Enhancing Potential of Trimethylamine Oxide on Atmospheric Particle Formation
Abstract
1. Introduction
2. Methods
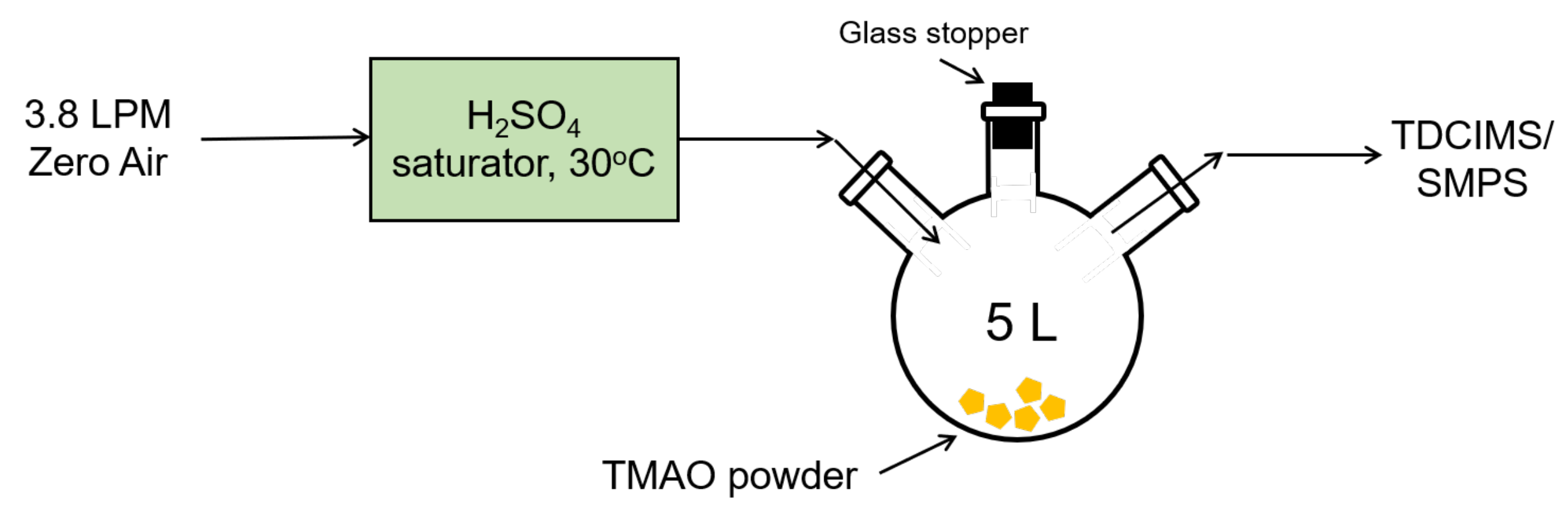
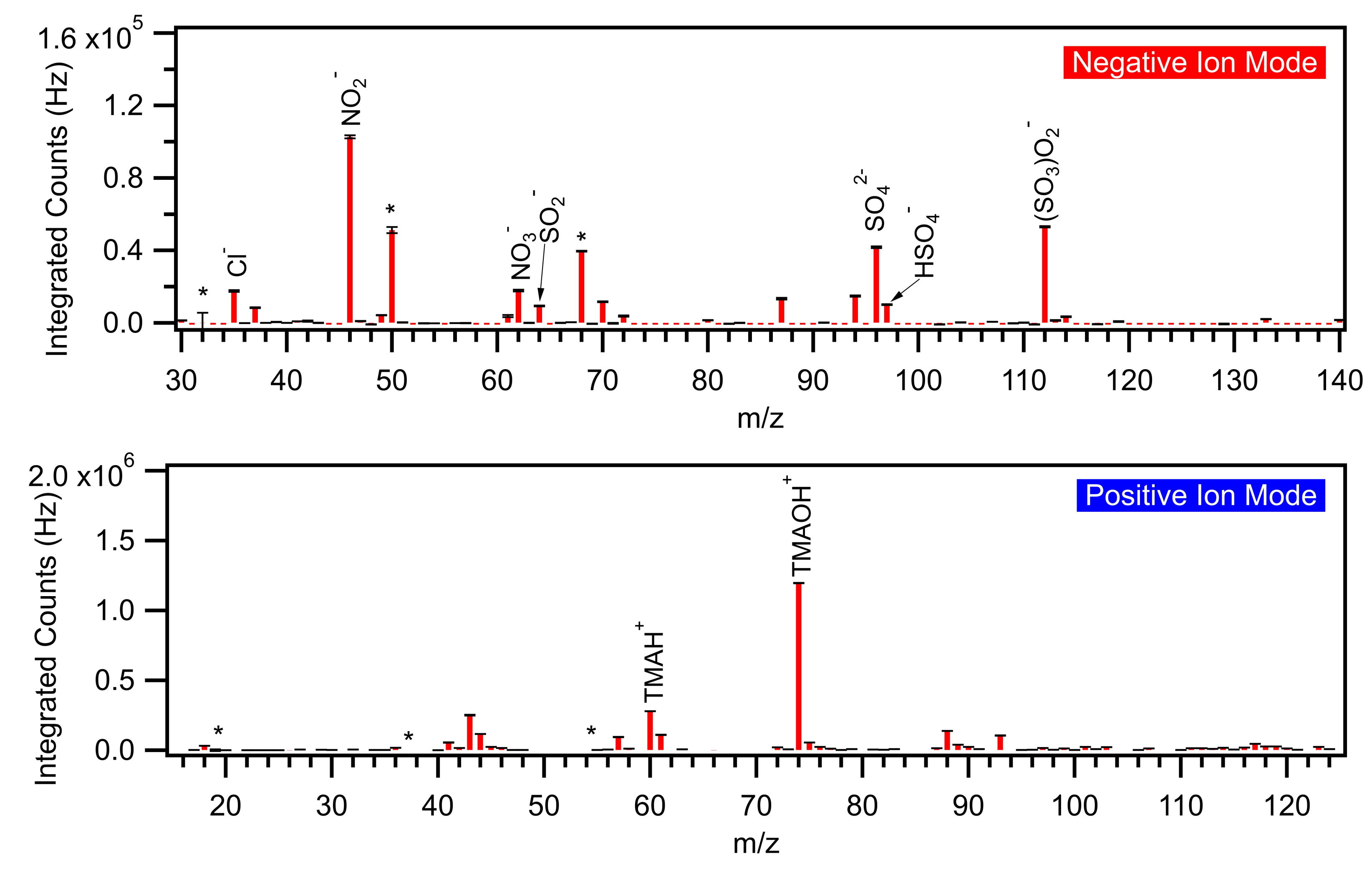
Production of SA-TMAO Particles and Measurement with TDCIMS
3. Results and Discussion
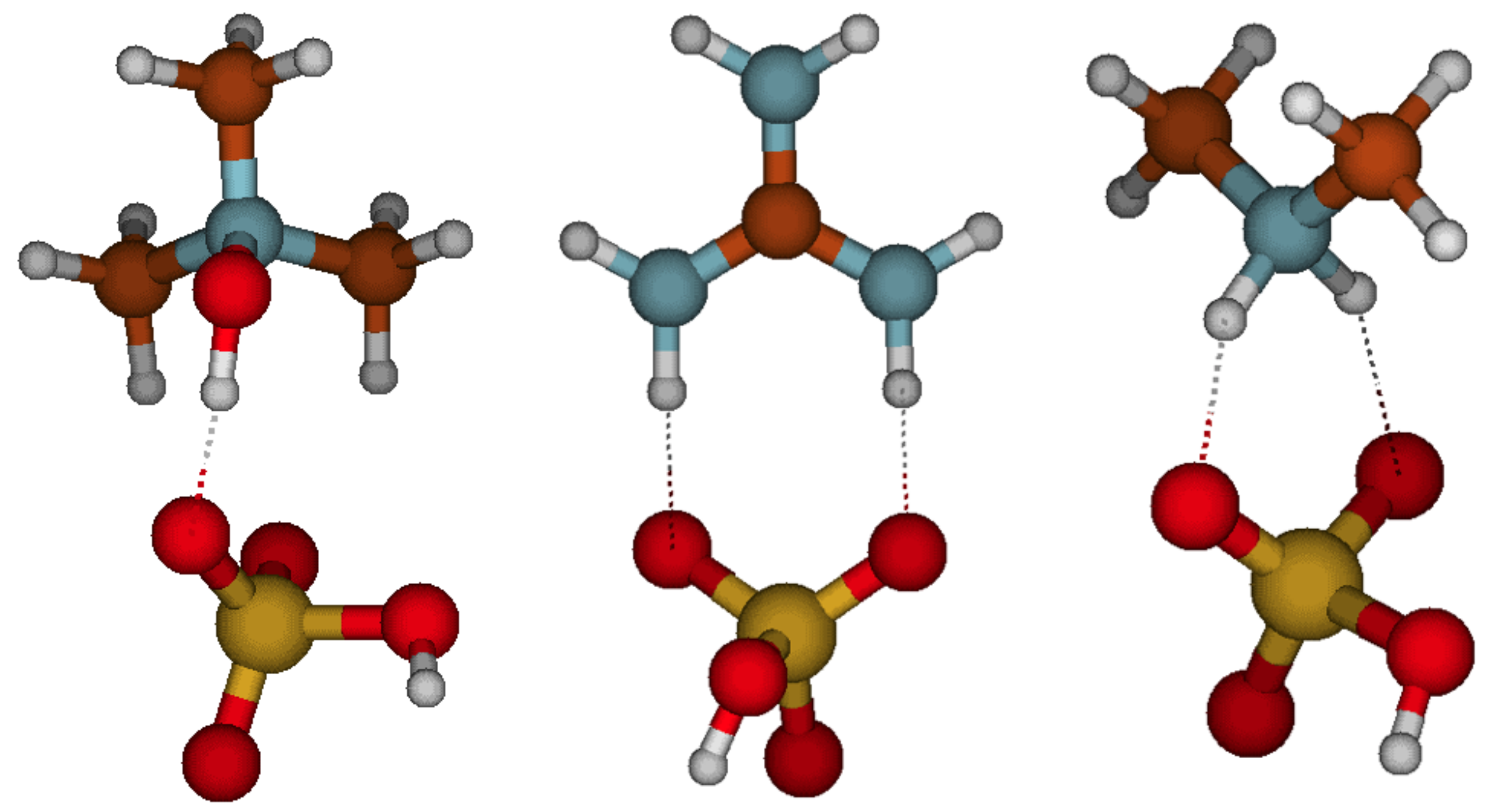
3.1. Acid–Base Heterodimer Stability
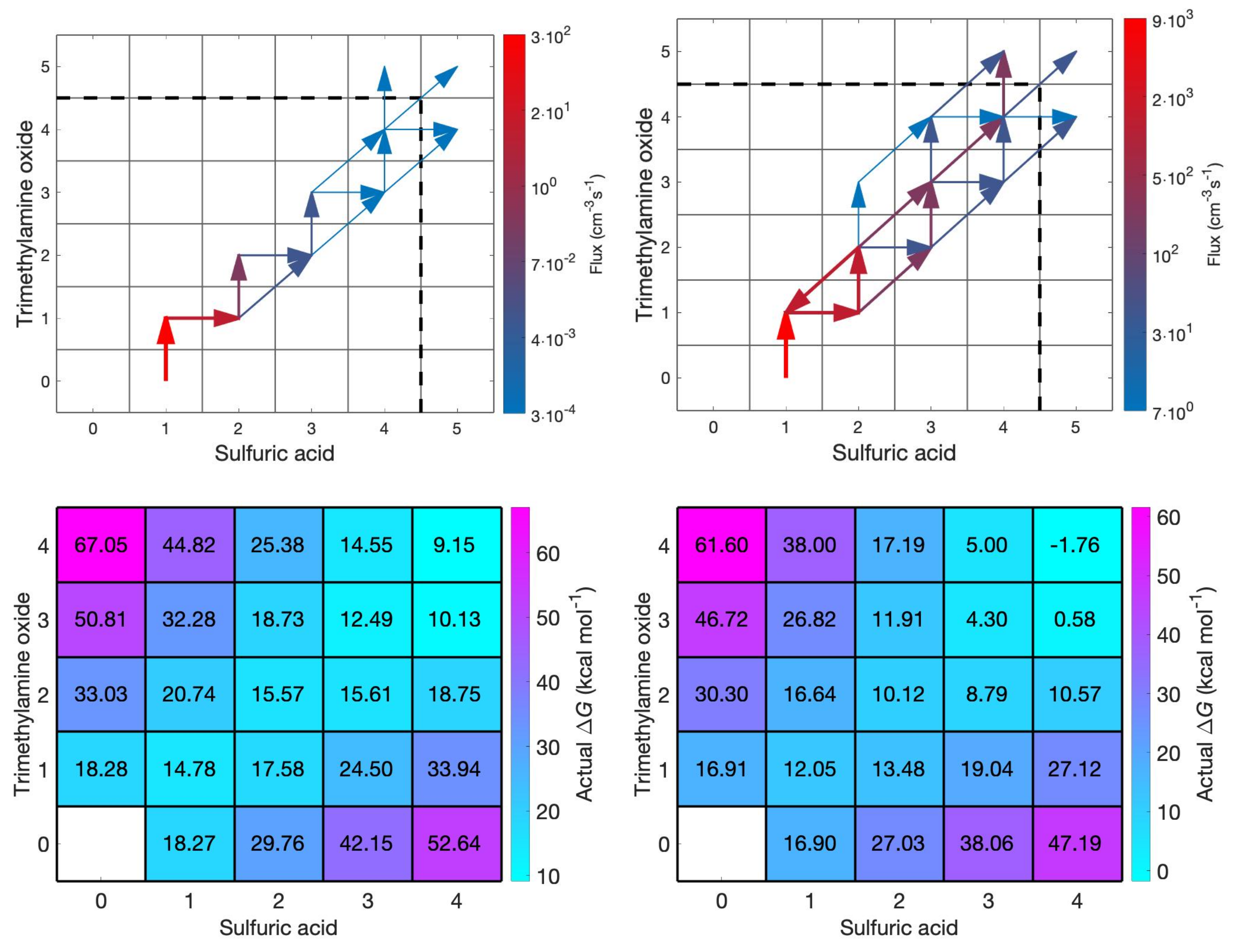
3.2. Cluster Growth Pathways
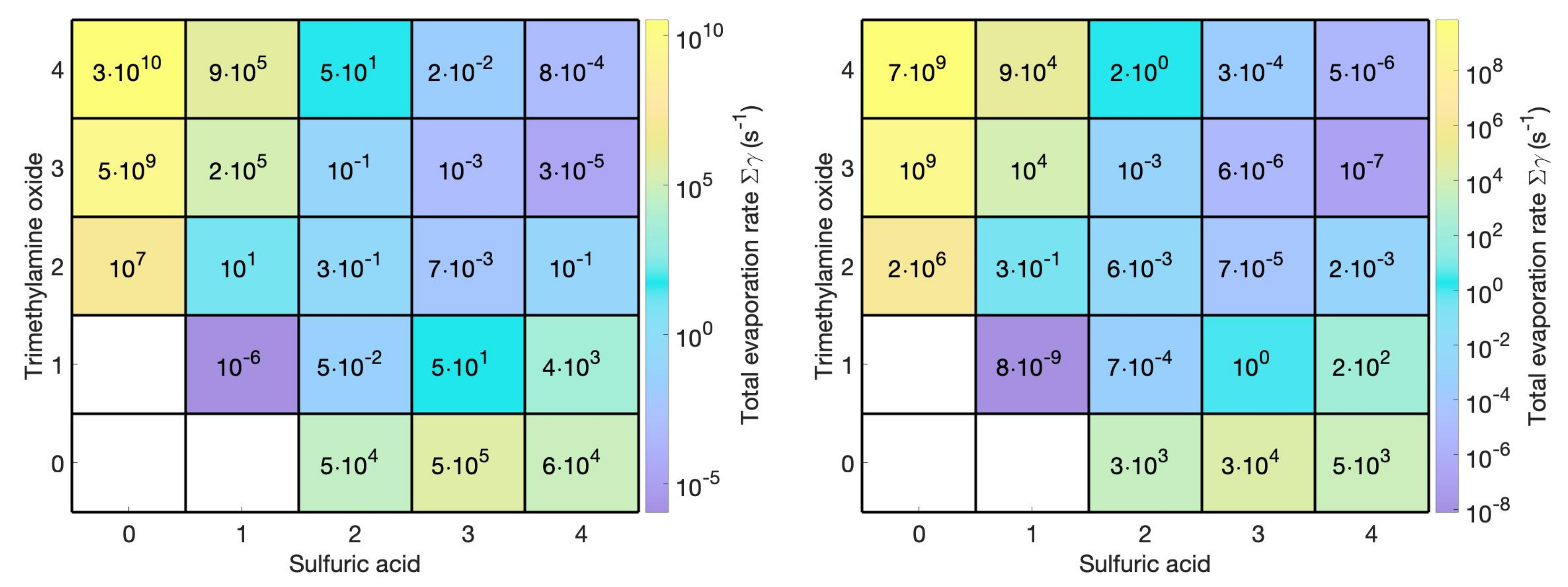
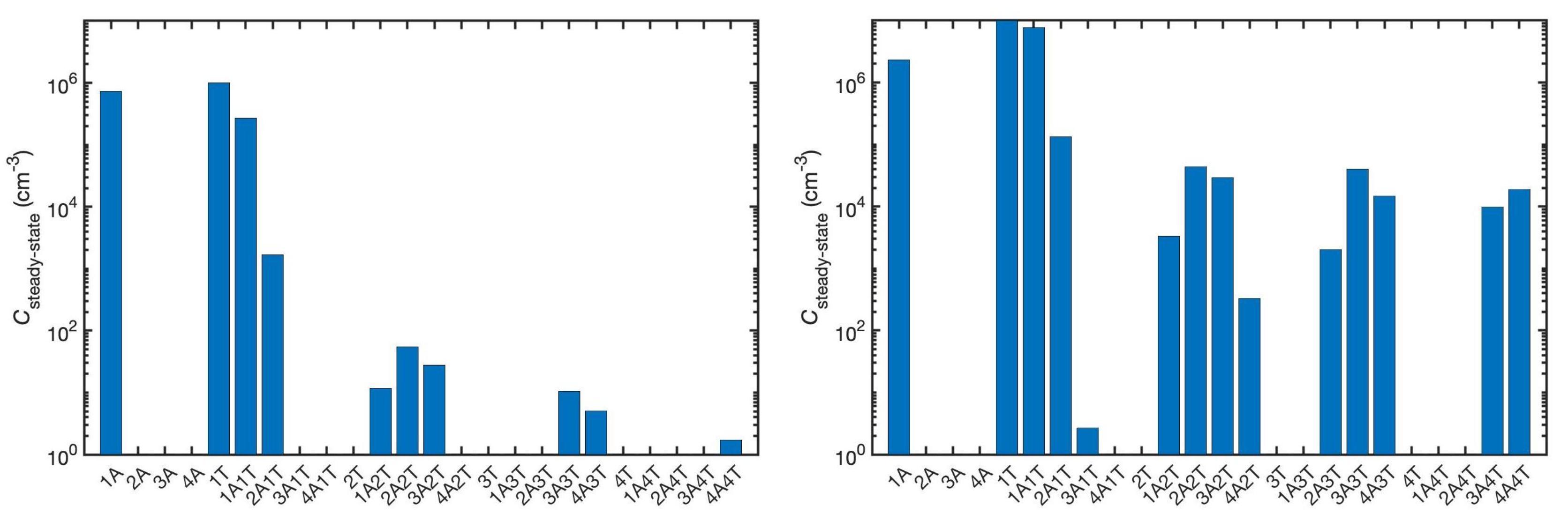
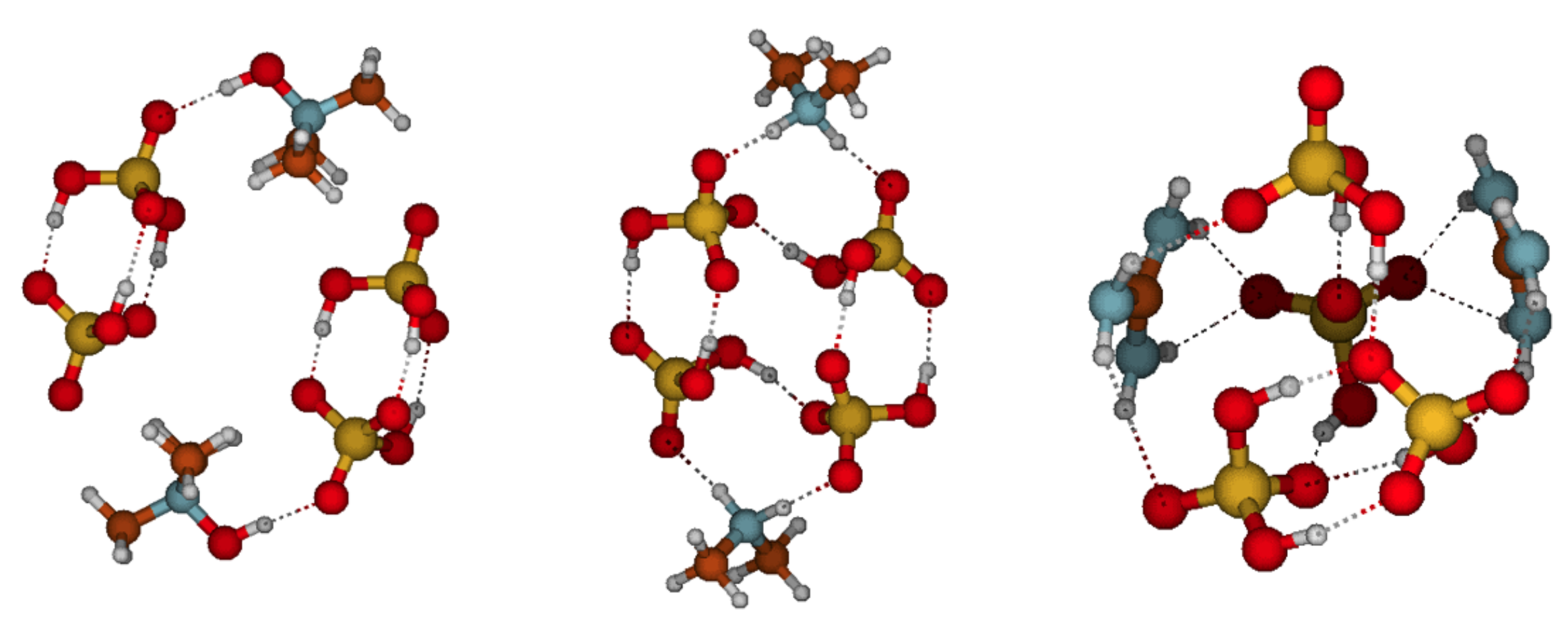
3.3. Cluster Structures, Stabilities, and Distributions
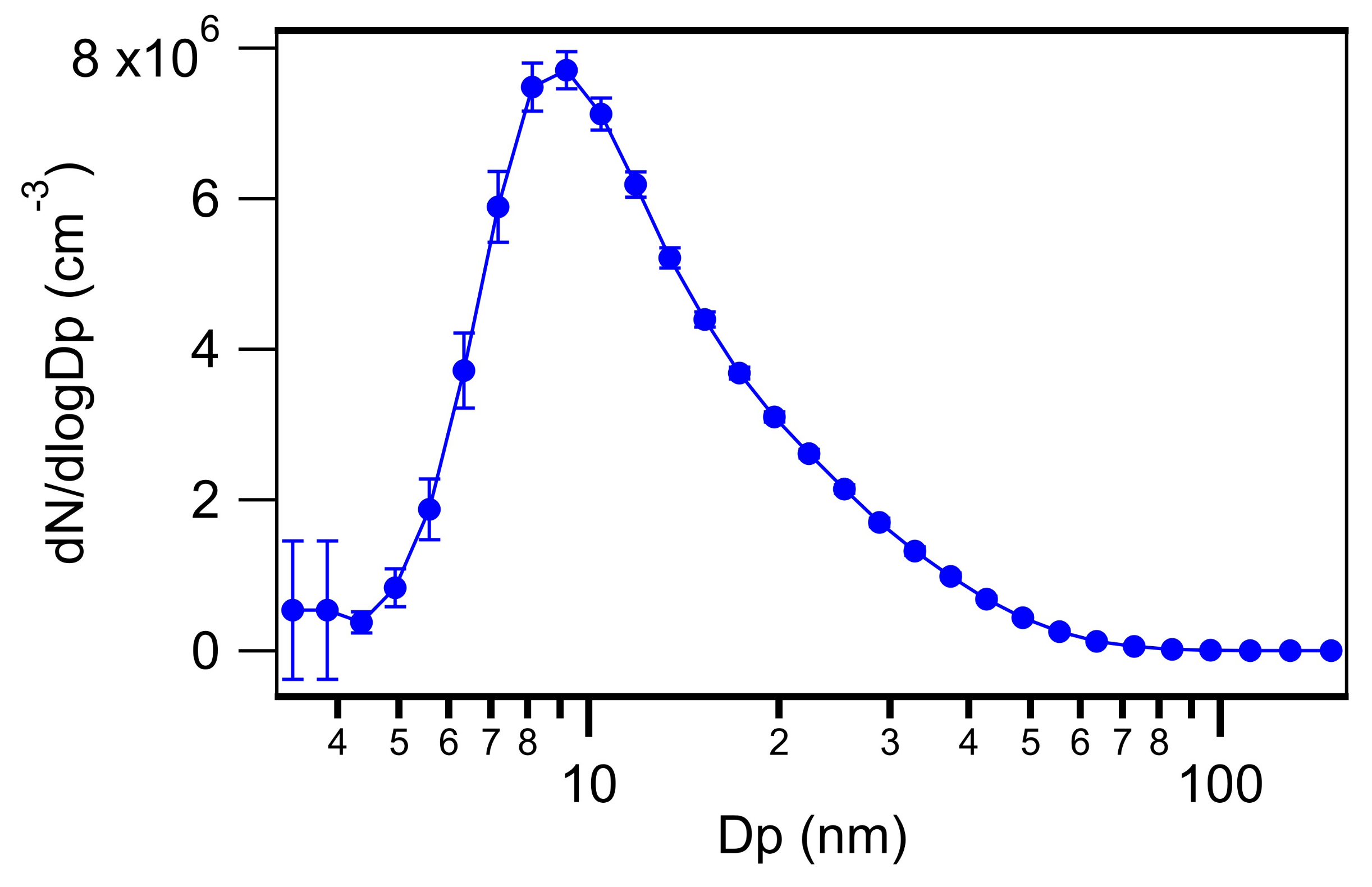
3.4. Particles Generated from SA and TMAO
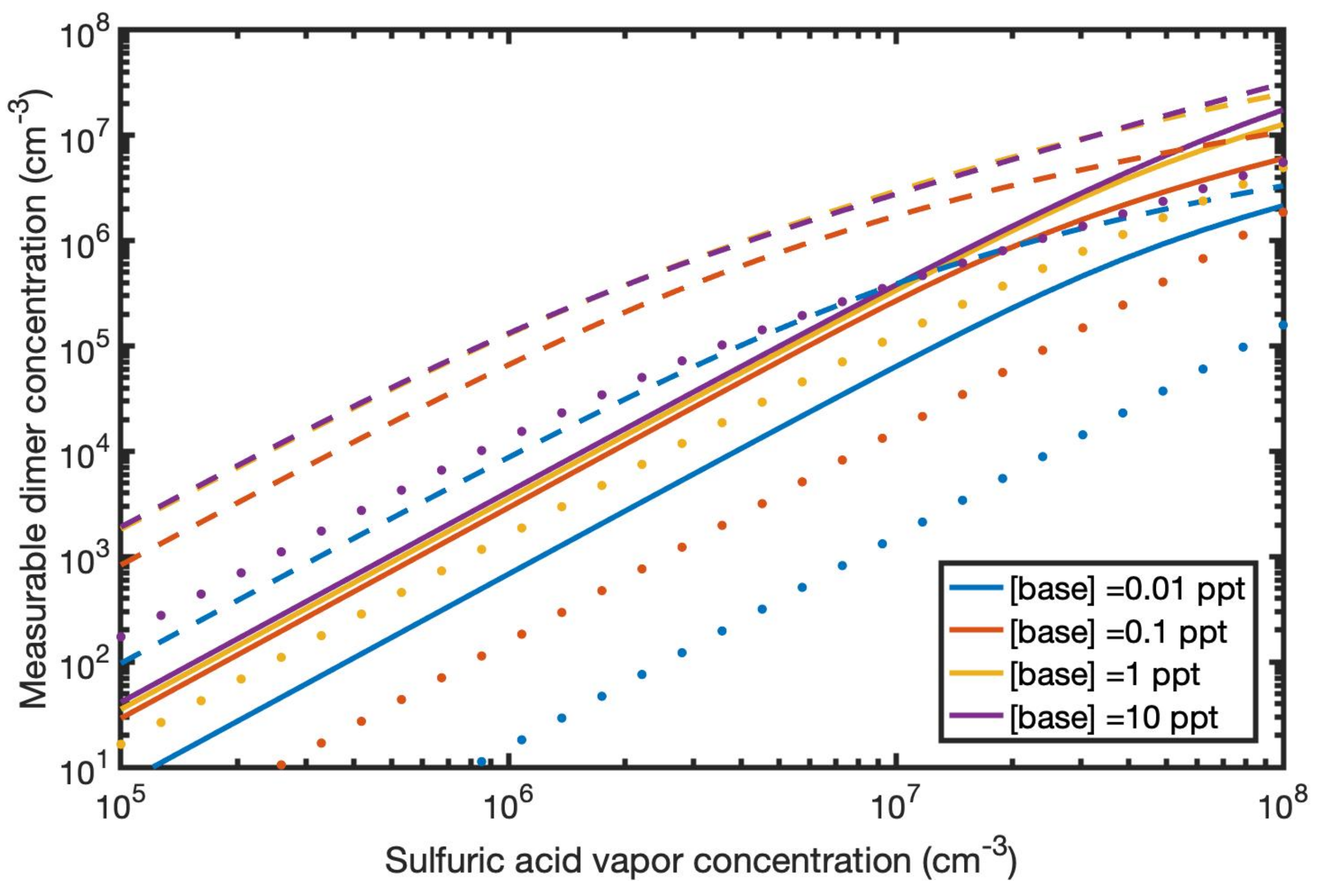
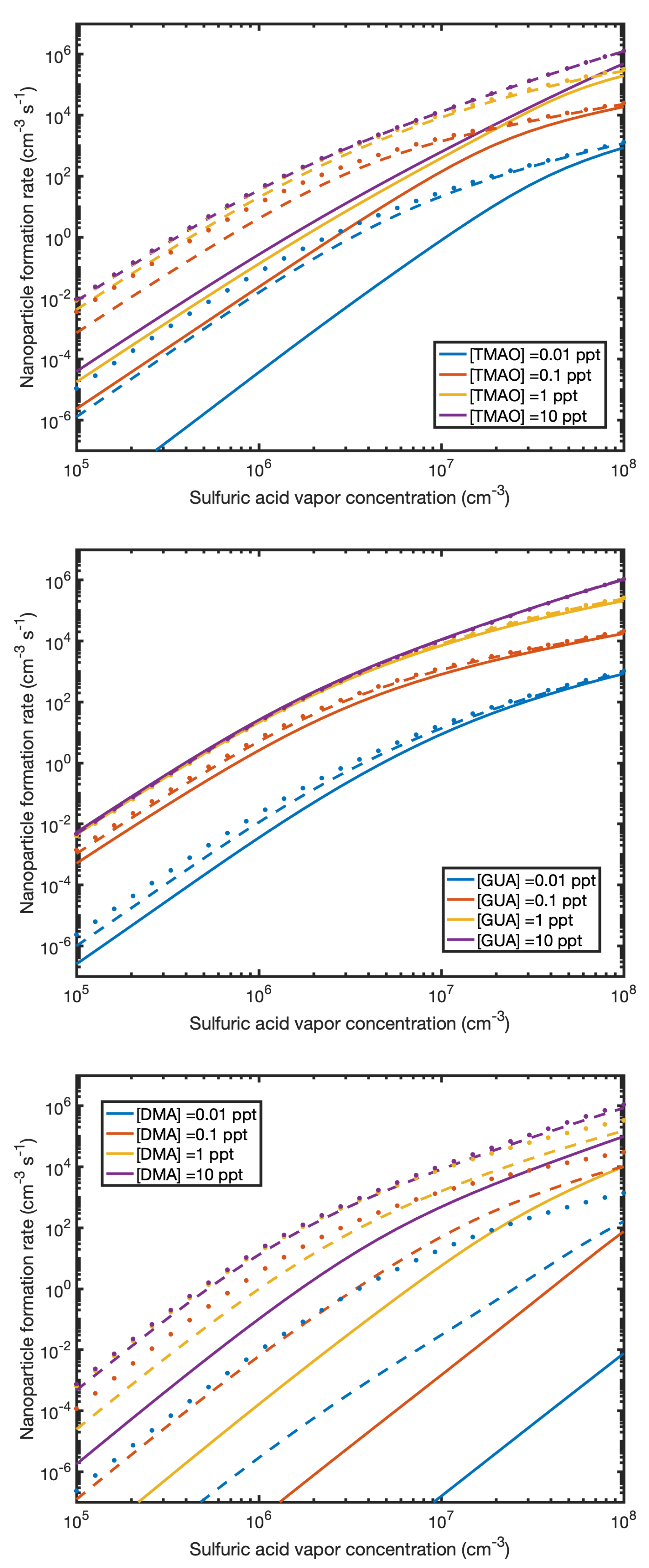
3.5. Measurable Dimer Concentrations and Nanoparticle Formation Rates
3.6. Base Strength and Structure versus Enhancing Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuang, C.; McMurry, P.H.; McCormick, A.V.; Eisele, F.L. Dependence of Nucleation Rates on Sulfuric Acid Vapor Concentration in Diverse Atmospheric Locations. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Anderson, N.; Strader, R.; Davidson, C. Airborne Reduced Nitrogen: Ammonia Emissions from Agriculture and Other Sources. Environ. Int. 2003, 29, 277–286. [Google Scholar] [CrossRef]
- Ball, S.; Hanson, D.; Eisele, F.; McMurry, P.H. Laboratory Studies of Particle Nucleation: Initial Results for H2SO4, H2O, and NH3 Vapors. J. Geophys. Res. Atmos. 1999, 104, 23709–23718. [Google Scholar] [CrossRef]
- Cape, J.; Cornell, S.; Jickells, T.; Nemitz, E. Organic Nitrogen in the Atmosphere—Where Does It Come from? A Review of Sources and Methods. Atmos. Res. 2011, 102, 30–48. [Google Scholar] [CrossRef]
- Kurtén, T.; Loukonen, V.; Vehkamäki, H.; Kulmala, M. Amines Are Likely to Enhance Neutral and Ion-Induced Sulfuric Acid-Water Nucleation in the Atmosphere More Effectively Than Ammonia. Atmos. Chem. Phys. 2008, 8, 4095–4103. [Google Scholar] [CrossRef]
- Almeida, J.; Schobesberger, S.; Kürten, A.; Ortega, I.K.; Kupiainen-Määttä, O.; Praplan, A.P.; Adamov, A.; Amorim, A.; Bianchi, F.; Breitenlechner, M.; et al. Molecular Understanding of Sulphuric Acid–Amine Particle Nucleation in the Atmosphere. Nature 2013, 502, 359–363. [Google Scholar] [CrossRef]
- Yu, H.; McGraw, R.; Lee, S.H. Effects of Amines on Formation of Sub-3 nm Particles and Their Subsequent Growth. Geophys. Res. Lett. 2012, 39, 2. [Google Scholar] [CrossRef]
- Yao, L.; Garmash, O.; Bianchi, F.; Zheng, J.; Yan, C.; Kontkanen, J.; Junninen, H.; Mazon, S.B.; Ehn, M.; Paasonen, P.; et al. Atmospheric New Particle Formation from Sulfuric Acid and Amines in a Chinese Megacity. Science 2018, 361, 278–281. [Google Scholar] [CrossRef]
- Erupe, M.E.; Viggiano, A.A.; Lee, S.H. The Effect of Trimethylamine on Atmospheric Nucleation Involving H2SO4. Atmos. Chem. Phys. 2011, 11, 4767–4775. [Google Scholar] [CrossRef]
- Myllys, N.; Chee, S.; Olenius, T.; Lawler, M.; Smith, J.N. Molecular-Level Understanding of Synergistic Effects in Sulfuric Acid–Amine–Ammonia Mixed Clusters. J. Phys. Chem. A 2019, 123, 2420–2425. [Google Scholar] [CrossRef]
- Kürten, A.; Jokinen, T.; Simon, M.; Sipilä, M.; Sarnela, N.; Junninen, H.; Adamov, A.; Almeida, J.; Amorim, A.; Bianchi, F.; et al. Neutral Molecular Cluster Formation of Sulfuric Acid–Dimethylamine Observed in Real Time Under Atmospheric Conditions. Proc. Natl. Acad. Sci. USA 2014, 111, 15019–15024. [Google Scholar] [CrossRef] [PubMed]
- Paasonen, P.; Olenius, T.; Kupiainen, O.; Kurtén, T.; Petäjä, T.; Birmili, W.; Hamed, A.; Hu, M.; Huey, L.G.; Plass-Duelmer, C.; et al. On the Formation of Sulphuric Acid–Amine Clusters in Varying Atmospheric Conditions and Its Influence on Atmospheric New Particle Formation. Atmos. Chem. Phys. 2012, 12, 9113–9133. [Google Scholar] [CrossRef]
- Temelso, B.; Morrison, E.F.; Speer, D.L.; Cao, B.C.; Appiah-Padi, N.; Kim, G.; Shields, G.C. Effect of Mixing Ammonia and Alkylamines on Sulfate Aerosol Formation. J. Phys. Chem. A 2018, 122, 1612–1622. [Google Scholar] [CrossRef] [PubMed]
- Bustos, D.J.; Temelso, B.; Shields, G.C. Hydration of the Sulfuric Acid–Methylamine Complex and Implications for Aerosol Formation. J. Phys. Chem. A 2014, 118, 7430–7441. [Google Scholar] [CrossRef] [PubMed]
- Hemmilä, M.; Hellén, H.; Virkkula, A.; Makkonen, U.; Praplan, A.P.; Kontkanen, J.; Ahonen, L.; Kulmala, M.; Hakola, H. Amines in Boreal Forest Air at SMEAR II Station in Finland. Atmos. Chem. Phys. 2018, 18, 6367–6380. [Google Scholar] [CrossRef]
- Olenius, T.; Halonen, R.; Kurtén, T.; Henschel, H.; Kupiainen-Määttä, O.; Ortega, I.K.; Jen, C.N.; Vehkamäki, H.; Riipinen, I. New Particle Formation from Sulfuric Acid and Amines: Comparison of Mono-, Di-, and Trimethylamines. J. Geophys. Res. Atmos. 2017, 122, 7103–7118. [Google Scholar] [CrossRef]
- Glasoe, W.; Volz, K.; Panta, B.; Freshour, N.; Bachman, R.; Hanson, D.; McMurry, P.; Jen, C. Sulfuric Acid Nucleation: An Experimental Study of the Effect of Seven Bases. J. Geophys. Res. Atmos. 2015, 120, 1933–1950. [Google Scholar] [CrossRef]
- Jen, C.N.; McMurry, P.H.; Hanson, D.R. Stabilization of Sulfuric Acid Dimers by Ammonia, Methylamine, Dimethylamine, and Trimethylamine. J. Geophys. Res. Atmos. 2014, 119, 7502–7514. [Google Scholar] [CrossRef]
- Schade, G.W.; Crutzen, P.J. Emission of Aliphatic Amines from Animal Husbandry and Their Reactions: Potential Source of N2O and HCN. J. Atmos. Chem. 1995, 22, 319–346. [Google Scholar] [CrossRef]
- Malloy, Q.G.J.; Qi, L.; Warren, B.; Cocker, D.R., III; Erupe, M.E.; Silva, P.J. Secondary Organic Aerosol Formation from Primary Aliphatic Amines with NO3 Radical. Atmos. Chem. Phys. 2009, 9, 2051–2060. [Google Scholar] [CrossRef]
- Erupe, M.E.; Liberman-Martin, A.; Silva, P.J.; Malloy, Q.G.; Yonis, N.; Cocker, D.R., III; Purvis-Roberts, K.L. Determination of Methylamines and Trimethylamine-N-oxide in Particulate Matter by Non-Suppressed Ion Chromatography. J. Chromatogr. A 2010, 1217, 2070–2073. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Luo, G. Modeling of Gaseous Methylamines in the Global Atmosphere: Impacts of Oxidation and Aerosol Uptake. Atmos. Chem. Phys. 2014, 14, 12455–12464. [Google Scholar] [CrossRef]
- Ho, K.L.; Chung, Y.C.; Lin, Y.H.; Tseng, C.P. Biofiltration of Trimethylamine, Dimethylamine, and Methylamine by Immobilized Paracoccus sp. CP2 and Arthrobacter sp. CP1. Chemosphere 2008, 72, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wexler, A.S.; Clegg, S.L. Atmospheric Amines–Part I. A Review. Atmos. Environ. 2011, 45, 524–546. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef]
- Baker, J.; Chaykin, S. The Biosynthesis of Trimethylamine-N-oxide. J. Biol. Chem. 1962, 237, 1309–1313. [Google Scholar] [CrossRef]
- Angelino, S.; Suess, D.T.; Prather, K.A. Formation of Aerosol Particles from Reactions of Secondary and Tertiary Alkylamines: Characterization by Aerosol Time-of-Flight Mass Spectrometry. Environ. Sci. Technol. 2001, 35, 3130–3138. [Google Scholar] [CrossRef]
- Silva, P.J.; Erupe, M.E.; Price, D.; Elias, J.; GJ Malloy, Q.; Li, Q.; Warren, B.; Cocker, D.R. Trimethylamine as Precursor to Secondary Organic Aerosol Formation via Nitrate Radical Reaction in the Atmosphere. Environ. Sci. Technol. 2008, 42, 4689–4696. [Google Scholar] [CrossRef]
- Murphy, S.; Sorooshian, A.; Kroll, J.; Ng, N.; Chhabra, P.; Tong, C.; Surratt, J.; Knipping, E.; Flagan, R.; Seinfeld, J. Secondary Aerosol Formation from Atmospheric Reactions of Aliphatic Amines. Atmos. Chem. Phys. 2007, 7, 2313–2337. [Google Scholar] [CrossRef]
- Atkinson, R.; Pitts, J.N. Kinetics of the Reactions of O(3P) Atoms with the Amines CH3NH2, C2H5NH2, (CH3)2NH, and (CH3)3N Over the Temperature Range 298–440 K. J. Chem. Phys. 1978, 68, 911–915. [Google Scholar] [CrossRef]
- Lidbury, I.; Murrell, J.C.; Chen, Y. Trimethylamine N-oxide Metabolism by Abundant Marine Heterotrophic Bacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 2710–2715. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Chen, X.L.; Shao, X.; Wei, T.D.; Wang, P.; Xie, B.B.; Qin, Q.L.; Zhang, X.Y.; Su, H.N.; Song, X.Y.; et al. Mechanistic Insight into Trimethylamine N-oxide Recognition by the Marine Bacterium Ruegeria Pomeroyi DSS-3. J. Bacteriol. 2015, 197, 3378–3387. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sintermann, J.; Schallhart, S.; Kajos, M.; Jocher, M.; Bracher, A.; Münger, A.; Johnson, D.; Neftel, A.; Ruuskanen, T. Trimethylamine Emissions in Animal Husbandry. Biogeosciences 2014, 11, 5073–5085. [Google Scholar] [CrossRef]
- Knölker, H.J. Trimethylamine N-Oxide—A Useful Oxidizing Reagent. J. Prakt. Chem. Chem. Ztg. 1996, 338, 190–192. [Google Scholar] [CrossRef]
- Åkesson, B.; Vinge, E.; Skerfving, S. Pharmacokinetics of Triethylamine and Triethylamine-N-oxide in Man. Toxicol. Appl. Pharm. 1989, 100, 529–538. [Google Scholar] [CrossRef]
- Rappert, S.; Müller, R. Odor Compounds in Waste Gas Emissions from Agricultural Operations and Food Industries. Waste Manag. 2005, 25, 887–907. [Google Scholar] [CrossRef]
- McGrath, M.J.; Olenius, T.; Ortega, I.K.; Loukonen, V.; Paasonen, P.; Kurtén, T.; Kulmala, M.; Vehkamäki, H. Atmospheric Cluster Dynamics Code: A Flexible Method for Solution of the Birth–Death Equations. Atmos. Chem. Phys. 2012, 12, 2345–2355. [Google Scholar] [CrossRef]
- Voisin, D.; Smith, J.N.; Sakurai, H.; McMurry, P.; Eisele, F.L. Thermal Desorption Chemical Ionization Mass Spectrometer for Ultrafine Particle Chemical Composition. Aerosol Sci. Technol. 2003, 37, 471–475. [Google Scholar] [CrossRef]
- Kubečka, J.; Besel, V.; Kurtén, T.; Myllys, N.; Vehkamäki, H. Configurational Sampling of Noncovalent (Atmospheric) Molecular Clusters: Sulfuric Acid and Guanidine. J. Phys. Chem. A 2019, 123, 6022–6033. [Google Scholar] [CrossRef]
- Zhang, J.; Dolg, M. ABCluster: The Artificial Bee Colony Algorithm for Cluster Global Optimization. Phys. Chem. Chem. Phys. 2015, 17, 24173–24181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dolg, M. Global Optimization of Clusters of Rigid Molecules using the Artificial Bee Colony Algorithm. Phys. Chem. Chem. Phys. 2016, 18, 3003–3010. [Google Scholar] [CrossRef] [PubMed]
- Karaboga, D.; Basturk, B. On the Performance of Artificial Bee Colony (ABC) Algorithm. Appl. Soft Comput. 2008, 8, 687–697. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom-Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Myllys, N.; Elm, J.; Kurtén, T. Density Functional Theory Basis Set Convergence of Sulfuric Acid-Containing Molecular Clusters. Comput. Theor. Chem. 2016, 1098, 1–12. [Google Scholar] [CrossRef]
- Riplinger, C.; Neese, F. An Efficient and Near Linear Scaling Pair Natural Orbital Based Local Coupled Cluster Method. J. Chem. Phys. 2013, 138, 034106. [Google Scholar] [CrossRef]
- Riplinger, C.; Sandhoefer, B.; Hansen, A.; Neese, F. Natural Triple Excitations in Local Coupled Cluster Calculations with Pair Natural Orbitals. J. Chem. Phys. 2013, 139, 134101. [Google Scholar] [CrossRef]
- Riplinger, C.; Pinski, P.; Becker, U.; Valeev, E.F.; Neese, F. Sparse Maps—A Systematic Infrastructure for Reduced-Scaling Electronic Structure Methods. II. Linear Scaling Domain Based Pair Natural Orbital Coupled Cluster Theory. J. Chem. Phys. 2016, 144, 024109. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron Affinities of the First-Row Atoms Revisited. Systematic Basis Sets and Wave Functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Liakos, D.G.; Sparta, M.; Kesharwani, M.K.; Martin, J.M.L.; Neese, F. Exploring the Accuracy Limits of Local Pair Natural Orbital Coupled-Cluster Theory. J. Chem. Theory Comput. 2015, 11, 1525–1539. [Google Scholar] [CrossRef]
- Myllys, N.; Elm, J.; Halonen, R.; Kurtén, T.; Vehkamäki, H. Coupled Cluster Evaluation of the Stability of Atmospheric Acid–Base Clusters with up to 10 Molecules. J. Phys. Chem. A 2016, 120, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian16 Revision A. 03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Neese, F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Smith, J.N.; Moore, K.F.; McMurry, P.H.; Eisele, F.L. Atmospheric Measurements of Sub-20 nm Diameter Particle Chemical Composition by Thermal Desorption Chemical Ionization Mass Spectrometry. Aerosol Sci. Technol. 2004, 38, 100–110. [Google Scholar] [CrossRef]
- Chen, D.R.; Pui, D.Y. A High Efficiency, High Throughput Unipolar Aerosol Charger for Nanoparticles. J. Nanoparticle Res. 1999, 1, 115–126. [Google Scholar] [CrossRef]
- Myllys, N.; Ponkkonen, T.; Passananti, M.; Elm, J.; Vehkamäki, H.; Olenius, T. Guanidine: A Highly Efficient Stabilizer in Atmospheric New-Particle Formation. J. Phys. Chem. A 2018, 122, 4717–4729. [Google Scholar] [CrossRef]
- Myllys, N.; Kubečka, J.; Besel, V.; Alfaouri, D.; Olenius, T.; Smith, J.N.; Passananti, M. Role of Base Strength, Cluster Structure and Charge in Sulfuric-Acid-Driven Particle Formation. Atmos. Chem. Phys. 2019, 19, 9753–9768. [Google Scholar] [CrossRef]
- Elm, J. Elucidating the Limiting Steps in Sulfuric Acid–Base New Particle Formation. J. Phys. Chem. A 2017, 121, 8288–8295. [Google Scholar] [CrossRef]
- Elm, J.; Jen, C.N.; Kurtén, T.; Vehkamäki, H. Strong Hydrogen Bonded Molecular Interactions between Atmospheric Diamines and Sulfuric Acid. J. Phys. Chem. A 2016, 120, 3693–3700. [Google Scholar] [CrossRef]
- Chen, H.; Chee, S.; Lawler, M.J.; Barsanti, K.C.; Wong, B.M.; Smith, J.N. Size Resolved Chemical Composition of Nanoparticles from Reactions of Sulfuric Acid with Ammonia and Dimethylamine. Aerosol Sci. Technol. 2018, 52, 1120–1133. [Google Scholar] [CrossRef]
- Chee, S.; Myllys, N.; Barsanti, K.C.; Wong, B.M.; Smith, J.N. An Experimental and Modeling Study of Nanoparticle Formation and Growth from Dimethylamine and Nitric Acid. J. Phys. Chem. A 2019, 123, 5640–5648. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.B.; Elm, J.; Halonen, R.; Myllys, N.; Kurtén, T.; Kulmala, M.; Vehkamäki, H. Atmospheric Fate of Monoethanolamine: Enhancing New Particle Formation of Sulfuric Acid as an Important Removal Process. Environ. Sci. Technol. 2017, 51, 8422–8431. [Google Scholar] [CrossRef] [PubMed]
- Lawler, M.J.; Whitehead, J.; O’Dowd, C.; Monahan, C.; McFiggans, G.; Smith, J.N. Composition of 15–85 nm Particles in Marine Air. Atmos. Chem. Phys. 2014, 14, 11557–11569. [Google Scholar] [CrossRef]
- Kürten, A.; Bergen, A.; Heinritzi, M.; Leiminger, M.; Lorenz, V.; Piel, F.; Simon, M.; Sitals, R.; Wagner, A.C.; Curtius, J. Observation of New Particle Formation and Measurement of Sulfuric Acid, Ammonia, Amines and Highly Oxidized Organic Molecules at a Rural Site in Central Germany. Atmos. Chem. Phys. 2016, 16, 12793–12813. [Google Scholar] [CrossRef]
- Rumble, J. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Waller, S.E.; Yang, Y.; Castracane, E.; Racow, E.E.; Kreinbihl, J.J.; Nickson, K.A.; Johnson, C.J. The Interplay Between Hydrogen Bonding and Coulombic Forces in Determining the Structure of Sulfuric Acid-Amine Clusters. J. Phys. Chem. Lett. 2018, 9, 1216–1222. [Google Scholar] [CrossRef]
- Yang, Y.; Waller, S.E.; Kreinbihl, J.J.; Johnson, C.J. Direct Link between Structure and Hydration in Ammonium and Aminium Bisulfate Clusters Implicated in Atmospheric New Particle Formation. J. Phys. Chem. Lett. 2018, 9, 5647–5652. [Google Scholar] [CrossRef]
- Elm, J.; Myllys, N.; Kurtén, T. What Is Required for Highly Oxidized Molecules To Form Clusters with Sulfuric Acid? J. Phys. Chem. A 2017, 121, 4578–4587. [Google Scholar] [CrossRef]
- Elm, J.; Fard, M.; Bilde, M.; Mikkelsen, K.V. Interaction of Glycine with Common Atmospheric Nucleation Precursors. J. Phys. Chem. A 2013, 117, 12990–12997. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myllys, N.; Ponkkonen, T.; Chee, S.; Smith, J. Enhancing Potential of Trimethylamine Oxide on Atmospheric Particle Formation. Atmosphere 2020, 11, 35. https://doi.org/10.3390/atmos11010035
Myllys N, Ponkkonen T, Chee S, Smith J. Enhancing Potential of Trimethylamine Oxide on Atmospheric Particle Formation. Atmosphere. 2020; 11(1):35. https://doi.org/10.3390/atmos11010035
Chicago/Turabian StyleMyllys, Nanna, Tuomo Ponkkonen, Sabrina Chee, and James Smith. 2020. "Enhancing Potential of Trimethylamine Oxide on Atmospheric Particle Formation" Atmosphere 11, no. 1: 35. https://doi.org/10.3390/atmos11010035
APA StyleMyllys, N., Ponkkonen, T., Chee, S., & Smith, J. (2020). Enhancing Potential of Trimethylamine Oxide on Atmospheric Particle Formation. Atmosphere, 11(1), 35. https://doi.org/10.3390/atmos11010035




