Tailpipe VOC Emissions from Late Model Gasoline Passenger Vehicles in the Japanese Market
Abstract
:1. Introduction
2. Methods
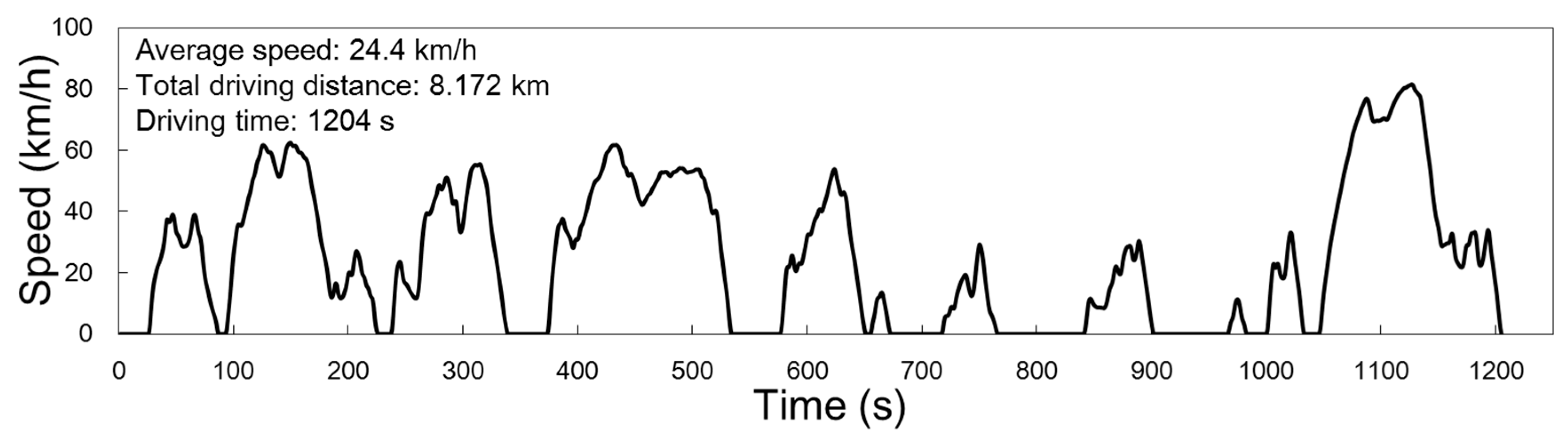
2.1. Chassis Dynamometer Experiments for Late Model Gasoline Passenger Vehicles
2.2. Composition Analysis of Non-Methane Hydrocarbons (Non-Methane VOCs)
3. Results and Discussion
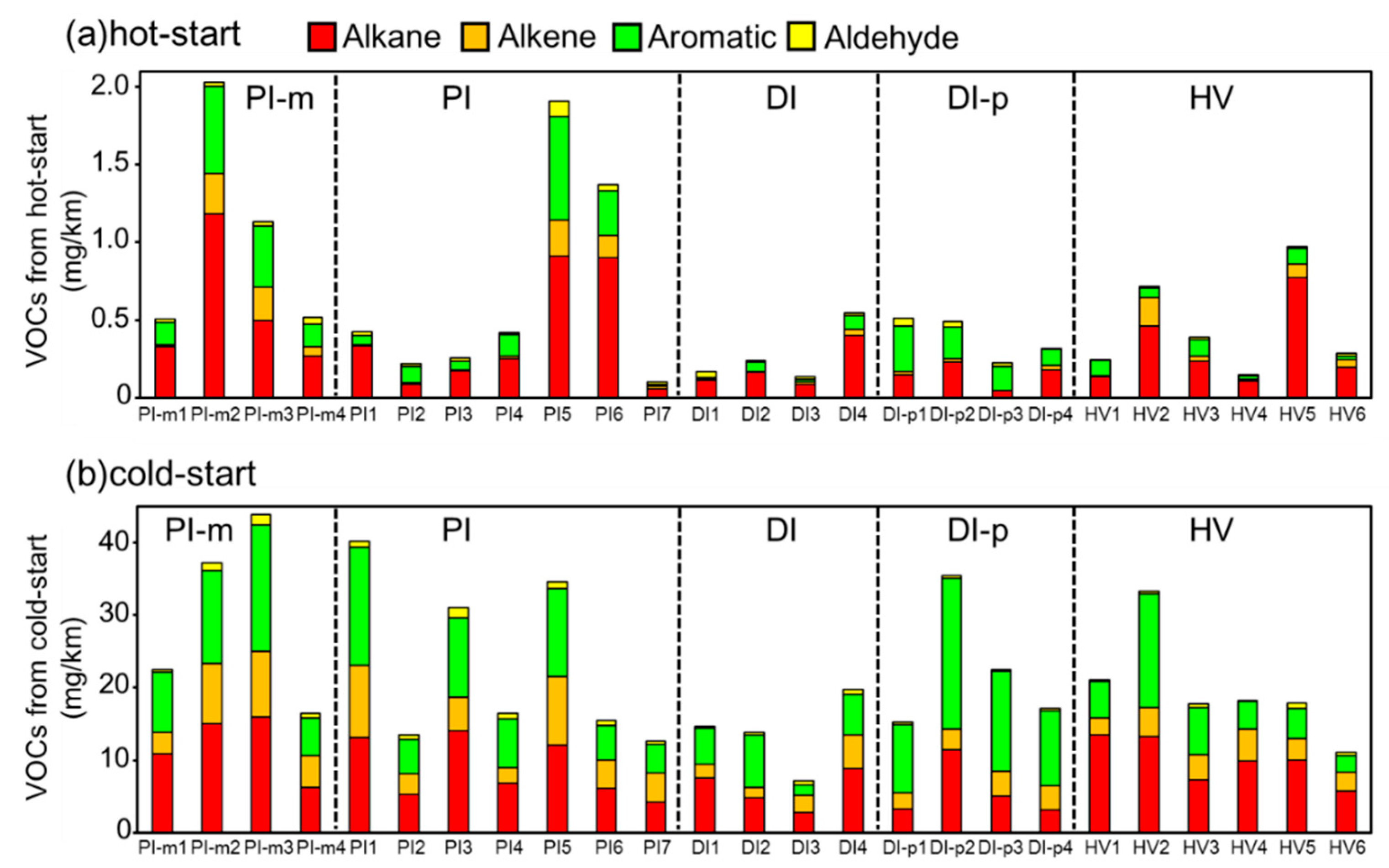
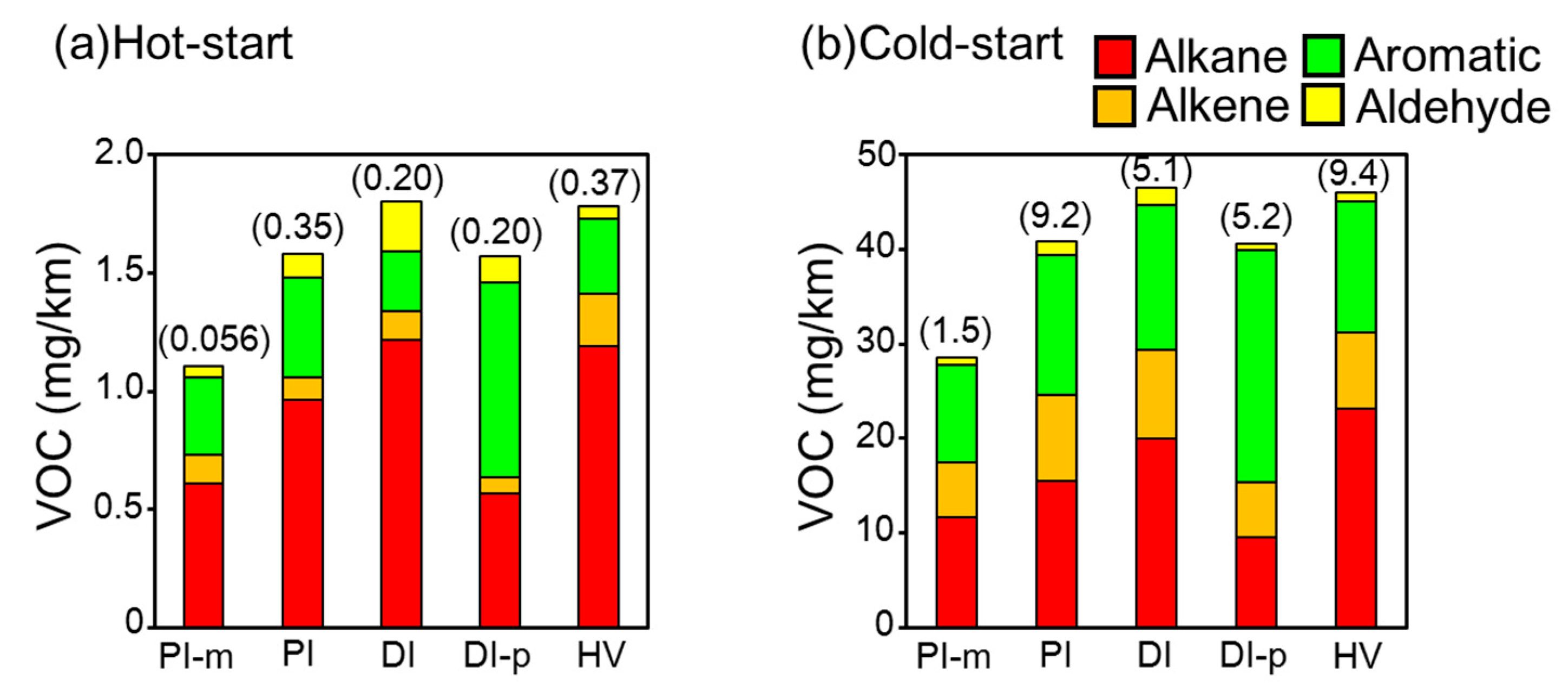
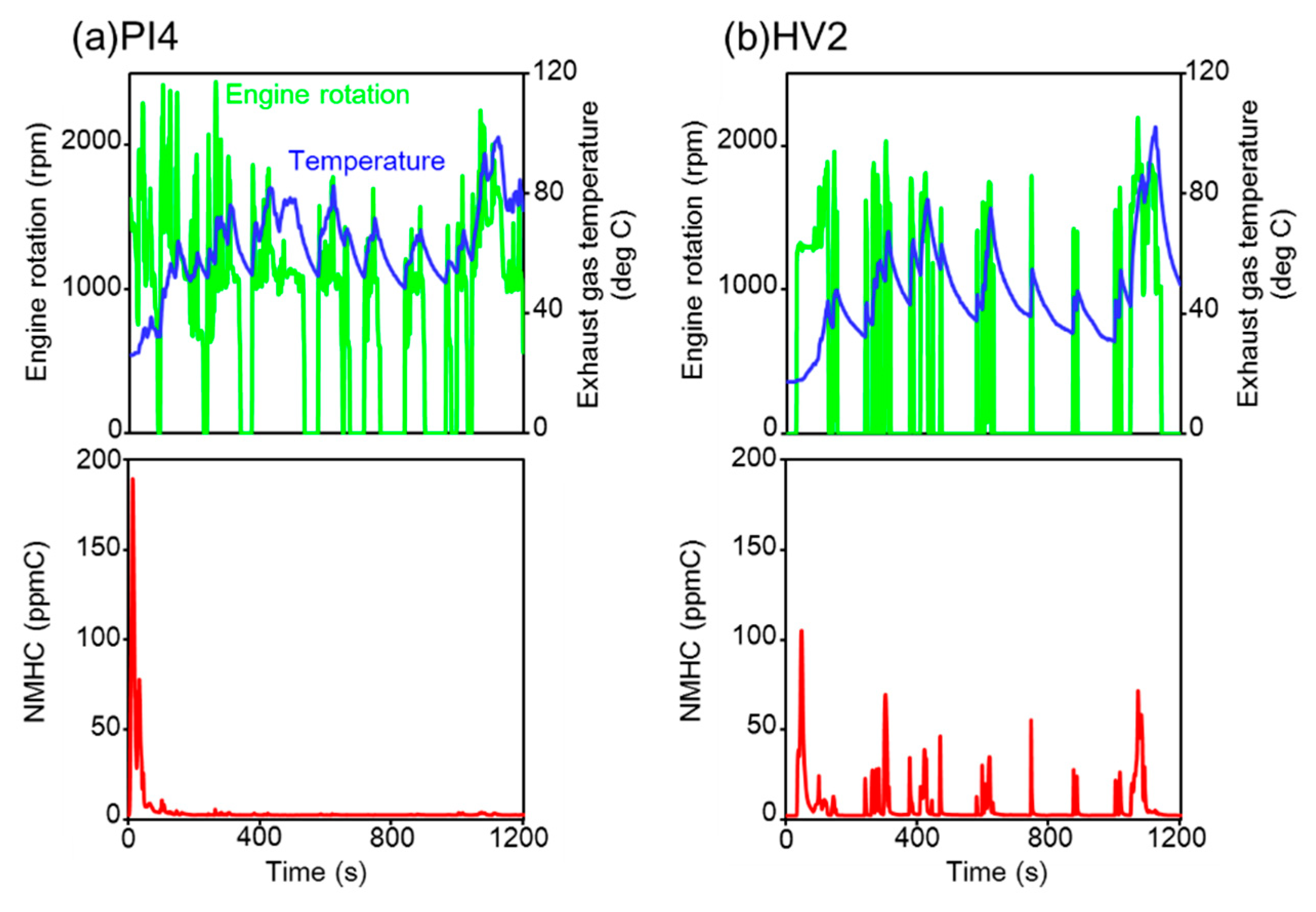
3.1. Trends of Tailpipe VOCs Emissions and Ozone Formation Potential for Hot- and Cold-Starts
3.2. Increased VOCs from Tailpipe Emissions Caused by the Deterioration of the Three-Way Catalyst
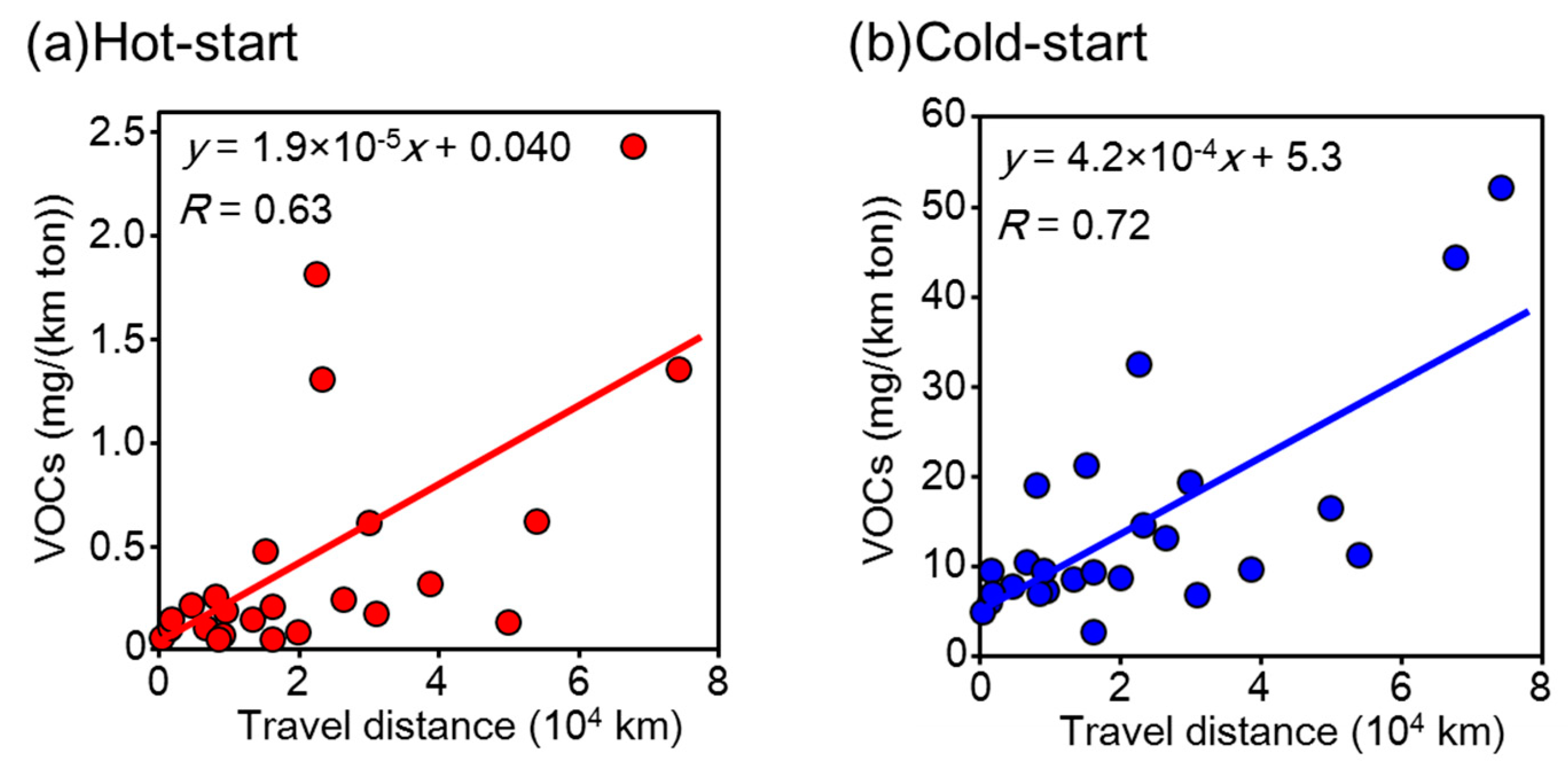
3.3. VOCs Emissions from Passenger Vehicles Normalized for Mileage
3.4. High VOC Emissions from a Specific Hybrid Vehicle
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Column Temperature rising Program | Type | TC-BOND Alumina/KCl (50 m, 0.53 mmID, 10 um) |
| Initial temp. (ºC) | 40 | |
| Initial temp. retention time (min.) | 5 | |
| First temp. rate of increase (ºC/min.) | 2.5 | |
| First retention temp. (ºC) | 130 | |
| First retention time (min.) | 0 | |
| Second temp. rate of increase (ºC/min.) | 20 | |
| Final retention temp. (ºC) | 200 | |
| Final retention time (min.) | 15.5 | |
| Carrier gas | Type | He |
| Pressure (kPa) | 400 | |
| Detector | Type | FID |
| Temp. (ºC) | 250 | |
| Fuel gas (mL/min.) | H2 40 | |
| Combustion gas (mL/min.) | Air 400 | |
| Additional gas (mL/min.) | N2 30 |
| Column Temperature riding Program | Type | DB-1 (60 m, 0.25 mmID, 1 um) |
| Initial temp. (ºC) | 40 | |
| Initial temp. rate of increase (min.) | 5 | |
| First temp. rising velocity (ºC/min.) | 3 | |
| First retention temp. (ºC) | 180 | |
| First retention time (min.) | 0 | |
| Second temp. rate of increase (ºC/min.) | 30 | |
| Final retention temp. (ºC) | 250 | |
| Final retention time (min.) | 6 | |
| Carrier gas | Type | He |
| Pressure (kPa) | 400 | |
| Detector | Type | Shimadzu GCMS-QP2020 |
| Column Temperature riding Program | Type | Poroshell 120 EC-C18 (2.1×100 mm, 2.7 um) |
| Mobile Phase A | 95:5 (v/v) water / acetonitrile | |
| Mobile Phase B | acetonitrile | |
| Gradient | Time (min) B (%) 0 20 2 20 8 40 12 50 22 60 23 100 | |
| Flow rate | 0.4 mL/min | |
| Column temp. (ºC) | 40 | |
| Injection part | Volume (uL) | 100 |
| wash solvent | acetonitrile | |
| Injection amount (uL) | 5 | |
| Detector | Type | Agilent 6120API-ES, Negative, SIM |
References
- Roman, R.; Cansino, M.; Rueda-Cantuche, M.J. A multi-regional input-output analysis of ozone precursor emissions embodied in Spanish international trade. J. Clean. Prod. 2016, 137, 1382–1392. [Google Scholar] [CrossRef]
- Velasco, E.; Retama, A. Ozone’s threat hits back Mexico City. Sustain. Cities. Soc. 2017, 31, 260–263. [Google Scholar] [CrossRef]
- Chen, J.; Luo, D. Ozone formation potentials of organic compounds from different emission sources in the South Coast Air Basin of California. Atmos. Environ. 2012, 55, 448–455. [Google Scholar] [CrossRef]
- National Air Quality: Status and Trends of Key Air Pollutants. Available online: https://www.epa.gov/air-trends (accessed on 19 June 2019).
- Air quality standards. Available online: https://www.eea.europa.eu/themes/air/air-quality-standards (accessed on 19 June 2019).
- The Air Pollution Trend in Japan. 2017. Available online: https://www.env.go.jp/press/106609.html (accessed on 19 June 2019).
- Zaveri, A.R.; Berkowltz, M.C.; Kleinman, I.L.; Springston, R.S.; Doskey, V.P.; Lonneman, A.W.; Spicer, W.C. Ozone production efficiency and NOx depletion in an urban plume: Interpretation of field observations and implications for evaluating O3-NOx-VOC sensitivity. J. Geophys. Res. 2003, 108, 4436. [Google Scholar] [CrossRef]
- Sillman, S. The relation between ozone, NOx and hydrocarbons in urban and polluted rural environments. Atmos. Environ. 1999, 33, 1821–1845. [Google Scholar] [CrossRef]
- Ou, J.; Yuan, Z.; Zheng, J.; Huang, Z.; Shao, M.; Li, Z.; Huang, X.; Guo, H.; Louie, K.K.P. Ambient ozone control in a photochemically active region: Short-term despiking or long-term attainment? Environ. Sci. Technol. 2016, 50, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Dhupeng, S.; Kinnon, M.M.; Shaffer, P.B.; Samuelsen, S.G.; Brouwer, J.; Dabdub, D. An uncertainty for clean air: Air quality modeling implications of underestimating VOC emissions in urban inventories. Atmos. Environ. 2019, 211, 256–267. [Google Scholar]
- Hata, H.; Tonokura, K. Impact of next-generation vehicles on tropospheric ozone estimated by chemical transport model in the Kanto region of Japan. Sci. Rep. 2019, 3573. [Google Scholar] [CrossRef] [PubMed]
- Sartelet, K.; Zhu, S.; Moukhtar, S.; Andre, M.; Andre, M.J.; Gros, V.; Favez, O.; Brasseur, A.; Redaelli, M. Emission of intermediate, semi and low volatile organic compounds from traffic and their impact on secondary organic aerosol concentrations over Greater Paris. Atmos. Environ. 2018, 180, 126–137. [Google Scholar] [CrossRef]
- Brandt, P.E.; Wang, Y.; Grizzle, W.J. Dynamic modeling of a three-way catalyst for SI engine exhaust emission control. IEEE Trans. Control Syst. Technol. 2000, 8, 767–776. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Zhou, M.; Huang, Z.; Gao, J.; Ma, Z.; Chen, J.; Tang, X. Enhanced performance of Ceria-based NOx reduction catalysts by optimal support effect. Environ. Sci. Technol. 2017, 51, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Martini, G.; Manfredi, U.; Mellios, G.; Krsenbrink, A.; De Santi, G.; McArragher, S.; Thompson, N.; Baro, J.; Zemroch, P.J.; Boggio, F.; et al. Effects of gasoline vapour pressure and ethanol content on evaporative emissions from modern European cars. SAE Technical Paper, 2007; 2007-01-1928. [Google Scholar] [CrossRef]
- Yamada, H.; Inomata, S.; Tanimoto, H. Refueling emissions from cars in Japan: Compositions, temperature dependence and effect of vapor liquefied collection system. Atmos. Environ. 2015, 120, 445–462. [Google Scholar] [CrossRef]
- Freda, F.; Bob, M. Onboard Refueling Vapor Recovery: Evaluation of the ORVR Program in the United States. Report from International Council on Clean Transportation. Available online: https://theicct.org/sites/default/files/publications/ORVR_v4_0.pdf (accessed on 16 July 2019).
- Guidance on Removing Stage 2 Gasoline Vapor Control Programs from State Implementation Plans and Assessing Comparable Measures. The report from United States Environmental Protection Agency. Available online: https://www3.epa.gov/ttn/naaqs/aqmguide/collection/cp2/20120807_page_stage2_removal_guidance.pdf (accessed on 16 July 2019).
- Hata, H.; Yamada, H.; Kokuryo, K.; Okada, M.; Funakubo, C.; Tonokura, K. Estimation model for evaporative emissions from gasoline vehicles based on thermodynamics. Sci. Total. Environ. 2018, 618, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Hata, H.; Yamada, H.; Yanai, K.; Kugata, M.; Noumura, G.; Tonokura, K. Modeling evaporative emissions from parked gasoline cars based on vehicle carbon canister experiments. Sci. Total. Environ. 2019, 675, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Inomata, S.; Tanimoto, H.; Hata, H.; Tonokura, K. Estimation of refueling emissions based on theoretical model and effects of E10 fuel on refueling and evaporative emissions from gasoline cars. Sci. Total. Environ. 2018, 622–623, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Nonaka, N.; Takada, I.; Ishimori, S. Mass spectrometric analysis for distinction between regular and premium motor gasolines. Analytical Sciences. 1993, 9, 261–266. [Google Scholar] [CrossRef]
- DieselNet. Available online: https://www.dieselnet.com/standards/cycles/jp_jc08.php (accessed on 7 October 2019).
- Statistical report of vehicle usage conducted by the Ministry of Land, Infrastructure, Transport and Tourism in Japan. Available online: http://www.mlit.go.jp/jidosha/iinkai/seibi/5th/5-2.pdf (accessed on 3 September 2019).
- Tables of Maximum Incremental Reactivity (MIR) Values. Available online: https://ww3.arb.ca.gov/regact/2009/mir2009/mir2009.htm (accessed on 7 October 2019).
- Shinichi, M.; Koji, Y.; Shi-aki, H.; Tadashi, S.; Hideo, S. Thermal Deterioration Mechanism of Pt/Rh Three-way Catalysts. SAE Trans. 1998, 107, 2174–2178. [Google Scholar]
- Moulijn, J.A.; van Diepen, A.E.; Kapteijn, F. Catalyst deactivation: Is it predictable? What to do? Appl. Catal. A General 2001, 212, 3–16. [Google Scholar] [CrossRef]

| Vehicle Type | PI-m | PI | DI | DI-p | HV |
|---|---|---|---|---|---|
| Number of tested vehicles | 4 | 7 | 4 | 4 | 6 |
| Fuel type | R-gasoline | R-gasoline | R-gasoline | P-gasoline | R-gasoline |
| Displacement (L) | 0.658–0.659 | 1.242–2.359 | 1.496–1.997 | 1.490–1.997 | 1.496–2.493 |
| Inertial weight (kg) | 910–1020 | 1020–1930 | 1250–1700 | 1130–1590 | 1250–2150 |
| Mileage before test (km) | 15131–74204 | 1363–49810 | 358–16067 | 8053–38593 | 1586–53814 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hata, H.; Okada, M.; Funakubo, C.; Hoshi, J. Tailpipe VOC Emissions from Late Model Gasoline Passenger Vehicles in the Japanese Market. Atmosphere 2019, 10, 621. https://doi.org/10.3390/atmos10100621
Hata H, Okada M, Funakubo C, Hoshi J. Tailpipe VOC Emissions from Late Model Gasoline Passenger Vehicles in the Japanese Market. Atmosphere. 2019; 10(10):621. https://doi.org/10.3390/atmos10100621
Chicago/Turabian StyleHata, Hiroo, Megumi Okada, Chikage Funakubo, and Junya Hoshi. 2019. "Tailpipe VOC Emissions from Late Model Gasoline Passenger Vehicles in the Japanese Market" Atmosphere 10, no. 10: 621. https://doi.org/10.3390/atmos10100621






