Near Real-Time Monitoring of Formaldehyde in a Low-Energy School Building
Abstract
:1. Introduction
2. Experiments
2.1. Reference Method ISO 16000-3 Using DNPH Tube
2.2. First Generation of Formaldehyde Analyzer
2.3. Formaldehyde Microanalyzer Based on a Microfluidic Device
2.4. Description of the School Building and the Chosen Classroom
2.5. Sampling Conditions
2.6. Ventilation
2.6.1. Description of the Ventilation System
2.6.2. Classroom Ventilation Conditions during the Field Measurements
3. Results
3.1. Instruments Calibration and Detection Limits
3.1.1. Reference Method ISO 16000-3 Using DNPH tube
3.1.2. First Generation of Formaldehyde Analyzer
3.1.3. Formaldehyde Microanalyzer Based on a Microfluidic Device
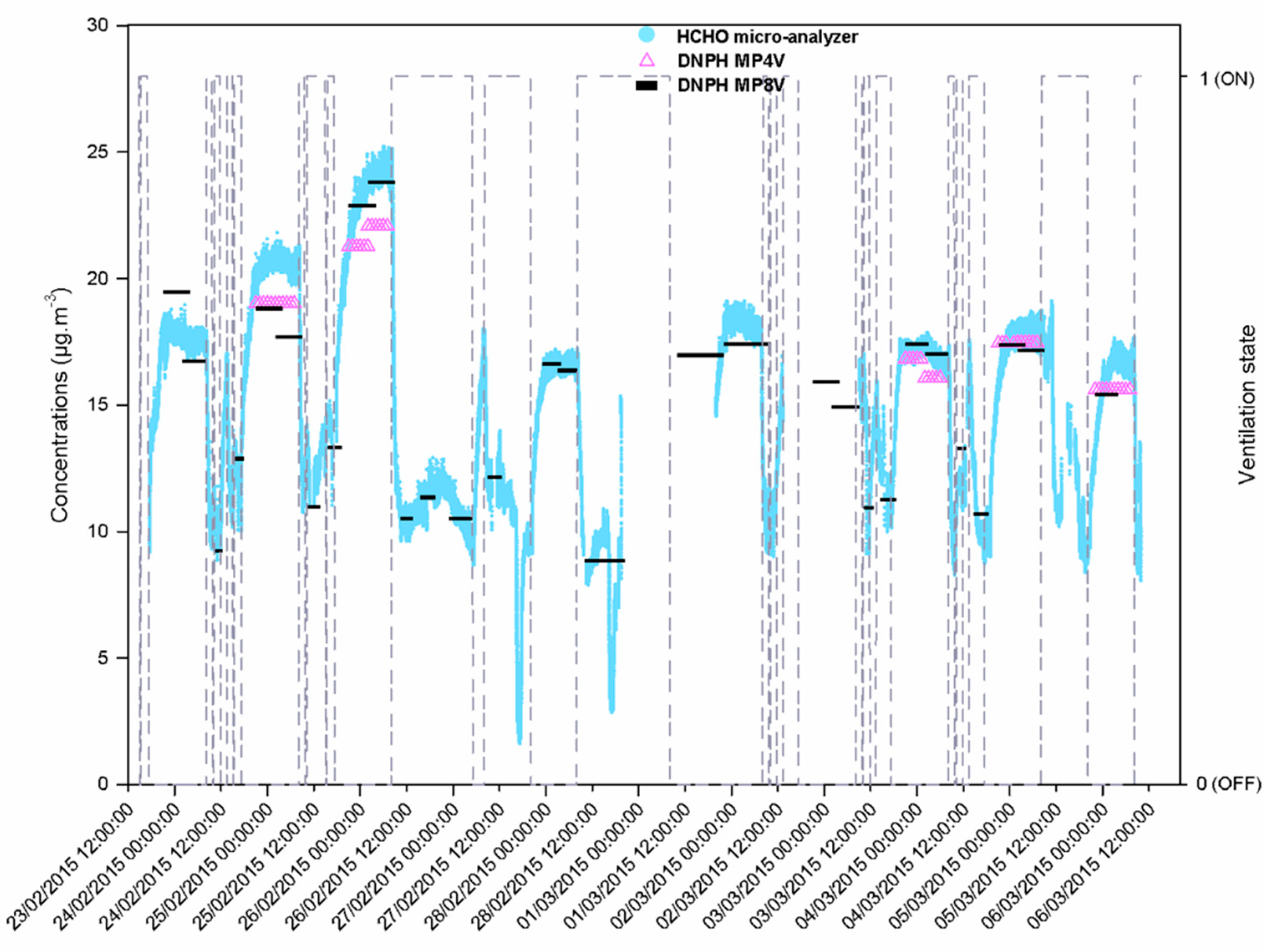
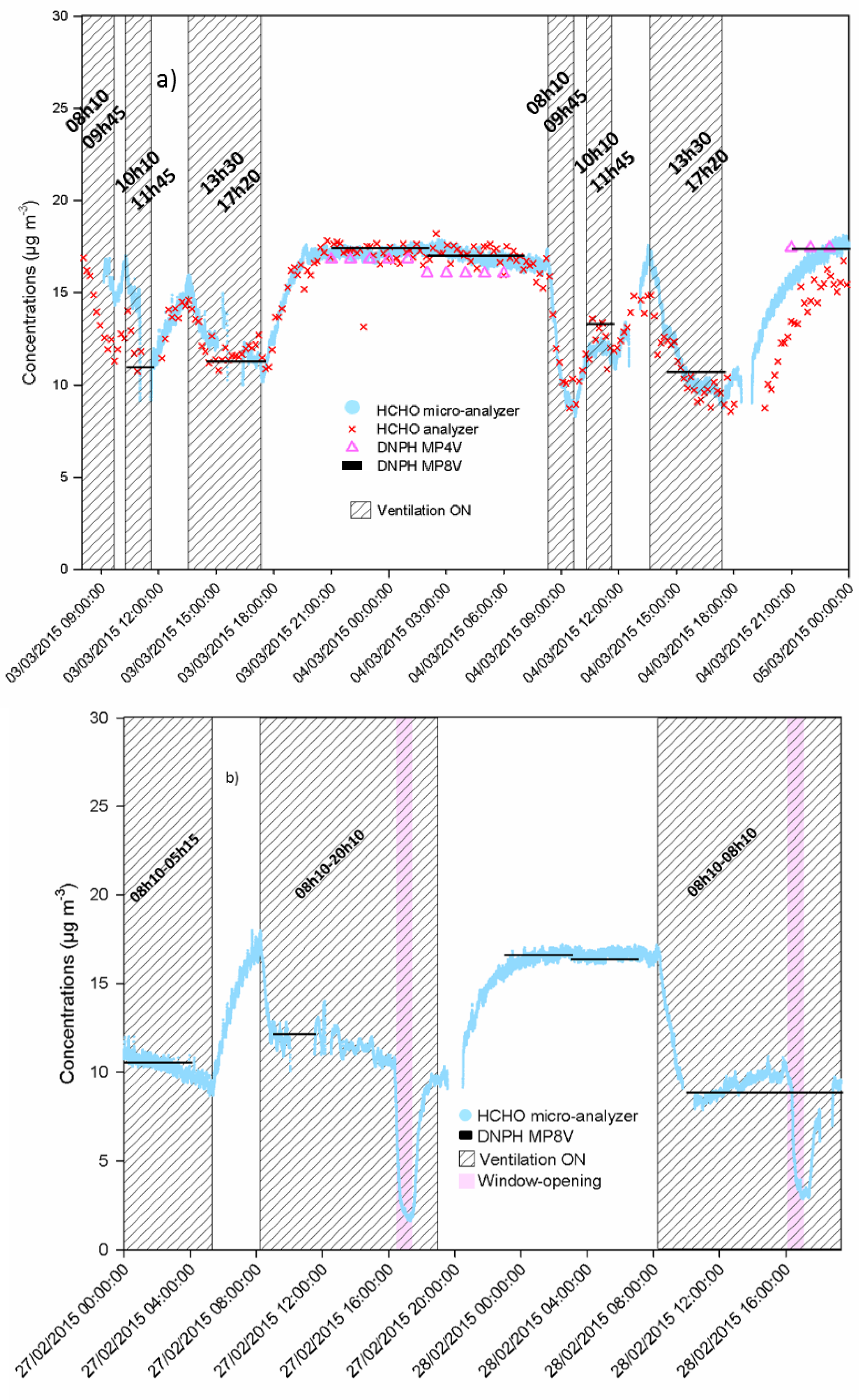
3.2. Formaldehyde Monitoring in the Classroom
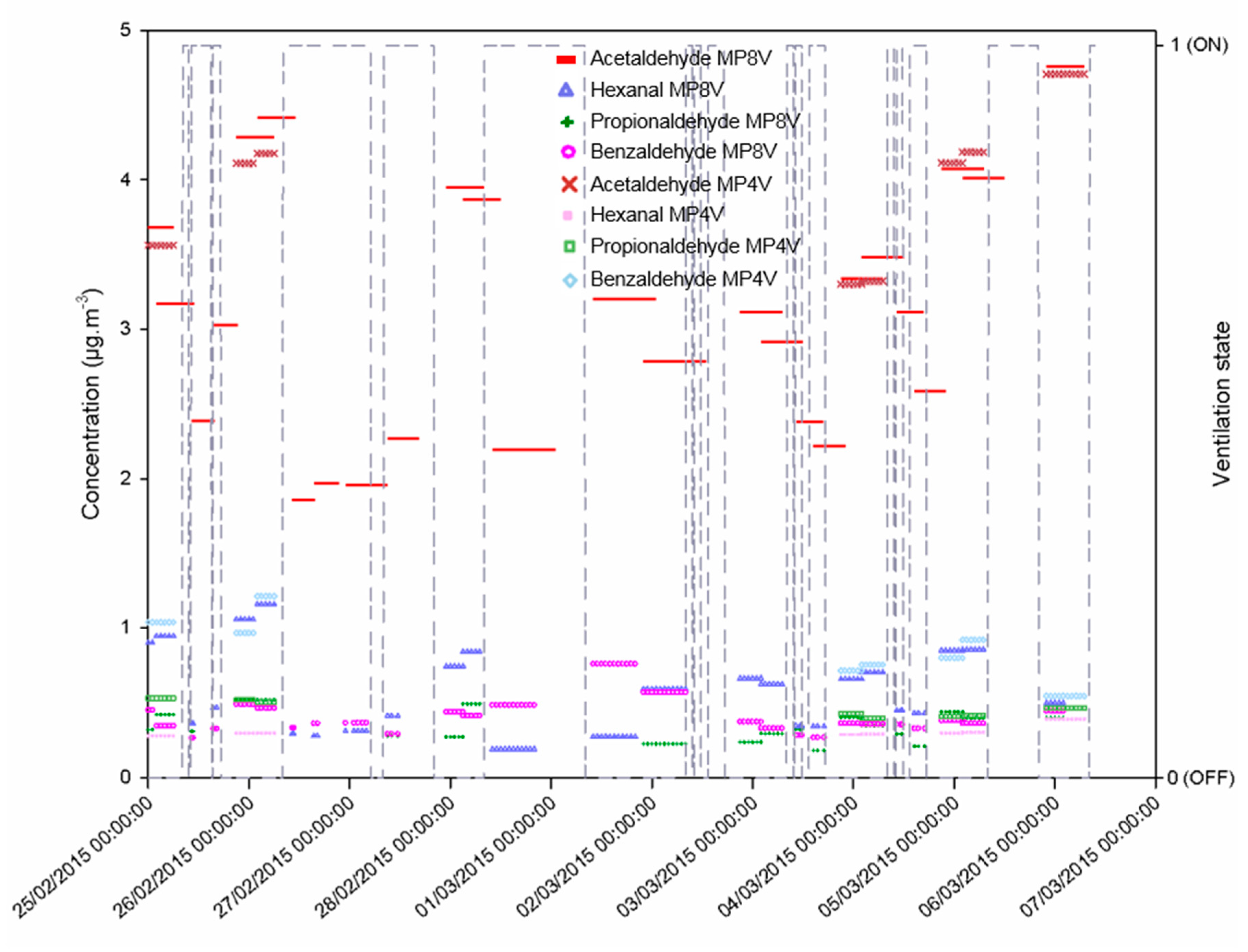
3.3. Monitoring of Other Aldehydes in the Classroom
3.4. Monitoring of Outdoor Aldehydes Concentrations
3.5. Monitoring of Aldehydes Concentrations at Ventilation Inlet
4. Discussion
4.1. Comparison of the Formaldehyde Concentrations Measured by Different Techniques
4.2. Comparison with Previous Aldehydes Measurements Performed in EUROPEAN Low-Energy Building
4.3. Application to Green Buildings Monitoring
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Omer, A.M. Energy and Environment: Applications and Sustainable Development. Int. J. Environ. Clim. Change 2011, 1, 118–158. [Google Scholar] [CrossRef] [PubMed]
- Billionnet, C.; Gay, E.; Kirchner, S.; Leynaert, B.; Annesi-Maesano, I. Quantitative assessments of indoor air pollution and respiratory health in a population-based sample of French dwellings. Environ. Res. 2011, 111, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Derbez, M.; Berthineau, B.; Cochet, V.; Lethrosne, M.; Pignon, C.; Riberon, J.; Kirchner, S. Indoor air quality and comfort in seven newly built, energy-efficient houses in France. Build. Environ. 2014, 72, 173–187. [Google Scholar] [CrossRef]
- Verriele, M.; Schoemaecker, C.; Hanoune, B.; Leclerc, N.; Germain, S.; Gaudion, V.; Locoge, N. The MERMAID study: Indoor and outdoor average pollutant concentrations in 10 low-energy school buildings in France. Indoor Air 2016, 26, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Salthammer, T. Formaldehyde in the Ambient Atmosphere: From an Indoor Pollutant to an Outdoor Pollutant? Angew. Chem. Int. Ed. 2013, 52, 3320–3327. [Google Scholar] [CrossRef]
- Kim, K.-W.; Kim, S.; Kim, H.-J.; Park, J.C. Formaldehyde and TVOC emission behaviors according to finishing treatment with surface materials using 20L chamber and FLEC. J. Hazard. Mater. 2010, 177, 90–94. [Google Scholar] [CrossRef]
- Paolacci, H.; Dagnelie, R.; Porterat, D.; Piuzzi, F.; Lepetit, F.; Tran-Thi, T.-H. A ppb Formaldehyde Gas Sensor for Fast Indoor Air Quality Measurements. Sens. Transducers J. 2007, 82, 1423–1430. [Google Scholar]
- Wang, B.; Lee, S.C.; Ho, K.F. Characteristics of carbonyls: Concentrations and source strengths for indoor and outdoor residential microenvironments in China. Atmos. Environ. 2007, 41, 2851–2861. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Wang, S. Ambient formaldehyde and its contributing factor to ozone and OH radical in a rural area. Atmos. Environ. 2010, 44, 2074–2078. [Google Scholar]
- Kim, J.; Kim, S.; Lee, K.; Yoon, D.; Lee, J.; Ju, D. Indoor aldehydes concentration and emission rate of formaldehyde in libraries and private reading rooms. Atmos. Environ. 2013, 71, 1–6. [Google Scholar] [CrossRef]
- Marchand, C.; Le Calvé, S.; Mirabel, Ph.; Glasser, N.; Casset, A.; Schneider, N.; de Blay, F. Concentrations and determinants of gaseous aldehydes in 162 homes in Strasbourg (France). Atmos. Environ. 2008, 42, 505–516. [Google Scholar] [CrossRef]
- Huang, J.; Feng, Y.; Li, J.; Xiong, B.; Feng, J.; Wen, S.; Sheng, G.; Fu, J.; Wu, M. Characteristics of carbonyl compounds in ambient air of Shanghai, China. J. Atmos. Chem. 2008, 61, 1–20. [Google Scholar] [CrossRef]
- Casset, A.; Marchand, C.; Purohit, A.; le Calve, S.; Uring-Lambert, B.; Donnay, C.; Meyer, P.; de Blay, F. Inhaled formaldehyde exposure: Effect on bronchial response to mite allergen in sensitized asthma patients. Allergy 2006, 61, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- IARC Monographs on the Evaluation Carcinogenic Risks on Humans; International Agency for Research on Cancer: Lyon, France, 2006.
- AFSSET Le Formaldéhyde. Valeurs Guides de Qualité d’air Intérieur; French Agency for Food, Environmental and Occupational Health & Safety: Maisons-Alfort, France, 2007.
- Décret n° 2011-1727 du 2 décembre 2011 relatif aux valeurs-guides pour l'air intérieur pour le formaldéhyde et le benzène, JORF n°0281 du 4 décembre 2011 page 20529, texte n° 4.
- Guglielmino, M.; Allouch, A.; Serra, C.A.; Calvé, S.L. Development of microfluidic analytical method for on-line gaseous Formaldehyde detection. Sens. Actuators B Chem. 2017, 243, 963–970. [Google Scholar] [CrossRef]
- Guglielmino, M.; Bernhardt, P.; Trocquet, C.; Serra, C.A.; Le Calvé, S. On-line gaseous formaldehyde detection by a microfluidic analytical method based on simultaneous uptake and derivatization in a temperature controlled annular flow. Talanta 2017, 172, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Tanaka, S.; Teshima, N.; Yasuda, S.; Ura, N. Fluorimetric flow injection analysis of trace amount of formaldehyde in environmental atmosphere with 5,5-dimethylcyclohexane-1,3-dione. Talanta 2002, 58, 1271–1278. [Google Scholar] [CrossRef]
- Bertoni, G.; Ciuchini, C.; Palo, V.Di.; Possanzini, M. Development of a Passive Sampler for Long-Term Measurements of Formaldehyde and Total Oxidants in Air. Chromatographia 2005, 61, 385–389. [Google Scholar] [CrossRef]
- Hak, C.; Pundt, I.; Trick, S.; Kern, C.; Platt, U.; Dommen, J.; Ordóñez, C.; Prévôt, A.S.H.; Junkermann, W.; Astorga-Lloréns, C.; et al. Intercomparison of four different in-situ techniques for ambient formaldehyde measurements in urban air. Atmos. Chem. Phys. 2005, 5, 2881–2900. [Google Scholar] [CrossRef]
- Hanoune, B.; LeBris, T.; Allou, L.; Marchand, C.; Le Calvé, S. Formaldehyde measurements in libraries: Comparison between infrared diode laser spectroscopy and a DNPH-derivatization method. Atmos. Environ. 2006, 40, 5768–5775. [Google Scholar] [CrossRef]
- Wisthaler, A.; Apel, E.C.; Bossmeyer, J.; Hansel, A.; Junkermann, W.; Koppmann, R.; Meier, R.; Müller, K.; Solomon, S.J.; Steinbrecher, R.; et al. Technical Note: Intercomparison of formaldehyde measurements at the atmosphere simulation chamber SAPHIR. Atmos. Chem. Phys. 2008, 8, 2189–2200. [Google Scholar] [CrossRef]
- Bono, R.; Degan, R.; Pazzi, M.; Romanazzi, V.; Rovere, R. Benzene and formaldehyde in air of two winter Olympic venues of “Torino 2006. ” Environ. Int. 2010, 36, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Pretto, A.; Milani, M.R.; Cardoso, A.A. Colorimetric determination of formaldehyde in air using a hanging drop of chromotropic acid. J. Environ. Monit. 2000, 2, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dasgupta, P.K.; Luke, W. Measurement of gaseous and aqueous trace formaldehyde: Revisiting the pentanedione reaction and field applications. Anal. Chim. Acta 2005, 531, 51–68. [Google Scholar] [CrossRef]
- Allouch, A.; Guglielmino, M.; Bernhardt, P.; Serra, C.A.; Le Calvé, S. Transportable, fast and high sensitive near real-time analyzers: Formaldehyde detection. Sens. Actuators B Chem. 2013, 181, 551–558. [Google Scholar] [CrossRef]
- Toda, K.; Yoshioka, K.-I.; Mori, K.; Hirata, S. Portable system for near-real time measurement of gaseous formaldehyde by means of parallel scrubber stopped-flow absorptiometry. Anal. Chim. Acta 2005, 531, 41–49. [Google Scholar] [CrossRef]
- Toda, K.; Tokunaga, W.; Gushiken, Y.; Hirota, K.; Nose, T.; Suda, D.; Nagai, J.; Ohira, S.-I. Mobile monitoring along a street canyon and stationary forest air monitoring of formaldehyde by means of a micro-gas analysis system. J. Environ. Monit. 2012, 14, 1462–1472. [Google Scholar] [CrossRef]
- Zheng, W. Développement d’un analyseur rapide et transportable du formaldéhyde dans l’air. Ph.D. Thesis, University of Strasbourg, Strasbourg, France, 2010. [Google Scholar]
- Schoemaecker, C.; Fèvre-Nollet, V.; Verriele, M.; Dusanter, S.; Le Calvé, S.; Trocquet, C.; Hanoune, B.; Locoge, N. Effect of occupancy controlled ventilation in schools on the exposure quantification. In Proceedings of the 14th International Conference of Indoor Air Quality and Climate-Indoor Air 2016, Ghent, Belgium, 3–7 July 2016. [Google Scholar]
- Bakó-Biró, Zs.; Clements-Croome, D.J.; Kochhar, N.; Awbi, H.B.; Williams, M.J. Ventilation rates in schools and pupils’ performance. Build. Environ. 2012, 48, 215–223. [Google Scholar] [CrossRef]
- Mendell, M.J.; Heath, G.A. Do indoor pollutants and thermal conditions in schools influence student performance? A critical review of the literature. Indoor Air 2005, 15, 27–52. [Google Scholar] [CrossRef]
- ASTM International Test Method for Determination of Formaldehyde and Other Carbonyl Compounds in Air (Active Sampler Methodology) 2009. Available online: https://www.astm.org/Standards/D5197.htm (accessed on 1 October 2019).
- Pinheiro, H.L.C.; de Andrade, M.V.; de Paula Pereira, P.A.; de Andrade, J.B. Spectrofluorimetric determination of formaldehyde in air after collection onto silica cartridges coated with Fluoral, P. Microchem. J. 2004, 78, 15–20. [Google Scholar] [CrossRef]
- Eisner, U.; Kuthan, J. Chemistry of dihydropyridines. Chem. Rev. 1972, 72, 1–42. [Google Scholar] [CrossRef]
- Triplett, K.A.; Ghiaasiaan, S.M.; Abdel-Khalik, S.I.; Sadowski, D.L. Gas–liquid two-phase flow in microchannels Part I: Two-phase flow patterns. Int. J. Multiph. Flow 1999, 25, 377–394. [Google Scholar] [CrossRef]
- Nasreddine, R.; Person, V.; Serra, C.A.; Schoemaecker, C.; Le Calvé, S. Portable novel micro-device for BTEX real-time monitoring: Assessment during a field campaign in a low consumption energy junior high school classroom. Atmos. Environ. 2016, 126, 211–217. [Google Scholar] [CrossRef]
- Barguil, S.; Le Moullec, Y.; Person, A.; Laurent, A.-M.; Festy, B. Chemical characterization of indoor air quality in Parisian homes. Aerobiologia 1990, 6, 28–31. [Google Scholar] [CrossRef]
- Kaunelienė, V.; Prasauskas, T.; Krugly, E.; Stasiulaitienė, I.; Čiužas, D.; Šeduikytė, L.; Martuzevičius, D. Indoor air quality in low energy residential buildings in Lithuania. Build. Environ. 2016, 108, 63–72. [Google Scholar] [CrossRef]
- Kolarik, B.; Gunnarsen, L.; Logadottir, A.; Funch, L.W. Concentrations of Formaldehyde in new Danish Residential Buildings in Relation to WHO Recommendations and CEN Requirements. Indoor Built Environ. 2011, 21, 552–561. [Google Scholar] [CrossRef]
- Langer, S.; Bekö, G.; Bloom, E.; Widheden, A.; Ekberg, L. Indoor air quality in passive and conventional new houses in Sweden. Build. Environ. 2015, 93, 92–100. [Google Scholar] [CrossRef]
- Järnström, H.; Saarela, K.; Kalliokoski, P.; Pasanen, A.-L. Reference values for indoor air pollutant concentrations in new, residential buildings in Finland. Atmos. Environ. 2006, 40, 7178–7191. [Google Scholar] [CrossRef]
- WELL v2 The Next Version of the WELL Building StandardTM, Concepts/Air/Feature A08, Air Quality Monitoring and Awareness. Available online: https://v2.wellcertified.com/v/en/air/feature/8 2019 (accessed on 1 October 2019).
- Rizk, M.; Guo, F.; Verriele, M.; Ward, M.; Dusanter, S.; Blond, N.; Locoge, N.; Schoemaecker, C. Impact of material emissions and sorption of volatile organic compounds on indoor air quality in a low energy building: Field measurements and modeling. Indoor Air 2018, 28, 924–935. [Google Scholar] [CrossRef]


| Method | LOD (µg m−3) | Time Resolution | Interferences | Reagent Consumption (µL min−1) | Weight (kg) | References | |
|---|---|---|---|---|---|---|---|
| Chromatographic and spectroscopic methods | DNPH tube + HPLC/UV-Vis 1 | 0.13 (S/N = 3) 0.12 (S/N = 5) | 30 days 1–2 h | O3, NOX, H2O | - - | >50 2 | [20,22] |
| GC-MS 3 | 0.02 (S/N = 3) | - | Overlapped peaks | - | >100 2 | [24] | |
| FTIR 4 | 0.5 | 5 min | Carbonyl species | - | >30 2 | [21] | |
| PTR/MS 5 | 0.2 (S/N = 2) | 2 s | H2O | - | >100 2 | [23] | |
| Chemical methods | Chromotropic acid + Colorimetry | 2.5 | 7 min | phenol, alcohols, aromatic hydrocarbons | Droplet of 15 µL | N/A | [25] |
| Aerolaser AL4021 | 0.19 | 1.5 min | H2O, H2O2, O3, Glyoxal | 500 + 500 6 | 20 2 | [21] | |
| Dimedone + fluorescence | 1.1 (S/N = 3) | - | Other aldehydes | 700 6 | N/A | [19] | |
| Fluoral-P + fluorescence | 0.09 (S/N = 3) | - | H2O, O3 | 110 + 30 + 30 7 | 17.4 2 | [26] | |
| Acetyl acetone + fluorescence (1st generation of formaldehyde analyser) | 0.5 (S/N = 3) | 6–10 min | None identified | 1100 6 | 8 2 | [30] | |
| Acetyl acetone + Colorimetric microfluidic device | 0.7 (S/N = 3) | 1–2 min | None identified | 35 | 9 8 | [17,18] | |
| Acetyl acetone + Fluorimetric microfluidic device | 1.2 (S/N = 3) | 2 s | None identified | 20 | 4 | This work |
| Characteristics/Performances | Reference Method ISO 16000-3 1 | Formaldehyde Analyzer 2 | Formaldehyde Microanalyzer 3 |
|---|---|---|---|
| Reagents | ACN/Water | Acetylacetone+ HNO3 0,1N | Acetylacetone |
| Reagents consumption (µL min−1) | - | 1200 | 17 |
| Autonomy (min) 4 | - | 83 | 5880 |
| Sampling flow (mL min−1) | 1000–2000 | 300 | 20 |
| Interferences | O3, NOX, H20 | - | - |
| LD (µg m−3) | 0.004 (S/N = 3) 5 | 1 (S/N = 3) | 1.2 (S/N = 3) |
| Response time (min) | - | 10 | 10 |
| Temporal resolution | 30 min–7 days 6 | 10 min | 2–120 s |
| Near Real-time | No | Yes | Yes |
| Weight | ≥50 kg | 8 kg | 4 kg |
| Portability | Sedentary | transportable | portable |
| Pollutants | Concentrations Ranges Mean (min-max) | Time Number of Buildings (Type of Buildings) | Technique | Location | Reference |
|---|---|---|---|---|---|
| Formaldehyde | 19 (0.8–26) µg m−3 a 21 (0.9–28) µg m−3 b 26 (0.8–37) µg m−3 c | August 2000–July 2003 n = 8 | 0.005M H2SO4 sol. at 2–4 L/min + spectrometric acetyl-acetone method | Finland | [43] |
| Formaldehyde | 40 µg m−3 | 2010 n = 20 (new buildings) | Passive DNPH tube + HPLC/UV | Denmark | [41] |
| Formaldehyde Acetaldehyde Hexanal | 22.8 (6–48) µg m−3 16.5 µg m−3 61.2 µg m−3 | July 2009–July 2010 N = 6 (new occupied houses) | Passive DNPH tube + HPLC/UV | France | [3] |
| Acetaldehyde Hexanal | 12.9 µg m−3 86.7 µg m−3 | February–December 2009 n = 3 (new unoccupied houses) | |||
| Formaldehyde Acetaldehyde Hexanal | 15.9 µg m−3 6.0 µg m−3 9.6 µg m−3 | 2014 n = 10 (Schools) | Passive DNPH tube + HPLC/UV | North and East of France | [4] |
| Formaldehyde | 11.1 µg m−3 | 2012/2013 and 2013/2014 heating seasons n = 20 (new buildings) | Passive DNPH tube + HPLC/UV | Sweden | [42] |
| Formaldehyde | 30.8 (3.3–52.3) µg m−3 | April–August 2014 n = 11 (new buildings) | Passive DNPH tube + UPLC/UV | Lithuania | [40] |
| Formaldehyde Acetaldehyde Hexanal | (2–25) µg m−3 (2–5) µg m−3 (≤1) µg m−3 | February–March 2015 n = 1 (School) | Active DNPH tube + HPLC/UV | North of France | This work |
| Formaldehyde | (2–25) µg m−3 | microanalyzer |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trocquet, C.; Bernhardt, P.; Guglielmino, M.; Malandain, I.; Liaud, C.; Englaro, S.; Le Calvé, S. Near Real-Time Monitoring of Formaldehyde in a Low-Energy School Building. Atmosphere 2019, 10, 763. https://doi.org/10.3390/atmos10120763
Trocquet C, Bernhardt P, Guglielmino M, Malandain I, Liaud C, Englaro S, Le Calvé S. Near Real-Time Monitoring of Formaldehyde in a Low-Energy School Building. Atmosphere. 2019; 10(12):763. https://doi.org/10.3390/atmos10120763
Chicago/Turabian StyleTrocquet, Claire, Pierre Bernhardt, Maud Guglielmino, Isabelle Malandain, Céline Liaud, Stéphanette Englaro, and Stéphane Le Calvé. 2019. "Near Real-Time Monitoring of Formaldehyde in a Low-Energy School Building" Atmosphere 10, no. 12: 763. https://doi.org/10.3390/atmos10120763
APA StyleTrocquet, C., Bernhardt, P., Guglielmino, M., Malandain, I., Liaud, C., Englaro, S., & Le Calvé, S. (2019). Near Real-Time Monitoring of Formaldehyde in a Low-Energy School Building. Atmosphere, 10(12), 763. https://doi.org/10.3390/atmos10120763