Characterization of Volatile Organic Compound (VOC) Emissions from Swine Manure Biogas Digestate Storage
Abstract
1. Introduction
2. Experiments
2.1. Biogas Digestate
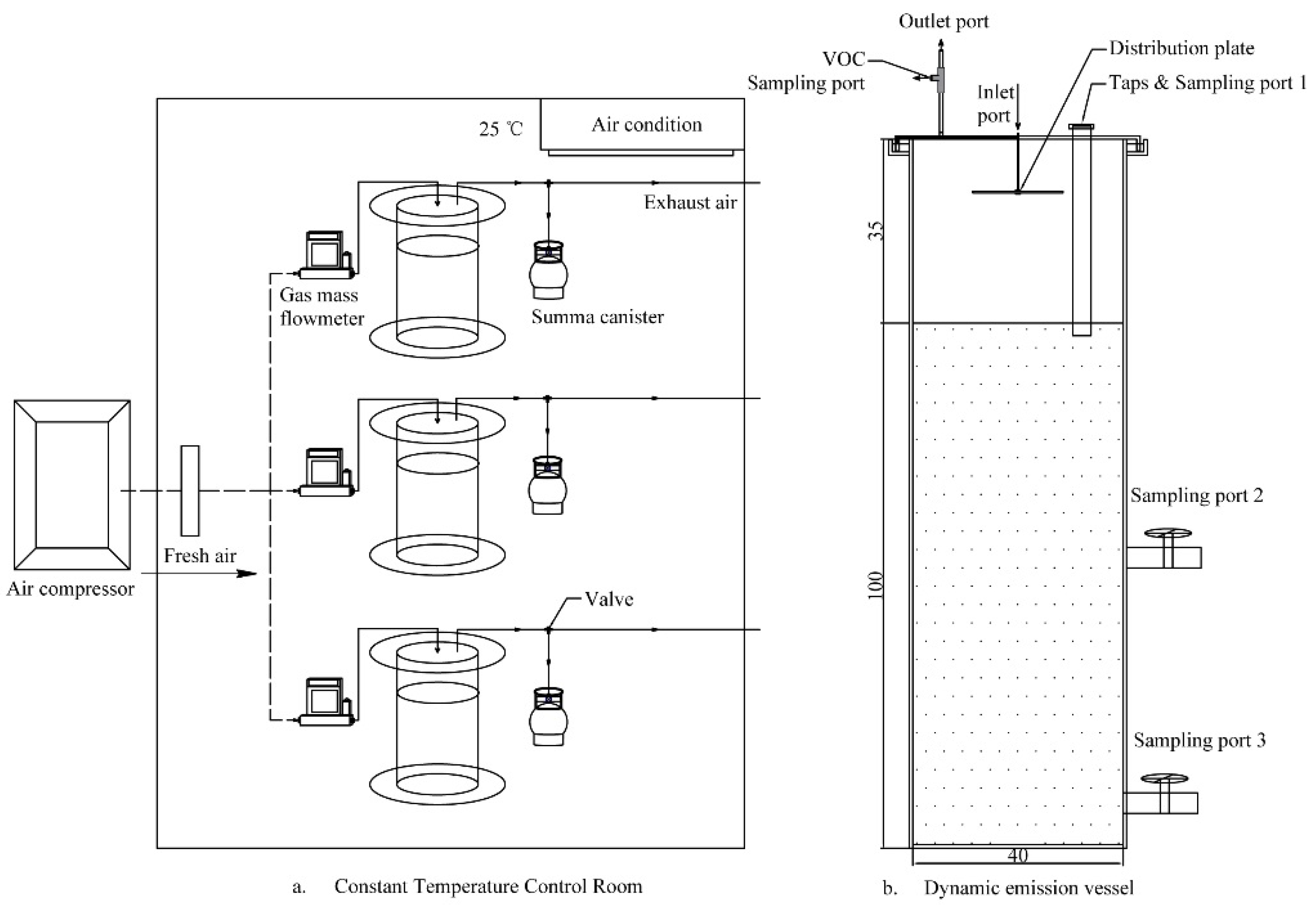
2.2. Experimental Set-Up and Methods
2.3. Sampling and Analysis
2.3.1. VOC Sampling and Analysis
2.3.2. Digestate Sample Collection and Analysis
2.3.3. Determination of VOC Flux
2.4. Statistical Analysis
3. Results and Discussion
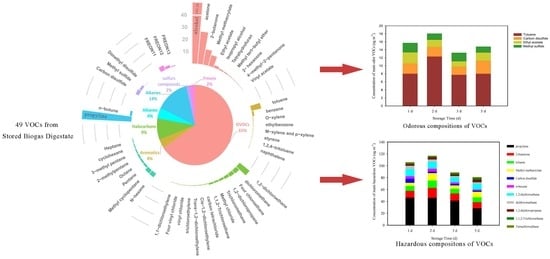
3.1. The Abundance of Main VOC Classes
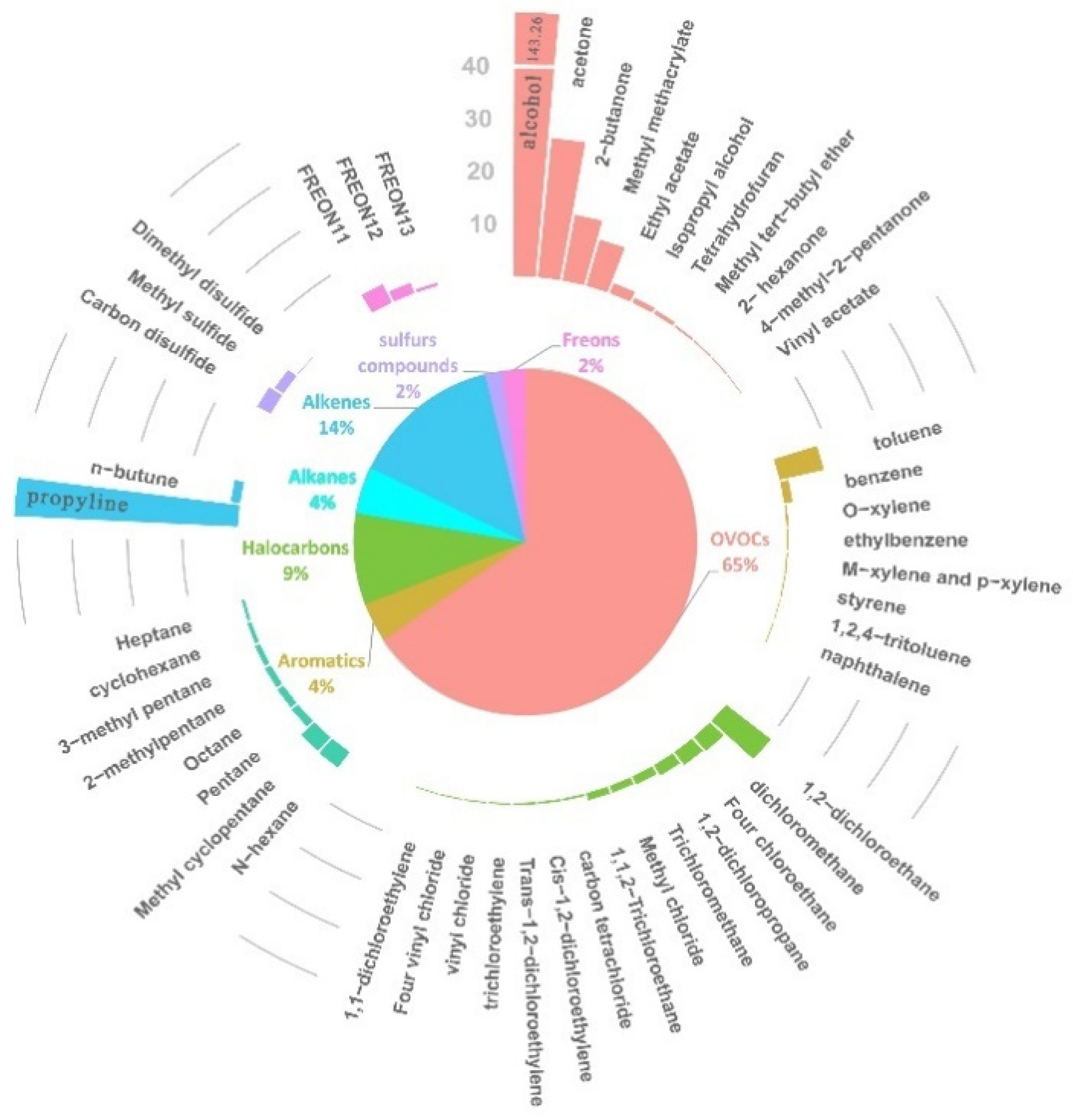
3.2. Main Compositions of VOCs
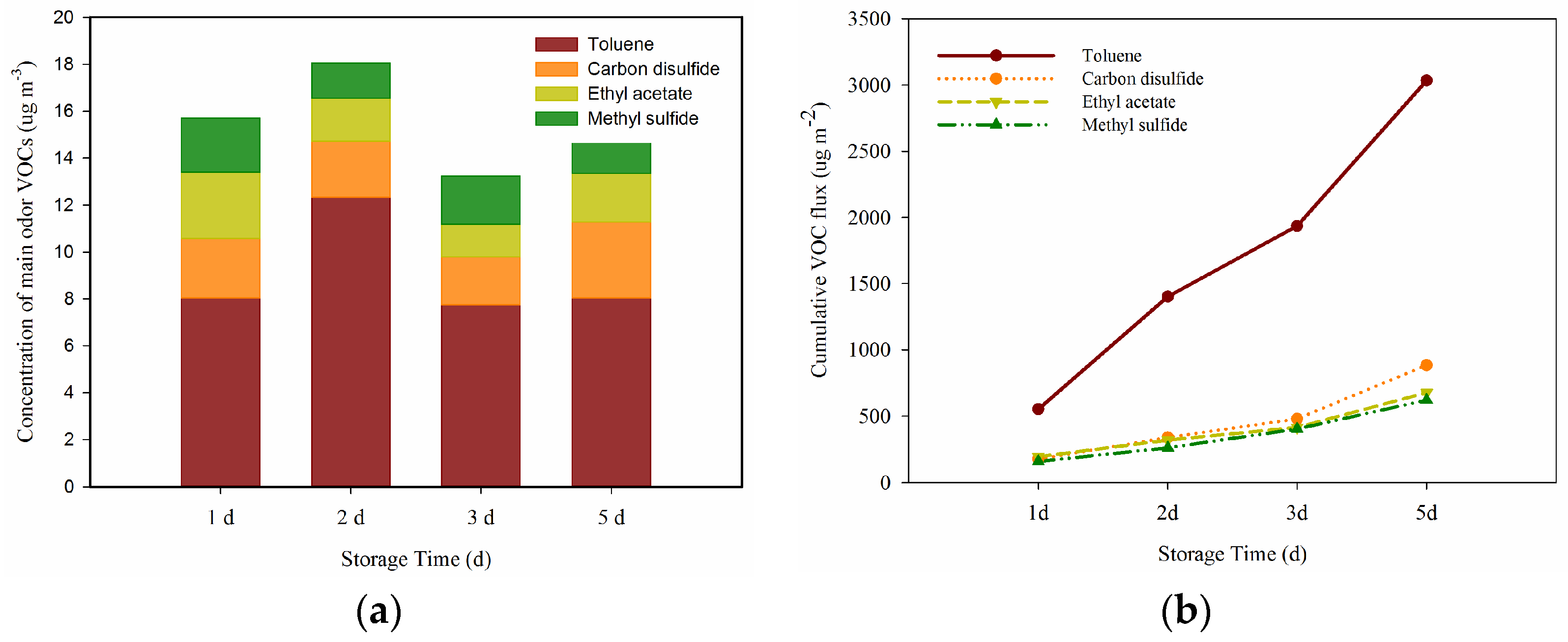
3.3. Odorous VOCs
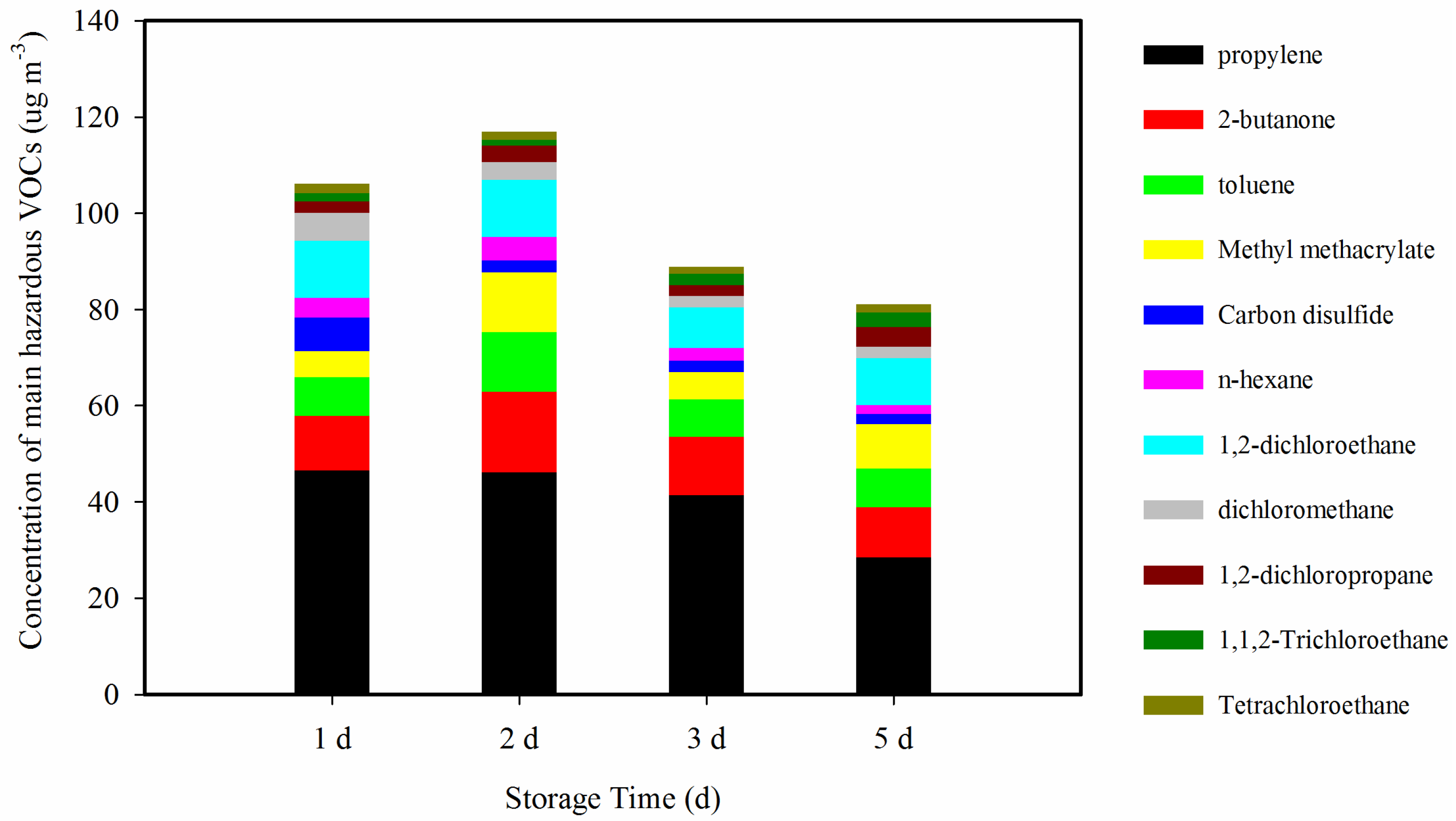
3.4. Hazardous VOCs
4. Conclusions
- A total of 49 compositions were identified, including 8 alkanes, 2 olefins, 14 halogenated hydrocarbons, 8 aromatic hydrocarbons, 11 OVOCs, 3 freons and 3 sulfur compounds. Many of the identified VOCs are classified as odorous or hazardous air pollutants.
- OVOCs, olefins and halogenated hydrocarbons dominated the compositions, accounting for 65.43%, 14.12% and 8.53% of the TVOCs, respectively.
- Ethanol, propylene, acetone and 2-butanone were the top four concentrated VOCs.
- Toluene, carbon disulfide, ethyl acetate and methyl sulfide were the dominant odorous substances, accounting for 5.15% of the TVOCs.
- Hazardous VOCs emitting from biogas digestate storage deserve more attention, especially from the standpoint of the potential impact on the atmospheric environment.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Delfino, R.J.; Gong, H., Jr.; Linn, W.S.; Pellizzari, E.D.; Hu, Y. Asthma symptoms in Hispanic children and daily ambient exposures to toxic and criteria air pollutants. Environ. Health Perspect. 2003, 111, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Salazar Gómez, J.I.; Lohmann, H.; Krassowski, J. Determination of volatile organic compounds from biowaste and co-fermentation biogas plants by single-sorbent adsorption. Chemosphere 2016, 153, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, L.; Zhao, X.; Ma, X.; Yang, J.; Liu, H.; Dou, S.; Zhou, M.; Xie, Z. Characteristics of volatile compounds removal in biogas slurry of pig manure by ozone oxidation and organic solvents extraction. J. Environ. Sci. 2013, 25, 1800. [Google Scholar] [CrossRef]
- Akhiar, A.; Battimelli, A.; Torrijos, M.; Carrere, H. Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manag. 2017, 59, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Giannadaki, D.; Giannakis, E.; Pozzer, A.; Lelieveld, J. Estimating health and economic benefits of reductions in air pollution from agriculture. Sci. Total Environ. 2018, 622–623, 1304–1316. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Yang, J.; Tian, Z.; Sun, Q.; Xue, W.; Dong, H. Mitigating Greenhouse Gas and Ammonia Emissions from Beef Cattle Feedlot Production: A System Meta-Analysis. Environ. Sci. Technol. 2018, 52, 11232–11242. [Google Scholar] [CrossRef]
- Anna, C. The concentration of volatile organic compounds (VOCs) in pig farm air. Ann. Agric. Environ. Med. 2009, 16, 249–256. [Google Scholar]
- Blunden, J.; Aneja, V.P.; Lonneman, W.A. Characterization of non-methane volatile organic compounds at swine facilities in eastern North Carolina. Atmos. Environ. 2005, 39, 6707–6718. [Google Scholar] [CrossRef]
- Cai, L.; Koziel, J.A.; Lo, Y.C.; Hoff, S.J. Characterization of volatile organic compounds and odorants associated with swine barn particulate matter using solid-phase microextraction and gas chromatography-mass spectrometry-olfactometry. J. Chromatogr. A 2006, 1102, 60–72. [Google Scholar] [CrossRef]
- Filipy, J.; Rumburg, B.; Mount, G.; Westberg, H.; Lamb, B. Identification and quantification of volatile organic compounds from a dairy. Atmos. Environ. 2006, 40, 1480–1494. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, H.; Zhu, Z.; Gerber, P.J.; Xin, H.; Smith, P.; Opio, C.; Steinfeld, H.; Chadwick, D. Mitigating Greenhouse Gas and Ammonia Emissions from Swine Manure Management: A System Analysis. Environ. Sci. Technol. 2017, 51, 4503–4511. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.S.; Lopes, N.L.; Veloso, C.M.; Jacovine, L.A.; Tomich, T.R.; Pereira, L.G.; Marcondes, M.I. Greenhouse gases inventory and carbon balance of two dairy systems obtained from two methane-estimation methods. Sci. Total Environ. 2016, 571, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Nicell, J.A. Assessment and regulation of odour impacts. Atmos. Environ. 2009, 43, 196–206. [Google Scholar] [CrossRef]
- Orzi, V.; Riva, C.; Scaglia, B.; D’Imporzano, G.; Tambone, F.; Adani, F. Anaerobic digestion coupled with digestate injection reduced odour emissions from soil during manure distribution. Sci. Total Environ. 2018, 621, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.V.; Cremades, L.V.; Calbo, J. Relationship between VOC and NOx emissions and chemical production of tropospheric ozone in the Aburra Valley (Colombia). Chemosphere 2006, 65, 881–888. [Google Scholar] [CrossRef]
- Ziemann, P.J.; Atkinson, R. Kinetics, products, and mechanisms of secondary organic aerosol formation. Chem. Soc. Rev. 2012, 41, 6582–6605. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Shao, M.; Che, W.W.; Zhang, L.J.; Zhong, L.J.; Zhang, Y.H.; Streets, D. Speciated VOC emission inventory and spatial patterns of ozone formation potential in the Pearl River Delta, China. Environ. Sci. Technol. 2009, 43, 8580–8586. [Google Scholar] [CrossRef]
- Klimont, Z.; Streets, D.G.; Gupta, S.; Cofala, J.; Fu, L.; Ichikawa, Y. Anthropogenic emissions of non-methane volatile organic compounds in China. Atmos. Environ. 2002, 36, 1309–1322. [Google Scholar] [CrossRef]
- Wei, W.; Wang, S.; Chatani, S.; Klimont, Z.; Cofala, J.; Hao, J. Emission and speciation of non-methane volatile organic compounds from anthropogenic sources in China. Atmos. Environ. 2008, 42, 4976–4988. [Google Scholar] [CrossRef]
- Guo, H.; Ling, Z.H.; Cheng, H.R.; Simpson, I.J.; Lyu, X.P.; Wang, X.M.; Shao, M.; Lu, H.X.; Ayoko, G.; Zhang, Y.L.; et al. Tropospheric volatile organic compounds in China. Sci. Total Environ. 2017, 574, 1021–1043. [Google Scholar] [CrossRef]
- Li, J.; Zhai, C.; Yu, J.; Liu, R.; Li, Y.; Zeng, L.; Xie, S. Spatiotemporal variations of ambient volatile organic compounds and their sources in Chongqing, a mountainous megacity in China. Sci. Total Environ. 2018, 627, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yu, Y.; Sun, J.; Zhang, J.; Wang, J.; Tang, G.; Wang, Y. Characteristics, source apportionment and reactivity of ambient volatile organic compounds at Dinghu Mountain in Guangdong Province, China. Sci. Total Environ. 2016, 548–549, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Trabue, S.; Scoggin, K.; Li, H.; Burns, R.; Xin, H.; Hatfield, J. Speciation of volatile organic compounds from poultry production. Atmos. Environ. 2010, 44, 3538–3546. [Google Scholar] [CrossRef]
- Zhou, T.; Shang, B.; Dong, H.; Tao, X.; Liu, T.; Wang, Y. Emission characteristics of volatile organic compounds during pilot swine manure composting. Trans. Chin. Soc. Agric. Eng. 2017, 33, 192–198. [Google Scholar]
- Shen, Y.; Zhang, B.; Zhao, L.; Meng, H.; Cheng, H. Component analysis of volatile organic compounds and determination of key odor in pig manure aerobic fermentation process. Trans. Chin. Soc. Agric. Eng. 2016, 32, 205–210. [Google Scholar]
- Shaw, S.L.; Mitloehner, F.M.; Jackson, W.; DePeters, E.J.; Fadel, J.G.; Robinson, P.H.; Holzinger, R.; Goldstein, A.H. Volatile Organic Compound Emissions from Dairy Cows and Their Waste as Measured by Proton-Transfer-Reaction Mass Spectrometry. Environ. Sci. Technol. 2007, 41, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.Q.; Robarge, W.P.; Xiao, C.; Heber, A.J. Volatile organic compounds at swine facilities: A critical review. Chemosphere 2012, 89, 769–788. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, P.J.; Webb, J.; Mottram, T.T.; Grant, B.; Misselbrook, T.M. Emissions of volatile organic compounds originating from UK livestock agriculture. J. Sci. Food Agric. 2004, 84, 1414–1420. [Google Scholar] [CrossRef]
- Ngwabie, N.M.; Schade, G.W.; Custer, T.G.; Linke, S.; Hinz, T. Abundances and Flux Estimates of Volatile Organic Compounds from a Dairy Cowshed in Germany. J. Environ. Qual. 2008, 37, 565–573. [Google Scholar] [CrossRef]
- Chen, J.; Luo, D. Ozone formation potentials of organic compounds from different emission sources in the South Coast Air Basin of California. Atmos. Environ. 2012, 55, 448–455. [Google Scholar] [CrossRef]
- Turan, N.G.; Akdemir, A.; Ergun, O.N. Emission of Volatile Organic Compounds during Composting of Poultry Litter. Water Air Soil Pollut. 2007, 184, 177–182. [Google Scholar] [CrossRef]
- Ryu, H.W.; Cho, K.S.; Lee, T.H. Reduction of ammonia and volatile organic compounds from food waste-composting facilities using a novel anti-clogging biofilter system. Bioresour. Technol. 2011, 102, 4654–4660. [Google Scholar] [CrossRef]
- Rasi, S.; Veijanen, A.; Rintala, J. Trace compounds of biogas from different biogas production plants. Energy 2007, 32, 1375–1380. [Google Scholar] [CrossRef]
- Rumsey, I.C.; Aneja, V.P.; Lonneman, W.A. Characterizing non-methane volatile organic compounds emissions from a swine concentrated animal feeding operation. Atmos. Environ. 2012, 47, 348–357. [Google Scholar] [CrossRef]
- Pang, X.; Guo, X.; Qin, Z.; Yao, Y.; Hu, X.; Wu, J. Identification of aroma-active compounds in Jiashi muskmelon juice by GC-O-MS and OAV calculation. J. Agric. Food Chem. 2012, 60, 4179–4185. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Wu, Y.D.; Zhang, Y.L.; Zeng, P.Y.; Tu, X.; Xu, M.Y.; Sun, G.P. Emission of odorous volatile organic compounds from a municipal manure treatment plant and their removal using a biotrickling filter. Environ. Technol. 2015, 36, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Carslaw, N.; Shaw, D. Secondary product creation potential (SPCP): A metric for assessing the potential impact of indoor air pollution on human health. Environ. Sci. Process. Impacts 2019. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (EPA). Available online: https://cfpub.epa.gov/ncea/iris/search/index.cfm (accessed on 5 July 2019).
| Property | Start | End | Property | Start | End |
|---|---|---|---|---|---|
| Chemical oxygen demand (COD, mg L−1) | 3816.67 (2.71) | 3748.33 (168.77) | pH | 5.91 (0.05) | 5.02 (0.01) |
| Total nitrogen (TN, mg L−1) | 1240.00 (13.25) | 1313.33 (16.78) | Redox potential (Rp, mV) | 102.90 (3.08) | 154.21 (0.91) |
| NH4+-N (mg L−1) | 896.00 (61.64) | 808.00 (45.63) | Electronic conductivity (Ec, ms cm−1) | 9.79 (0.53) | 11.86 (0.58) |
| NO3-N (mg L−1) | 0.234 (0.02) | 0.120 (0.01) | Dissolved oxygen (DO, mg L−1) | 0.01 (0.02) | 0.03 (0.02) |
| Total solid (TS, %) | 1.50 (0.37) | 1.56 (0.69) | T (°C) | 20.27 (0.14) | 23.49 (0.14) |
| Volatile solid (VS, %) | 0.89 (0.22) | 0.90 (0.43) | Total dissolved solid (TDS, ppt) | 4.80 (0.26) | 5.81 (0.28) |
| Property | Instruments/Methods | Instrument/Vendor Information |
|---|---|---|
| TN | Potassium persulphate method, method 10071 (Test’N Tube TM tube) | DR 6000, HACH, US |
| COD | Reactor digestion method, method 8000 (Test’N Tube TM tube) | DR 6000, HACH, US |
| NH4+-N | Salicylic acid method, method 10031 (Test’N Tube TM tube) | DR 6000, HACH, US |
| NOx−-N | N-(1-naphthyl)-ethylenediamine colorimetric method (GB13580.7-92) | FIAstar 5000 Flow injection analyzer |
| TS, VS | Constant weight test | Oven (Japan YAMATO DN60), Muffle furnace (China), One-thousandth balance (Sartorius, Germany) |
| T, DO, pH, Rp, TDS and Ec | ORION STAR A329 PH /ISE/ Conductivity /RDO/DO Portable multi-parameter measuring instrument | Thermo Scientific, Singapore |
| Source | Number of VOCs | Methods | Main VOC Types | Concentration | Reference |
|---|---|---|---|---|---|
| Barn | - | SummaTM canisters–GC/FID system and GC/MS | Oxygenated hydrocarbons, i.e., ethanol, methanol, acetaldehyde, acetone | B1: 0.8–249.97 ppb B2: 62.9–88.3 ppb B3: 310.8–437.9 ppb B4: 124.6–368.6 ppb B5: −3.6–281.2 ppb | Blunden et al. [8] |
| Summa canisters–GC/MS | Methanol, ethanol, acetaldehyde, acetone, 2,3-butanedione, 4-methylphenol | 2.77–16.12 ppb (single) | Rumsey et al. [34] | ||
| Compost | 81 | Summa canisters–GC/MS | Sulfur compounds, alkanes | - | Zhou et al. [24] |
| 31 | GC-MS | Acetone, methyl sulfide, dimethyl disulfide, dimethyl trisulfide | 0.52–36.68 mg m−3 | Shen et al. [25] | |
| Anaerobic Lagoon | - | Summa canisters–GC/MS | Ethanol 2-ethyl-1-hexanol, methanol, acetone, methyl ethyl ketone, acetaldehyde | 0.18–2.11 μg m−2 min−1 (single) | Rumsey et al. [34] |
| Biogas Plant | 15 | Nalophan NAC bags–GC/MAS | Sulphur compounds | 5–8 mg m−3 | Rasi et al. [33] |
| Sorbent tubes | Terpenes, ketones | 35–1731 mg Nm−3 | Salazar Gómez et al. [2] | ||
| Biogas Digestate | 48 | Summa canisters–GC/MS | OVOCs, alkenes, halogenated hydrocarbon | 171.35–523.71 μg m−3 (61.36–231.71 ppbV) | This study (2019) |
| VOC Compositions | Concentration Mean (SD) | VOCs Compositions | Concentration Mean (SD) |
|---|---|---|---|
| Ethanol | 143.26 (150.37) | n-Hexane | 3.38 (1.36) |
| Propylene | 40.66 (8.42) | 1,2-Dichloropropane | 3.00 (0.86) |
| Acetone | 26.50 (3.19) | Methyl cyclopentane | 2.80 (2.07) |
| 2-Butanone | 12.64 (2.81) | Ethyl acetate | 2.27 (0.79) |
| 1,2-Dichloroethane | 10.47 (1.66) | 1,1,2-Trichloroethane | 2.08 (0.81) |
| Toluene | 9.05 (2.20) | FREON12 | 2.01 (0.05) |
| Methyl methacrylate | 8.24 (3.30) | Methyl sulfide | 1.90 (0.35) |
| FREON11 | 3.85 (2.55) | Four chloroethane | 1.69 (0.21) |
| Dichloromethane | 3.60 (1.65) | n-Butune | 1.66 (0.68) |
| Carbon disulfide | 3.46 (2.29) | Trichloromethane | 1.53 (0.40) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhu, Z.; Zheng, Y.; Chen, Y.; Yin, F.; Zhang, W.; Dong, H.; Xin, H. Characterization of Volatile Organic Compound (VOC) Emissions from Swine Manure Biogas Digestate Storage. Atmosphere 2019, 10, 411. https://doi.org/10.3390/atmos10070411
Zhang Y, Zhu Z, Zheng Y, Chen Y, Yin F, Zhang W, Dong H, Xin H. Characterization of Volatile Organic Compound (VOC) Emissions from Swine Manure Biogas Digestate Storage. Atmosphere. 2019; 10(7):411. https://doi.org/10.3390/atmos10070411
Chicago/Turabian StyleZhang, Yu, Zhiping Zhu, Yunhao Zheng, Yongxing Chen, Fubin Yin, Wanqin Zhang, Hongmin Dong, and Hongwei Xin. 2019. "Characterization of Volatile Organic Compound (VOC) Emissions from Swine Manure Biogas Digestate Storage" Atmosphere 10, no. 7: 411. https://doi.org/10.3390/atmos10070411
APA StyleZhang, Y., Zhu, Z., Zheng, Y., Chen, Y., Yin, F., Zhang, W., Dong, H., & Xin, H. (2019). Characterization of Volatile Organic Compound (VOC) Emissions from Swine Manure Biogas Digestate Storage. Atmosphere, 10(7), 411. https://doi.org/10.3390/atmos10070411