Application of DPPH Assay for Assessment of Particulate Matter Reducing Properties
Abstract
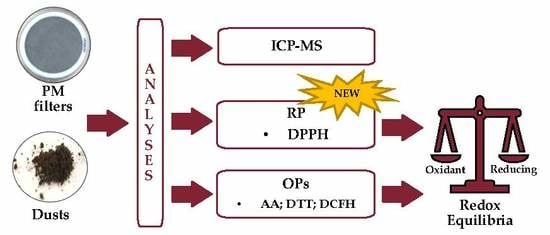
1. Introduction
2. Materials and Methods
2.1. Reagents and DPPH Assay
2.2. Collection and Chemical Characterization of Samples
2.3. Oxidative and Reducing Potential Assays on Selected Types of PM
2.3.1. DPPH Assay on EtOH Extracted Samples
2.3.2. DPPH Assay on H2O Extracted Samples
2.3.3. DPPH Assay on H2O Extracted Samples with EtOH Addition
2.3.4. DPPH Assay on whole Dusts Samples
2.4. Oxidative and Reducing Potential Assays on PM2.5 Filters
3. Results and Discussion
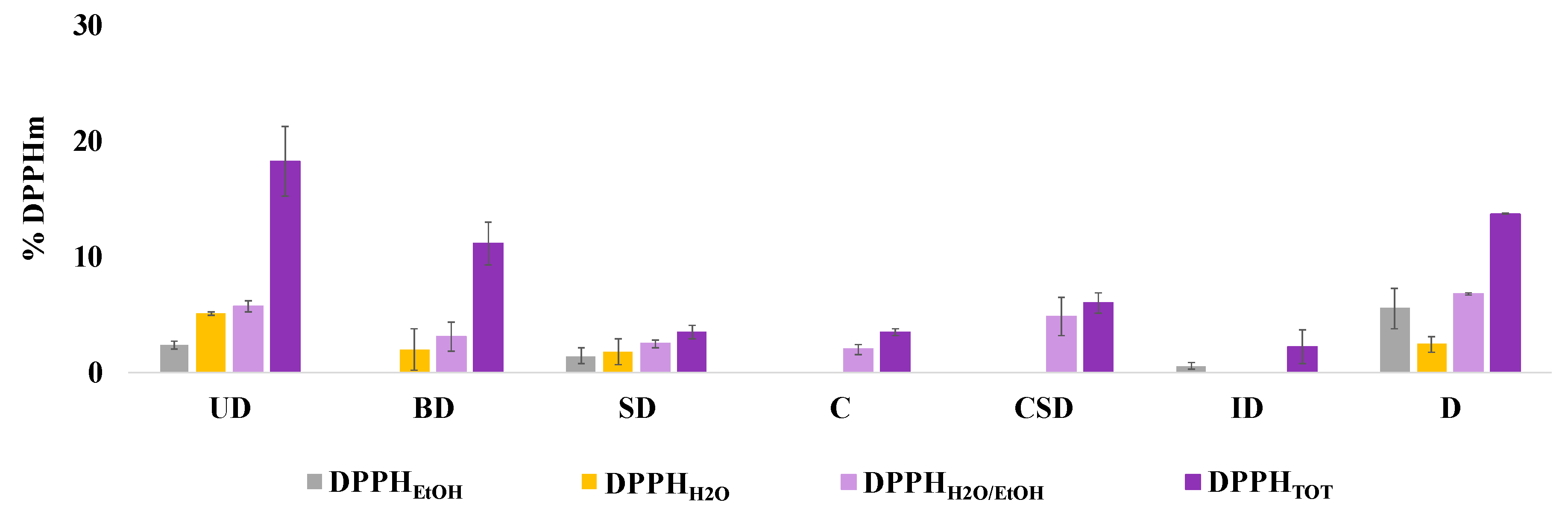
3.1. DPPH Assay Application on Selected Types of PM
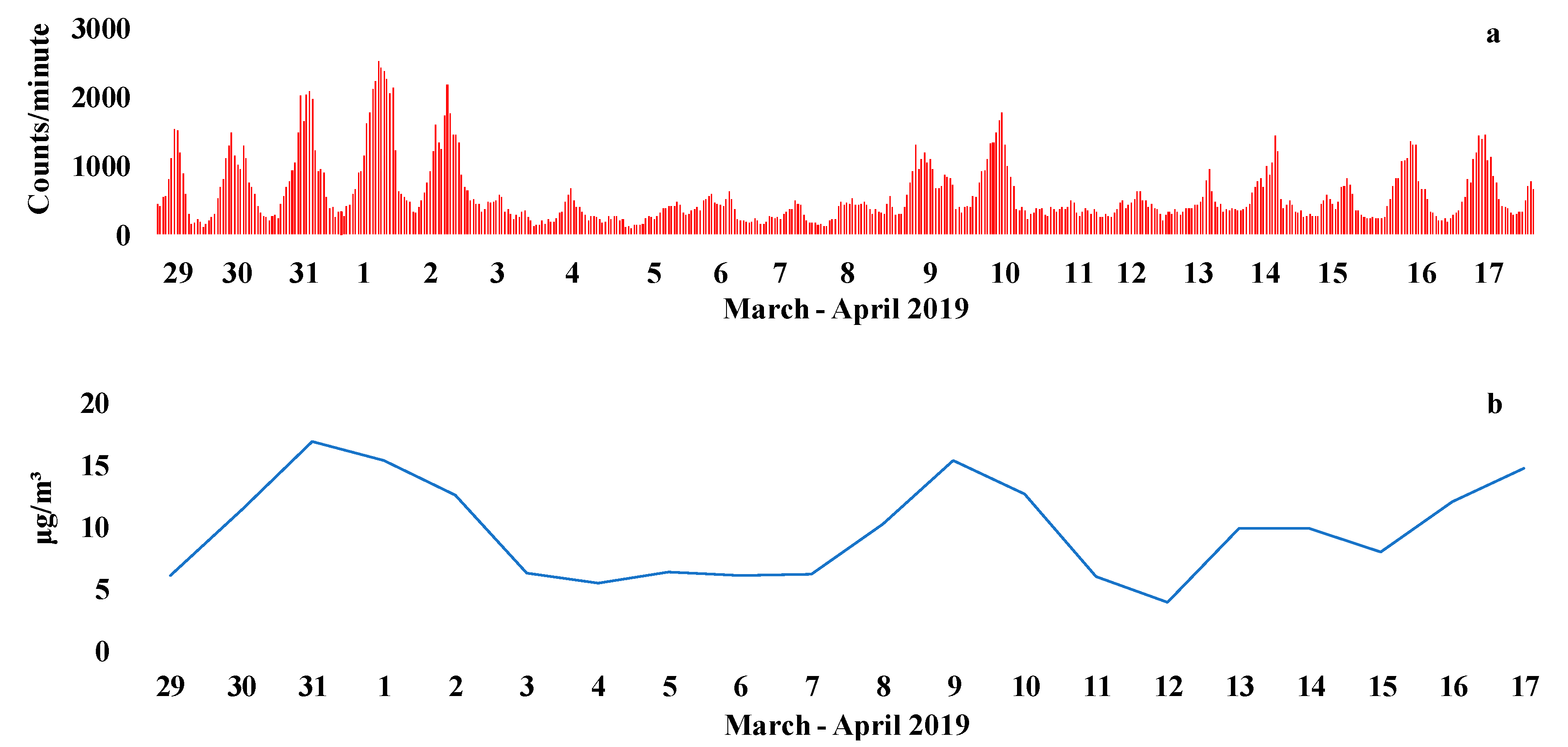
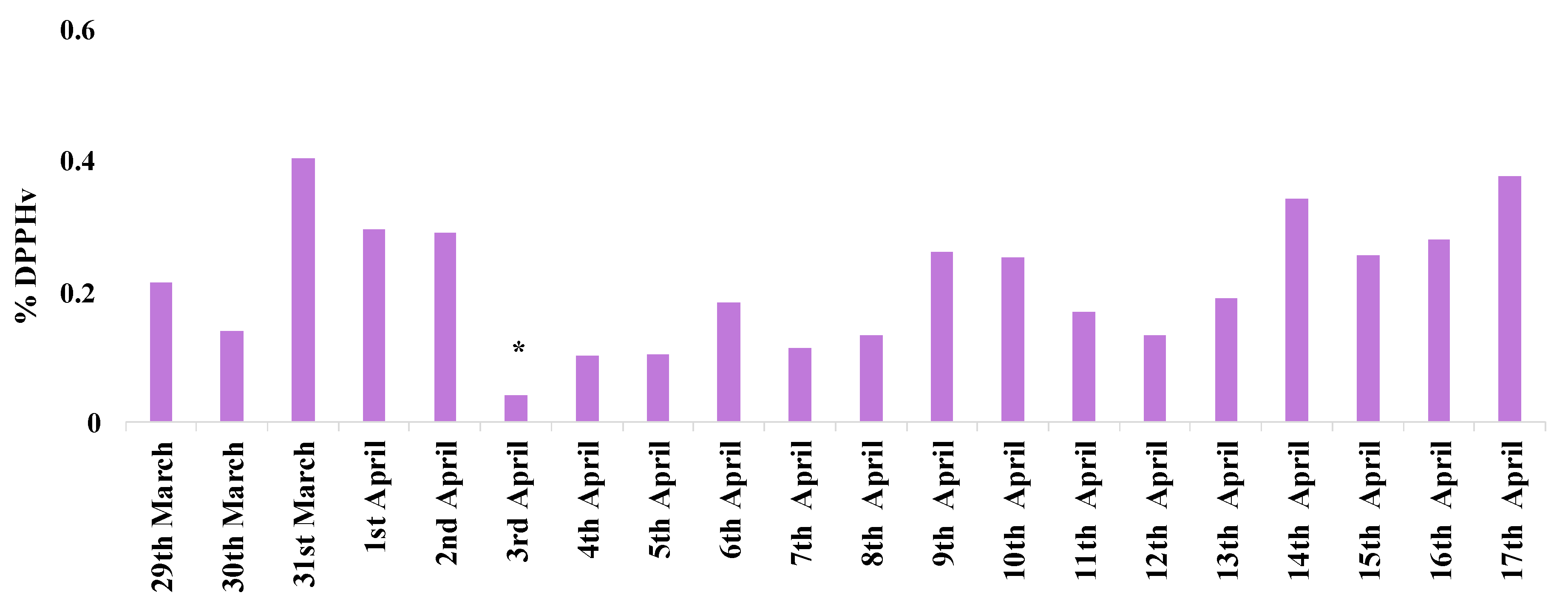
3.2. Oxidative and Reducing Potential Assays on PM2.5 Field Filters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Yu, X.; Mo, L.; Xu, Y.; Bao, L.; Lun, X. Atmospheric particulate matter accumulation on trees: A comparison of boles, branches and leaves. J. Clean. Prod. 2019, 226, 349–356. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367. [Google Scholar] [CrossRef] [PubMed]
- Crobeddu, B.; Aragao-Santiago, L.; Bui, L.C.; Boland, S.; Squiban, A.B. Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environ. Pollut. 2017, 230, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Kioumourtzoglou, M.A.; Schwartz, J.D.; Weisskopf, M.G.; Melly, S.J.; Wang, Y.; Dominici, F.; Zanobetti, A. Long-term PM2.5 exposure and neurological hospital admissions in the northeastern United States. Environ. Health Perspect. 2015, 124, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Øvrevik, J. Oxidative potential versus biological effects: A review on the relevance of cell-free/abiotic assays as predictors of toxicity from airborne particulate matter. Int. J. Mol. Sci. 2019, 20, 4772. [Google Scholar] [CrossRef]
- Calas, A.; Uzu, G.; Kelly, F.J.; Houdier, S.; Martins, J.M.; Thomas, F.; Jacob, V. Comparison between five acellular oxidative potential measurement assays performed with detailed chemistry on PM10 samples from the city of Chamonix (France). Atmos. Chem. Phys. 2018, 18, 7863–7875. [Google Scholar] [CrossRef]
- Perrone, M.G.; Zhou, J.; Malandrino, M.; Sangiorgi, G.; Rizzi, C.; Ferrero, L.; Bolzacchini, E. PM chemical composition and oxidative potential of the soluble fraction of particles at two sites in the urban area of Milan, Northern Italy. Atmos. Environ. 2016, 128, 104–113. [Google Scholar] [CrossRef]
- Yang, A.; Janssen, N.A.; Brunekreef, B.; Cassee, F.R.; Hoek, G.; Gehring, U. Children’s respiratory health and oxidative potential of PM2.5: The PIAMA birth cohort study. Occup. Environ. Med. 2016, 73, 154–160. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Russo, M.; Zagatti, E. Review of PM Oxidative Potential Measured with Acellular Assays in Urban and Rural Sites across Italy. Atmosphere 2019, 10, 626. [Google Scholar] [CrossRef]
- Ayres, J.G.; Borm, P.; Cassee, F.R.; Castranova, V.; Donaldson, K.; Ghio, A.; Marano, F. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential—A workshop report and consensus statement. Inhal. Toxicol. 2008, 20, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Stoeger, T.; Takenaka, S.; Frankenberger, B.; Ritter, B.; Karg, E.; Maier, K.; Schmid, O. Deducing in vivo toxicity of combustion-derived nanoparticles from a cell-free oxidative potency assay and metabolic activation of organic compounds. Environ. Health Perspect. 2008, 117, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.K.; Sioutas, C.; Miguel, A.H.; Kumagai, Y.; Schmitz, D.A.; Singh, M.; Froines, J.R. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ. Res. 2005, 99, 40–47. [Google Scholar] [CrossRef]
- Hung, H.F.; Wang, C.S. Experimental determination of reactive oxygen species in Taipei aerosols. J. Aerosol Sci. 2001, 32, 1201–1211. [Google Scholar] [CrossRef]
- Fang, T.; Verma, V.; Bates, J.T.; Abrams, J.; Klein, M.; Strickland, M.J.; Russell, A.G. Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: Contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos. Chem. Phys. 2016, 16, 3865–3879. [Google Scholar] [CrossRef]
- Bates, J.T.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J.; Tolbert, P.E.; Russell, A.G. Review of acellular assays of ambient particulate matter oxidative potential: Methods and relationships with composition, sources, and health effects. Environ. Sci. Technol. 2019, 53, 4003–4019. [Google Scholar] [CrossRef]
- Yang, A.; Jedynska, A.; Hellack, B.; Kooter, I.; Hoek, G.; Brunekreef, B.; Janssen, N.A. Measurement of the oxidative potential of PM2.5 and its constituents: The effect of extraction solvent and filter type. Atmos. Environ. 2014, 83, 35–42. [Google Scholar] [CrossRef]
- Tayo, L.L.; Lin, Y.H.; Lin, S.L.; Gou, Y.Y.; Hsu10, Y.C.; Hou, W.C.; Chao, H.R. Fine particulate matter-induced toxic effects in an animal model of caenorhabditis elegans. Aerosol Air Qual. Res. 2019, 19, 1068–1078. [Google Scholar] [CrossRef]
- Marcoccia, M.; Ronci, L.; De Matthaeis, E.; Setini, A.; Perrino, C.; Canepari, S. In-vivo assesment of the genotoxic and oxidative stress effects of particulate matter on Echinogammarus veneris. Chemosphere 2017, 173, 124–134. [Google Scholar] [CrossRef]
- Piacentini, D.; Falasca, G.; Canepari, S.; Massimi, L. Potential of PM-selected components to induce oxidative stress and root system alteration in a plant model organism. Environ. Int. 2019, 132, 105094. [Google Scholar] [CrossRef]
- Costabile, F.; Gualtieri, M.; Canepari, S.; Tranfo, G.; Consales, C.; Grollino, M.G.; Simonetti, G. Evidence of association between aerosol properties and in-vitro cellular oxidative response to PM1, oxidative potential of PM2.5, a biomarker of RNA oxidation, and its dependency on combustion sources. Atmos. Environ. 2019, 213, 444–455. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Bertoli, I.; Manarini, F.; Russo, M. Ascorbate assay as a measure of oxidative potential for ambient particles: Evidence for the importance of cell-free surrogate lung fluid composition. Atmos. Environ. 2019, 211, 103–112. [Google Scholar] [CrossRef]
- Khurshid, S.S.; Siegel, J.A.; Kinney, K.A. Indoor particulate reactive oxygen species concentrations. Environ. Res. 2014, 132, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Miljevic, B.; Hedayat, F.; Stevanovic, S.; Fairfull-Smith, K.E.; Bottle, S.E.; Ristovski, Z.D. To sonicate or not to sonicate PM filters: Reactive oxygen species generation upon ultrasonic irradiation. Aerosol Sci. Technol. 2014, 48, 1276–1284. [Google Scholar] [CrossRef]
- Wei, J.; Yu, H.; Wang, Y.; Verma, V. Complexation of iron and copper in ambient particulate matter and its effect on the oxidative potential measured in a surrogate lung fluid. Environ. Sci. Technol. 2018, 53, 1661–1671. [Google Scholar] [CrossRef]
- Oh, S.M.; Kim, H.R.; Park, Y.J.; Lee, S.Y.; Chung, K.H. Organic extracts of urban air pollution particulate matter (PM2.5)-induced genotoxicity and oxidative stress in human lung bronchial epithelial cells (BEAS-2B cells). Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2011, 723, 142–151. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Miller, D.J.; Langenfeld, J.J.; Krieger, M.S. PM-10 high-volume collection and quantitation of semi-and nonvolatile phenols, methoxylated phenols, alkanes, and polycyclic aromatic hydrocarbons from winter urban air and their relationship to wood smoke emissions. Environ. Sci. Technol. 1992, 26, 2251–2262. [Google Scholar] [CrossRef]
- Simoneit, B.R.; Bi, X.; Oros, D.R.; Medeiros, P.M.; Sheng, G.; Fu, J. Phenols and hydroxy-PAHs (arylphenols) as tracers for coal smoke particulate matter: Source tests and ambient aerosol assessments. Environ. Sci. Technol. 2007, 41, 7294–7302. [Google Scholar] [CrossRef]
- Buiarelli, F.; Sonego, E.; Uccelletti, D.; Bruni, E.; Di Filippo, P.; Pomata, D.; Simonetti, G. Determination of the main bioaerosol components using chemical markers by liquid chromatography–tandem mass spectrometry. Microchem. J. 2019, 103974. [Google Scholar] [CrossRef]
- Menetrez, M.Y.; Foarde, K.K.; Esch, R.K.; Schwartz, T.D.; Dean, T.R.; Hays, M.D.; Moore, S.A. An evaluation of indoor and outdoor biological particulate matter. Atmos. Environ. 2009, 43, 5476–5483. [Google Scholar] [CrossRef]
- Nemmar, A.; Holme, J.A.; Rosas, I.; Schwarze, P.E.; Alfaro-Moreno, E. Recent advances in particulate matter and nanoparticle toxicology: A review of the in vivo and in vitro studies. Biomed Res. Int. 2013, 2013, 279371. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Sendra, J.M.; Sentandreu, E.; Navarro, J.L. Reduction kinetics of the free stable radical 2, 2-diphenyl-1-picrylhydrazyl (DPPH•) for determination of the antiradical activity of citrus juices. Eur. Food Res. Technol. 2006, 223, 615. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Sahu, R.K.; Kar, M.; Routray, R. DPPH free radical scavenging activity of some leafy vegetables used by tribals of odisha, India. J. Med. Plants 2013, 1, 21–27. [Google Scholar]
- Chedea, V.S.; Pop, R.M. Total polyphenols content and antioxidant DPPH assays on biological samples. In Polyphenols in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 169–183. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef]
- Hasan, S.R.; Hossain, M.M.; Akter, R.; Jamila, M.; Mazumder, M.E.H.; Rahman, S. DPPH free radical scavenging activity of some Bangladeshi medicinal plants. J. Med. Plants Res. 2009, 3, 875–879. [Google Scholar] [CrossRef]
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. Paper-based DPPH assay for antioxidant activity analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Chen, Y.X.; Liu, X.Y.; Xiao, Z.; Huang, Y.F.; Liu, B. Antioxidant activities of polysaccharides obtained from Chlorella pyrenoidosa via different ethanol concentrations. Int. J. Biol. Macromol. 2016, 91, 505–509. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol 2004, 26, 211–219. [Google Scholar]
- Thorpe, A.; Harrison, R.M. Sources and properties of non-exhaust particulate matter from road traffic: A review. Sci. Total Environ. 2008, 400, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Pant, P.; Harrison, R.M. Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: A review. Atmos. Environ. 2013, 77, 78–97. [Google Scholar] [CrossRef]
- Taioli, E.; Sram, R.J.; Garte, S.; Kalina, I.; Popov, T.A.; Farmer, P.B. Effects of polycyclic aromatic hydrocarbons (PAHs) in environmental pollution on exogenous and oxidative DNA damage (EXPAH project): Description of the population under study. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2007, 620, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Besombes, J.L.; Maître, A.; Patissier, O.; Marchand, N.; Chevron, N.; Stoklov, M.; Masclet, P. Particulate PAHs observed in the surrounding of a municipal incinerator. Atmos. Environ. 2001, 35, 6093–6104. [Google Scholar] [CrossRef]
- Brines, M.; Dall’Osto, M.; Amato, F.; Minguillón, M.C.; Karanasiou, A.; Grimalt, J.O.; van Drooge, B.L. Source apportionment of urban PM 1 in Barcelona during SAPUSS using organic and inorganic components. Environ. Sci. Pollut. Res. 2019, 26, 32114–32127. [Google Scholar] [CrossRef]
- Dugenest, S.; Casabianca, H.; Grenier-Loustalot, M.F. Municipal solid waste incineration bottom ash: Physicochemical characterization of organic matter. Analusis 1999, 27, 75–80. [Google Scholar] [CrossRef]
- Simonetti, G.; Conte, E.; Massimi, L.; Frasca, D.; Perrino, C.; Canepari, S. Oxidative potential of particulate matter components generated by specific emission sources. J. Aerosol Sci. 2018, 126, 99–109. [Google Scholar] [CrossRef]
- Massimi, L.; Ristorini, M.; Eusebio, M.; Florendo, D.; Adeyemo, A.; Brugnoli, D.; Canepari, S. Monitoring and evaluation of Terni (Central Italy) air quality through spatially resolved analyses. Atmosphere 2017, 8, 200. [Google Scholar] [CrossRef]
- Canepari, S.; Cardarelli, E.; Giuliano, A.; Pietrodangelo, A. Determination of metals, metalloids and non-volatile ions in airborne particulate matter by a new twostep sequential leaching procedure part a: Experimental design and optimization. Talanta 2006, 69, 581–587. [Google Scholar] [CrossRef]
- Canepari, S.; Cardarelli, E.; Pietrodangelo, A.; Strincone, M. Determination of metals, metalloids and non-volatile ions in airborne particulate matter by a new two-step sequential leaching procedure: Part B: Validation on equivalent real samples. Talanta 2006, 69, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Singh, P.N.; Dubey, S.D. Evaluation of antioxidant activities in ethanolic extract of Capparis Zeylanica Linn. Root. Rev. Latinoam. Química 2013, 41, 7–20. [Google Scholar]
- Scalzo, R.L. Organic acids influence on DPPH scavenging by ascorbic acid. Food Chem. 2008, 107, 40–43. [Google Scholar] [CrossRef]
- Shao, L.; Hu, Y.; Shen, R.; Schäfer, K.; Wang, J.; Wang, J.; Suppan, P. Seasonal variation of particle-induced oxidative potential of airborne particulate matter in Beijing. Sci. Total Environ. 2017, 579, 1152–1160. [Google Scholar] [CrossRef]
- Chirizzi, D.; Cesari, D.; Guascito, M.R.; Dinoi, A.; Giotta, L.; Donateo, A.; Contini, D. Influence of Saharan dust outbreaks and carbon content on oxidative potential of water-soluble fractions of PM2. 5 and PM10. Atmos. Environ. 2017, 163, 1–8. [Google Scholar] [CrossRef]
- Simonetti, G.; Conte, E.; Perrino, C.; Canepari, S. Oxidative potential of size-segregated PM in an urban and an industrial area of Italy. Atmos. Environ. 2018, 187, 292–300. [Google Scholar] [CrossRef]
- Noipa, T.; Srijaranai, S.; Tuntulani, T.; Ngeontae, W. New approach for evaluation of the antioxidant capacity based on scavenging DPPH free radical in micelle systems. Food Res. Int. 2011, 44, 798–806. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Pękal, A. Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Anal. Methods 2013, 5, 4288–4295. [Google Scholar] [CrossRef]
- Fang, T.; Zeng, L.; Gao, D.; Verma, V.; Stefaniak, A.B.; Weber, R.J. Ambient size distributions and lung deposition of aerosol dithiothreitol-measured oxidative potential: Contrast between soluble and insoluble particles. Environ. Sci. Technol. 2017, 51, 6802–6811. [Google Scholar] [CrossRef]
- Baulig, A.; Garlatti, M.; Bonvallot, V.; Marchand, A.; Barouki, R.; Marano, F.; Baeza-Squiban, A. Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L671–L679. [Google Scholar] [CrossRef]
- Boland, S.; Baeza-Squiban, A.; Bonvallot, V.; Houcine, O.; Pain, C.; Meyer, M.; Marano, F. Similar cellular effects induced by diesel exhaust particles from a representative diesel vehicle recovered from filters and standard reference material 1650. Toxicol. Vitr. 2001, 15, 379–385. [Google Scholar] [CrossRef]
- Campbell, S.J.; Utinger, B.; Lienhard, D.M.; Paulson, S.E.; Shen, J.; Griffiths, P.T.; Kalberer, M. Development of a physiologically relevant online chemical assay to quantify aerosol oxidative potential. Anal. Chem. 2019, 91, 13088–13095. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, A.; Saitoh, K.; Hayashi, K.; Ono, K.; Fujitani, Y.; Villalobos, A.M.; Schauer, J.J. Chemical characterization and oxidative potential of particles emitted from open burning of cereal straws and rice husk under flaming and smoldering conditions. Atmos. Environ. 2017, 163, 118–127. [Google Scholar] [CrossRef]
- Perrino, C.; Pietrodangelo, A.; Febo, A. An atmospheric stability index based on radon progeny measurements for the evaluation of primary urban pollution. Atmos. Environ. 2001, 35, 5235–5244. [Google Scholar] [CrossRef]
- Perrino, C.; Catrambone, M.; Dalla Torre, S.; Rantica, E.; Sargolini, T.; Canepari, S. Seasonal variations in the chemical composition of particulate matter: A case study in the Po Valley. Part I: Macro-components and mass closure. Environ. Sci. Pollut. Res. 2014, 21, 3999–4009. [Google Scholar] [CrossRef] [PubMed]
- Canepari, S.; Astolfi, M.L.; Farao, C.; Maretto, M.; Frasca, D.; Marcoccia, M.; Perrino, C. Seasonal variations in the chemical composition of particulate matter: A case study in the Po Valley. Part II: Concentration and solubility of micro-and trace-elements. Environ. Sci. Pollut. Res. 2014, 21, 4010–4022. [Google Scholar] [CrossRef]
| Soluble Fraction | LOD (ng/m³) | Mean (ng/m³) | Min–Max (ng/m³) | Residual Fraction | LOD (ng/m³) | Mean (ng/m³) | Min–Max (ng/m³) |
|---|---|---|---|---|---|---|---|
| Al | 2.5 | 2.2 * | 2.5–7.1 | Al | 11 | 13 * | 11–91 |
| As | 0.29 | 0.24 * | 0.29–0.48 | - | - | - | - |
| Bi | 0.011 | 0.017 * | 0.011–0.049 | Bi | 0.004 | 0.036 * | 0.004–0.078 |
| Cd | 0.022 | 0.068 * | 0.022–0.19 | Cd | 0.051 | 0.046 * | 0.051–0.22 |
| Ce | 0.004 | 0.005 * | 0.004–0.012 | Ce | 0.047 | 0.025 * | 0.047–0.051 |
| Co | 0.005 | 0.011 * | 0.005–0.024 | - | - | - | - |
| Cr | 0.054 | 0.063 * | 0.054–0.13 | Cr | 4.1 | 2.4 | 1.6–3.5 |
| Cs | 0.002 | 0.006 | 0.002–0.016 | Cs | 0.006 | 0.003 * | 0.006–0.013 |
| Cu | 0.057 | 0.69 | 0.22–1.5 | Cu | 0.87 | 0.69 * | 0.87–1.8 |
| Fe | 9.2 | 8.1 * | 9.2–36 | Fe | 21 | 18 * | 21–44 |
| La | 0.005 | 0.004 * | 0.005–0.018 | La | 0.029 | 0.015 * | 0.029–0.034 |
| Li | 0.015 | 0.013 * | 0.015–0.026 | Li | 0.011 | 0.013 * | 0.011–0.019 |
| Mg | 6.1 | 6.2 * | 6.1–26 | Mg | 13 | 7.1 * | 13–16 |
| Mn | 0.12 | 0.66 | 0.19–1.4 | Mn | 0.35 | 0.66 * | 0.35–1.1 |
| Mo | 0.004 | 0.11 | 0.031–0.26 | Mo | 0.029 | 0.096 | 0.029–0.24 |
| Na | 7.9 | 28 | 11–75 | - | - | - | - |
| Ni | 0.58 | 0.33 * | 0.58–0.73 | Ni | 0.82 | 0.51 * | 0.82–1.8 |
| Pb | 0.21 | 0.92 | 0.21–2.4 | Pb | 0.17 | 1.1 | 0.44–3.1 |
| Rb | 0.11 | 0.17 * | 0.11–0.36 | - | |||
| Sb | 0.004 | 0.25 | 0.038–0.63 | Sb | 0.17 | 0.24 * | 0.17–0.39 |
| Sn | 0.006 | 0.071 | 0.026–0.19 | Sn | 0.11 | 0.39 | 0.17–0.71 |
| Sr | 0.22 | 0.22 * | 0.22–0.78 | - | - | - | - |
| Ti | 0.046 | 0.075 * | 0.075–0.18 | Ti | 0.79 | 0.42 * | 0.79–0.81 |
| Tl | 0.0005 | 0.002 | 0.0004–0.011 | Tl | 0.002 | 0.003 * | 0.002–0.006 |
| V | 0.015 | 0.33 | 0.031–1.8 | V | 0.079 | 0.097 * | 0.079–0.38 |
| Zn | 2.7 | 9.6 | 4.1–40 | Zn | 15 | 5.3 | 1.5–12 |
| Zr | 0.006 | 0.012 | 0.006–0.032 | Zr | 0.028 | 0.071 | 0.027–0.32 |
| Parameters | PM | RP | AA | DTT | DCFH |
|---|---|---|---|---|---|
| PM | - | ||||
| RP | 0.77 | - | |||
| AA | 0.19 | 0.056 | - | ||
| DTT | 0.63 | 0.47 | 0.14 | - | |
| DCFH | 0.33 | 0.52 | −0.23 | 0.34 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frezzini, M.A.; Castellani, F.; De Francesco, N.; Ristorini, M.; Canepari, S. Application of DPPH Assay for Assessment of Particulate Matter Reducing Properties. Atmosphere 2019, 10, 816. https://doi.org/10.3390/atmos10120816
Frezzini MA, Castellani F, De Francesco N, Ristorini M, Canepari S. Application of DPPH Assay for Assessment of Particulate Matter Reducing Properties. Atmosphere. 2019; 10(12):816. https://doi.org/10.3390/atmos10120816
Chicago/Turabian StyleFrezzini, Maria Agostina, Federica Castellani, Nayma De Francesco, Martina Ristorini, and Silvia Canepari. 2019. "Application of DPPH Assay for Assessment of Particulate Matter Reducing Properties" Atmosphere 10, no. 12: 816. https://doi.org/10.3390/atmos10120816
APA StyleFrezzini, M. A., Castellani, F., De Francesco, N., Ristorini, M., & Canepari, S. (2019). Application of DPPH Assay for Assessment of Particulate Matter Reducing Properties. Atmosphere, 10(12), 816. https://doi.org/10.3390/atmos10120816