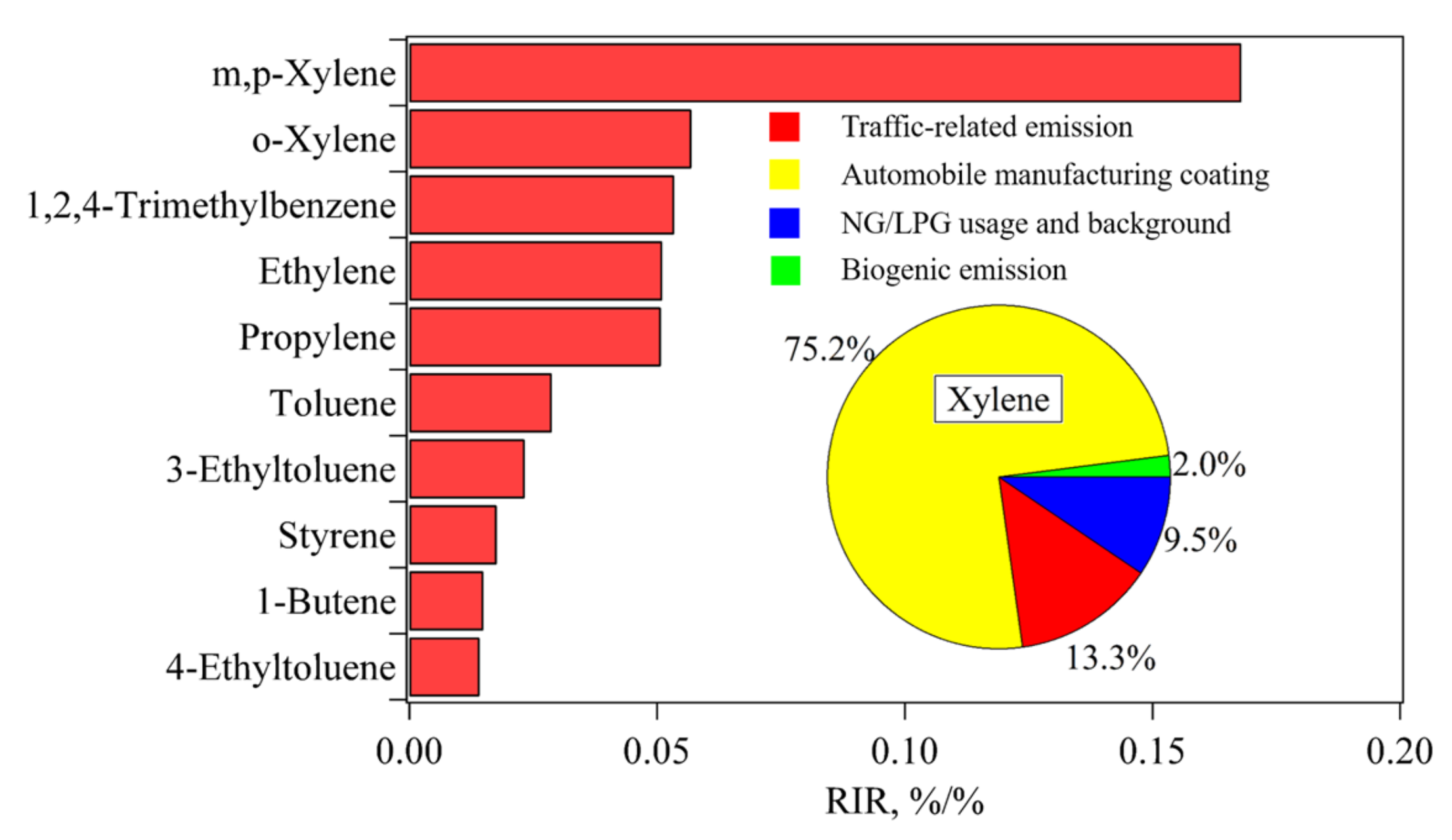
3.3.3. Identification of Key Anthropogenic NMHC Species in O3 Formation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, H.; Ling, Z.; Cheng, H.; Simpson, I.J.; Lyu, X.; Wang, X.; Shao, M.; Lu, H.; Ayoko, G.; Zhang, Y.; et al. Tropospheric volatile organic compounds in China. Sci. Total Environ. 2017, 574, 1021–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Z.; Lu, K.; Dong, H.; Hu, M.; Li, X.; Liu, Y.; Lu, S.; Shao, M.; Su, R.; Wang, H.; et al. Explicit diagnosis of the local ozone production rate and the ozone-NOx-VOC sensitivities. Sci. Bull. 2018, 63, 1067–1076. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Lyu, X.; Cheng, H.; Ling, Z.; Guo, H. Overview on the spatial–temporal characteristics of the ozone formation regime in China. Environ. Sci. Process. Impacts 2019, 21, 916–929. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Gu, D.; Zhao, C.; Huey, L.G.; Stickel, R.; Liao, J.; Shao, M.; Zhu, T.; Zeng, L.; et al. Summertime photochemistry during CAREBeijing-2007: ROx budgets and O3 formation. Atmos. Chem. Phys. 2012, 12, 7737–7752. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Zhang, Y.; Su, H.; Brauers, T.; Chou, C.; Hofzumahaus, A.; Liu, S.; Kita, K.; Kondo, Y.; Shao, M.; et al. Oxidant (O3 + NO2) production processes and formation regimes in Beijing. J. Geophys. Res. Atmos. 2010, 115, D07303. [Google Scholar]
- Zhang, Q.; Yuan, B.; Shao, M.; Wang, X.; Lu, S.; Lu, K.; Wang, M.; Chen, L.; Chang, C.C.; Liu, S.C. Variations of ground-level O3 and its precursors in Beijing in summertime between 2005 and 2011. Atmos. Chem. Phys. 2014, 14, 6089–6101. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Wang, Z.; Zhu, J.; Gbaguidi, A.E.; Wu, Q.; Li, J.; Zhu, T. Sensitivity of ozone to precursor emissions in urban Beijing with a Monte Carlo scheme. Atmos. Environ. 2010, 44, 3833–3842. [Google Scholar] [CrossRef]
- Wang, M.; Shao, M.; Chen, W.; Lu, S.; Liu, Y.; Yuan, B.; Zhang, Q.; Zhang, Q.; Chang, C.C.; Wang, B.; et al. Trends of non-methane hydrocarbons (NMHC) emissions in Beijing during 2002–2013. Atmos. Chem. Phys. 2015, 15, 1489–1502. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Zhang, J.; Li, H.; Li, L.; Xu, L.; Zhang, Y.; Wang, Z.; Wang, X.; Zhang, W.; Chen, Y.; et al. Comparative study of volatile organic compounds in ambient air using observed mixing ratios and initial mixing ratios taking chemical loss into account—A case study in a typical urban area in Beijing. Sci. Total Environ. 2018, 628–629, 791–804. [Google Scholar] [CrossRef]
- Xue, L.; Wang, T.; Louie, P.K.K.; Luk, C.W.Y.; Blake, D.R.; Xu, Z. Increasing External Effects Negate Local Efforts to Control Ozone Air Pollution: A Case Study of Hong Kong and Implications for Other Chinese Cities. Environ. Sci. Technol. 2014, 48, 10769–10775. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zeng, L.; Lu, S.; Shao, M.; Liu, X.; Yu, X.; Chen, W.; Yuan, B.; Zhang, Q.; Hu, M.; et al. Development and validation of a cryogen-free automatic gas chromatograph system (GC-MS/FID) for online measurements of volatile organic compounds. Anal. Methods 2014, 6, 9424–9434. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, M.; Lu, S.; Liao, C.; Wang, J.; Chen, G. Volatile organic compound (VOC) measurements in the pearl river delta (PRD) region, China. Atmos. Chem. Phys. 2008, 8, 1531–1545. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Shao, M.; Lu, S.; Yuan, B.; Zhao, Y.; Wang, M.; Zhang, S.; Wu, D. Variation of ambient non-methane hydrocarbons in Beijing city in summer 2008. Atmos. Chem. Phys. 2010, 10, 5911–5923. [Google Scholar] [CrossRef] [Green Version]
- Cardelino, C.A.; Chameides, W.L. An Observation-Based Model for Analyzing Ozone Precursor Relationships in the Urban Atmosphere. J. Air Waste Manage. Assoc. 1995, 45, 161–180. [Google Scholar] [CrossRef]
- EPA. EPA Positive Matrix Factorization (PMF) 5.0 Fundamentals and User Guide; United States Environmental Protection Agency: Washington, DC, USA, 2014.
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Crowley, J.N.; Hampson, R.F.; Hynes, R.G.; Jenkin, M.E.; Rossi, M.J.; Troe, J.; Subcommittee, I. Evaluated kinetic and photochemical data for atmospheric chemistry: Volume II—Gas phase reactions of organic species. Atmos. Chem. Phys. 2006, 6, 3625–4055. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Shao, M.; Liu, Y.; Lu, S.; Kuster, W.; Goldan, P.; Xie, S. Source Apportionment of Ambient Volatile Organic Compounds in Beijing. Environ. Sci. Technol. 2007, 41, 4348–4353. [Google Scholar] [CrossRef]
- Cai, C.; Geng, F.; Tie, X.; Yu, Q.; An, J. Characteristics and source apportionment of VOCs measured in Shanghai, China. Atmos. Environ. 2010, 44, 5005–5014. [Google Scholar] [CrossRef]
- Shao, P.; An, J.; Xin, J.; Wu, F.; Wang, J.; Ji, D.; Wang, Y. Source apportionment of VOCs and the contribution to photochemical ozone formation during summer in the typical industrial area in the Yangtze River Delta, China. Atmos. Res. 2016, 176-177, 64–74. [Google Scholar] [CrossRef]
- Lyu, X.; Chen, N.; Guo, H.; Zhang, W.; Wang, N.; Wang, Y.; Liu, M. Ambient volatile organic compounds and their effect on ozone production in Wuhan, central China. Sci. Total Environ. 2016, 541, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Shao, M.; Lu, S.; Niu, H.; Zhou, M.; Sun, J. Characterization of non-methane hydrocarbons and their sources in an industrialized coastal city, Yangtze River Delta, China. Sci. Total Environ. 2017, 593-594, 641–653. [Google Scholar] [CrossRef]
- Tang, J.; Chan, L.; Chan, C.; Li, Y.; Chang, C.; Liu, S.; Wu, D.; Li, Y. Characteristics and diurnal variations of NMHCs at urban, suburban, and rural sites in the Pearl River Delta and a remote site in South China. Atmos. Environ. 2007, 41, 8620–8632. [Google Scholar] [CrossRef]
- Leuchner, M.; Rappenglück, B. VOC source–receptor relationships in Houston during TexAQS-II. Atmos. Environ. 2010, 44, 4056–4067. [Google Scholar] [CrossRef]
- Na, K.; Kim, Y.P.; Moon, K.-C.; Moon, I.; Fung, K. Concentrations of volatile organic compounds in an industrial area of Korea. Atmos. Environ. 2001, 35, 2747–2756. [Google Scholar] [CrossRef]
- Wang, M.; Chen, W.; Zhang, L.; Qin, W.; Zhang, Y.; Zhang, X.; Xie, X. Ozone pollution characteristics and sensitivity analysis using an observation-based model in Nanjing, Yangtze River Delta Region of China. J. Environ. Sci. 2020, 93, 13–22. [Google Scholar] [CrossRef]
- Wang, Y. An Open Source Software Suite for Multi-Dimensional Meteorological Data Computation and Visualisation. J. Open Res. Softw. 2019, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Shao, M.; Fu, L.; Lu, S.; Zeng, L.; Tang, D. Source profiles of volatile organic compounds (VOCs) measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar] [CrossRef]
- Chan, L.; Chu, K.; Zou, S.; Chan, C.; Wang, X.; Barletta, B.; Blake, D.R.; Guo, H.; Tsai, W. Characteristics of non-methane hydrocarbons (NMHCs) in industrial, industrial-urban, and industrial-suburban atmospheres of the Pearl River Delta (PRD) region of south China. J. Geophys. Res. 2006, 111. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; Shao, M.; Lu, S.; Wang, B. Source profiles of volatile organic compounds associated with solvent use in Beijing, China. Atmos. Environ. 2010, 44, 1919–1926. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, L.; Fang, X.; Liu, M.; Zhang, J.; Shao, M.; Lu, S.; Mao, H. Emission factors of volatile organic compounds (VOCs) based on the detailed vehicle classification in a tunnel study. Sci. Total Environ. 2018, 624, 878–886. [Google Scholar] [CrossRef]
- Song, C.; Liu, Y.; Sun, L.; Zhang, Q.; Mao, H. Emissions of volatile organic compounds (VOCs) from gasoline- and liquified natural gas (LNG)-fueled vehicles in tunnel studies. Atmos. Environ. 2020, 234, 117626. [Google Scholar] [CrossRef]
- Wang, M.; Li, S.; Zhu, R.; Zhang, R.; Zu, L.; Wang, Y.; Bao, X. On-road tailpipe emission characteristics and ozone formation potentials of VOCs from gasoline, diesel and liquefied petroleum gas fueled vehicles. Atmos. Environ. 2020, 223, 117294. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, L.; Liu, M.; Fang, X.; Zhang, J.; Mao, H.; Shao, M.; Lu, S. Pollution characteristics and health risk assessment of VOCs from vehicle exhaust in Nanjing, China. China Environ. Sci. 2016, 36, 3118–3125. (In Chinese) [Google Scholar]
- Fu, L.; Shao, M.; Liu, Y.; Liu, Y.; Lu, S.; Tang, D. Tunnel experiment of characteristics and emission factors of volatile organic compounds (VOCs) from vehicles. Acta Sci. Circum. 2005, 25, 79–885. (In Chinese) [Google Scholar]
- Li, B.; Ho, S.S.H.; Xue, Y.; Huang, Y.; Wang, L.; Cheng, Y.; Dai, W.; Zhong, H.; Cao, J.; Lee, S. Characterizations of volatile organic compounds (VOCs) from vehicular emissions at roadside environment: The first comprehensive study in Northwestern China. Atmos. Environ. 2017, 161, 1–12. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Wang, X.; Ling, Z.; Zhao, J.; Guo, H.; Shao, M.; Wang, Z. Contributions of different anthropogenic volatile organic compound sources to ozone formation at a receptor site in the Pearl River Delta region and its policy implications. Atmos. Chem. Phys. 2019, 19, 8801–8816. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.T.; Shao, M.; Lu, S.H.; Wang, M.; Zeng, L.M.; Yuan, B.; Liu, Y. Understanding primary and secondary sources of ambient carbonyl compounds in Beijing using the PMF model. Atmos. Chem. Phys. 2014, 14, 3047–3062. [Google Scholar] [CrossRef] [Green Version]
- Blake, D.R.; Rowland, F.S. Urban Leakage of Liquefied Petroleum Gas and Its Impact on Mexico City Air Quality. Science 1995, 269, 953–956. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, H.; Jing, S.; Gao, Y.; Peng, Y.; Lou, S.; Cheng, T.; Tao, S.; Li, L.; Li, Y.; et al. Characteristics and sources of volatile organic compounds (VOCs) in Shanghai during summer: Implications of regional transport. Atmos. Environ. 2019, 215, 116902. [Google Scholar] [CrossRef]
- Melike, D.; Odabasi, M.; Yenisoy-Karakaş, S. Source apportionment of biogenic and anthropogenic VOCs in Bolu plateau. Sci. Total Environ. 2020, 731, 139201. [Google Scholar]
- Fan, M.; Zhang, Y.; Lin, Y.; Li, L.; Xie, F.; Hu, J.; Mozaffar, A.; Cao, F. Source apportionments of atmospheric volatile organic compounds in Nanjing, China during high ozone pollution season. Chemosphere 2020, 263, 128025. [Google Scholar] [CrossRef]
- Zhao, Q.; Bi, J.; Liu, Q.; Ling, Z.; Shen, G.; Chen, F.; Qiao, Y.; Li, C.; Ma, Z. Sources of volatile organic compounds and policy implications for regional ozone pollution control in an urban location of Nanjing, East China. Atmos. Chem. Phys. 2020, 20, 3905–3919. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.; Lu, K.; Jiang, M.; Su, R.; Wang, H.; Lou, S.; Fu, Q.; Zhai, C.; Tan, Q.; Yue, D.; et al. Daytime atmospheric oxidation capacity in four Chinese megacities during the photochemically polluted season: A case study based on box model simulation. Atmos. Chem. Phys. 2019, 19, 3493–3513. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Ding, A.; Gao, J.; Zheng, B.; Zhou, D.; Qi, X.; Tang, R.; Wang, J.; Ren, C.; Nie, W.; et al. Enhanced secondary pollution offset reduction of primary emissions during COVID-19 lockdown in China. Natl. Sci. Rev. 2020, 7, nwaa137. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Wang, M.; Sun, Y.; Wang, Z. A Case Study of Investigating Secondary Organic Aerosol Formation Pathways in Beijing using an Observation-based SOA Box Model. Aerosol Air Qual. Res. 2018, 18, 1606–1616. [Google Scholar] [CrossRef] [Green Version]


| City | Observation Period | Type of Site | Propane | i-Pentane | Toluene | References |
|---|---|---|---|---|---|---|
| Baoding | 2015-09 | urban | 4.0 ± 2.9 | 1.9 ± 1.3 | 4.2 ± 4.7 | This study |
| Beijing | 2005-08 | urban | 3.6 ± 2.2 | 4.1 ± 2.5 | 3.3 ± 1.7 | [17] |
| Shanghai | 2007-01–2010-03 | urban | 4.8 ± 2.1 | 2.3 ± 1.4 | 4.7 ± 4.2 | [18] |
| Nanjing | 2013-08 | suburb/industrial | 2.0 ± 1.7 | 1.1 ± 1.0 | 1.2 ± 1.5 | [19] |
| Guangzhou | 2005-04 | urban | 11.3 ± 5.7 | 3.8 ± 1.9 | 10.0 ± 4.7 | [22] |
| Wuhan | 2014-02–10 | urban | 1.9 | 1.0 | 2.0 | [20] |
| Ningbo | 2012-11–2013-10 | urban/industrial | 2.6 ± 2.1 | 1.3 ± 1.7 | 1.7 ± 2.4 | [21] |
| Houston, US | 2006-08–09 | industrial | 13.2 | 7.2 | 5.1 | [23] |
| Ulsan, Korea | 1997-06 | industrial | 9.5 ± 3.5 | 2.7 ± 1.2 | 3.9 ± 0.9 | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Hu, K.; Chen, W.; Shen, X.; Li, W.; Lu, X. Ambient Non-Methane Hydrocarbons (NMHCs) Measurements in Baoding, China: Sources and Roles in Ozone Formation. Atmosphere 2020, 11, 1205. https://doi.org/10.3390/atmos11111205
Wang M, Hu K, Chen W, Shen X, Li W, Lu X. Ambient Non-Methane Hydrocarbons (NMHCs) Measurements in Baoding, China: Sources and Roles in Ozone Formation. Atmosphere. 2020; 11(11):1205. https://doi.org/10.3390/atmos11111205
Chicago/Turabian StyleWang, Ming, Kun Hu, Wentai Chen, Xueyong Shen, Wenjing Li, and Xingdong Lu. 2020. "Ambient Non-Methane Hydrocarbons (NMHCs) Measurements in Baoding, China: Sources and Roles in Ozone Formation" Atmosphere 11, no. 11: 1205. https://doi.org/10.3390/atmos11111205
APA StyleWang, M., Hu, K., Chen, W., Shen, X., Li, W., & Lu, X. (2020). Ambient Non-Methane Hydrocarbons (NMHCs) Measurements in Baoding, China: Sources and Roles in Ozone Formation. Atmosphere, 11(11), 1205. https://doi.org/10.3390/atmos11111205




