Complement Factor C3 Methylation and mRNA Expression Is Associated to BMI and Insulin Resistance in Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. RNA Isolation and Gene Expression
2.2. Methylation Analysis DNA
2.3. Statistical Analysis
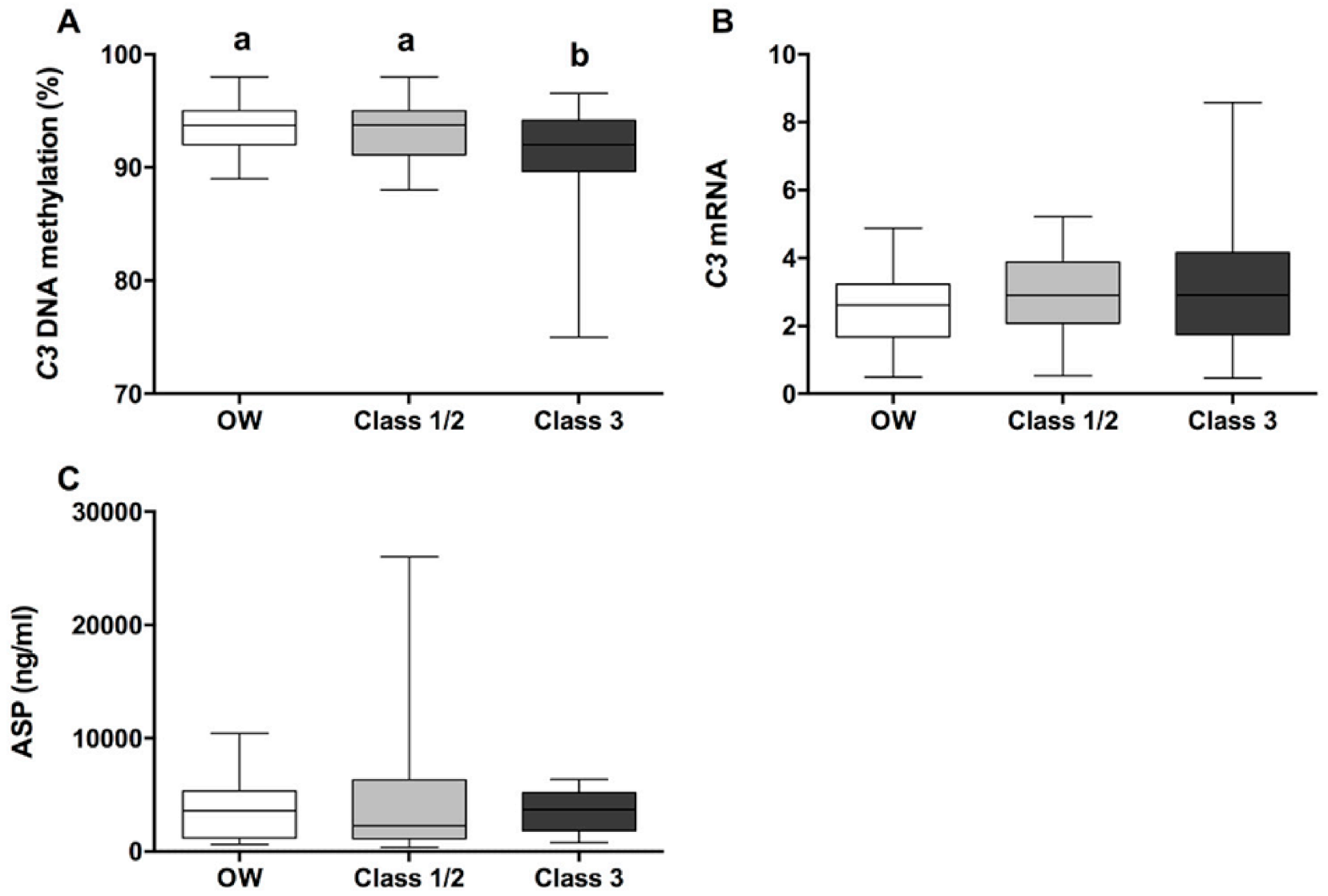
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [CrossRef] [PubMed]
- Barbu, A.; Hamad, O.A.; Lind, L.; Ekdahl, K.N.; Nilsson, B. The role of complement factor C3 in lipid metabolism. Mol. Immunol. 2015, 67, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hertle, E.; Stehouwer, C.D.; van Greevenbroek, M.M. The complement system in human cardiometabolic disease. Mol. Immunol. 2014, 61, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Choy, L.N.; Rosen, B.S.; Spiegelman, B.M. Adipsin and an endogenous pathway of complement from adipose cells. J. Boil. Chem. 1992, 267, 12736–12741. [Google Scholar]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, J.A.; Peake, P.W.; Campbell, L.V.; Pussell, B.A.; O’Grady, S.; Tzilopoulos, T. The influence of oral lipid loads on acylation stimulating protein (ASP) in healthy volunteers. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Faraj, M.; Jones, P.; Sniderman, A.D.; Cianflone, K. Enhanced dietary fat clearance in postobese women. J. Lipid Res. 2001, 42, 571–580. [Google Scholar] [PubMed]
- Scantlebury, T.; Maslowska, M.; Cianflone, K. Chylomicron-specific enhancement of acylation stimulating protein and precursor protein C3 production in differentiated human adipocytes. J. Boil. Chem. 1998, 273, 20903–20909. [Google Scholar] [CrossRef]
- Fisette, A.; Lapointe, M.; Cianflone, K. Obesity-inducing diet promotes acylation stimulating protein resistance. Biochem. Biophys. Res. Commun. 2013, 437, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Onat, A.; Can, G.; Rezvani, R.; Cianflone, K. Complement C3 and cleavage products in cardiometabolic risk. Clin. Chim. Acta 2011, 412, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Wlazlo, N.; van Greevenbroek, M.M.; Ferreira, I.; Feskens, E.J.; van der Kallen, C.J.; Schalkwijk, C.G.; Bravenboer, B.; Stehouwer, C.D. Complement factor 3 is associated with insulin resistance and with incident type 2 diabetes over a 7-year follow-up period: The codam study. Diabetes Care 2014, 37, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Dick, K.J.; Nelson, C.P.; Tsaprouni, L.; Sandling, J.K.; Aissi, D.; Wahl, S.; Meduri, E.; Morange, P.E.; Gagnon, F.; Grallert, H.; et al. DNA methylation and body-mass index: A genome-wide analysis. Lancet 2014, 383, 1990–1998. [Google Scholar] [CrossRef]
- Castellano-Castillo, D.; Moreno-Indias, I.; Fernandez-Garcia, J.C.; Alcaide-Torres, J.; Moreno-Santos, I.; Ocana, L.; Gluckman, E.; Tinahones, F.; Queipo-Ortuno, M.I.; Cardona, F. Adipose tissue LPL methylation is associated with triglyceride concentrations in the metabolic syndrome. Clin. Chem. 2018, 64, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Alegría-Torres, J.A.; Baccarelli, A.; Bollati, V. Epigenetics and lifestyle. Epigenomics 2011, 3, 267–277. [Google Scholar]
- He, Z.; Zhang, R.; Jiang, F.; Hou, W.; Hu, C. Role of genetic and environmental factors in DNA methylation of lipid metabolism. Genes Dis. 2018, 5, 9–15. [Google Scholar] [CrossRef]
- Barajas-Olmos, F.; Centeno-Cruz, F.; Zerrweck, C.; Imaz-Rosshandler, I.; Martínez-Hernández, A.; Cordova, E.J.; Rangel-Escareño, C.; Gálvez, F.; Castillo, A.; Maydón, H.; et al. Altered DNA methylation in liver and adipose tissues derived from individuals with obesity and type 2 diabetes. BMC Med. Genet. 2018, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Bollepalli, S.; Kaye, S.; Heinonen, S.; Kaprio, J.; Rissanen, A.; Virtanen, K.A.; Pietilainen, K.H.; Ollikainen, M. Subcutaneous adipose tissue gene expression and DNA methylation respond to both short- and long-term weight loss. Int. J. Obes. 2018, 42, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Oliva-Olivera, W.; Omiste, A.; Castellano-Castillo, D.; Lhamyani, S.; Camargo, A.; Tinahones, F.J. Adipose tissue infiltration in normal-weight subjects and its impact on metabolic function. Transl. Res. 2016, 172, 6–17.e13. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Postigo, M.; Queipo-Ortuno, M.I.; Fernandez-Garcia, D.; Gomez-Huelgas, R.; Tinahones, F.J.; Cardona, F. Adipose tissue gene expression of factors related to lipid processing in obesity. PLoS ONE 2011, 6, e24783. [Google Scholar] [CrossRef] [PubMed]
- Muscari, A.; Antonelli, S.; Bianchi, G.; Cavrini, G.; Dapporto, S.; Ligabue, A.; Ludovico, C.; Magalotti, D.; Poggiopollini, G.; Zoli, M. Serum C3 is a stronger inflammatory marker of insulin resistance than c-reactive protein, leukocyte count, and erythrocyte sedimentation rate: Comparison study in an elderly population. Diabetes Care 2007, 30, 2362–2368. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rezvani, R.; Lapointe, M.; Poursharifi, P.; Marceau, P.; Tiwari, S.; Tchernof, A.; Cianflone, K. Downregulation of complement C3 and C3aR expression in subcutaneous adipose tissue in obese women. PLoS ONE 2014, 9, e95478. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsson, B.G.; Johansson, J.M.; Lonn, M.; Jernas, M.; Olbers, T.; Peltonen, M.; Larsson, I.; Lonn, L.; Sjostrom, L.; Carlsson, B.; et al. High expression of complement components in omental adipose tissue in obese men. Obes. Res. 2003, 11, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Kalant, D.; Phelis, S.; Fielding, B.A.; Frayn, K.N.; Cianflone, K.; Sniderman, A.D. Increased postprandial fatty acid trapping in subcutaneous adipose tissue in obese women. J. Lipid Res. 2000, 41, 1963–1968. [Google Scholar] [PubMed]
- Paglialunga, S.; Julien, P.; Tahiri, Y.; Cadelis, F.; Bergeron, J.; Gaudet, D.; Cianflone, K. Lipoprotein lipase deficiency is associated with elevated acylation stimulating protein plasma levels. J. Lipid Res. 2009, 50, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Saleh, J.; Summers, L.K.; Cianflone, K.; Fielding, B.A.; Sniderman, A.D.; Frayn, K.N. Coordinated release of acylation stimulating protein (ASP) and triacylglycerol clearance by human adipose tissue in vivo in the postprandial period. J. Lipid Res. 1998, 39, 884–891. [Google Scholar] [PubMed]
- Barbarroja, N.; Lopez-Pedrera, R.; Mayas, M.D.; Garcia-Fuentes, E.; Garrido-Sanchez, L.; Macias-Gonzalez, M.; El Bekay, R.; Vidal-Puig, A.; Tinahones, F.J. The obese healthy paradox: Is inflammation the answer? Biochem. J. 2010, 430, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Yudkin, J.S.; Stehouwer, C.D.; Emeis, J.J.; Coppack, S.W. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arter. Thromb. Vasc. Biol. 1999, 19, 972–978. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Spiegelman, B.M. Tumor necrosis factor α: A key component of the obesity-diabetes link. Diabetes 1994, 43, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Tataranni, P.A.; Pratley, R.E. Insulin action and insulinemia are closely related to the fasting complement C3, but not acylation stimulating protein concentration. Diabetes Care 2000, 23, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Van Greevenbroek, M.M.J.; Ghosh, S.; van der Kallen, C.J.H.; Brouwers, M.C.G.J.; Schalkwijk, C.G.; Stehouwer, C.D.A. Up-regulation of the complement system in subcutaneous adipocytes from nonobese, hypertriglyceridemic subjects is associated with adipocyte insulin resistance. J. Clin. Endocrinol. Metab. 2012, 97, 4742–4752. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.J.; Morrell, C.N.; Bao, C.; Swaim, A.F.; Rodriguez, A.; Lowenstein, C.J. A novel anti-inflammatory effect for high density lipoprotein. PLoS ONE 2015, 10, e0144372. [Google Scholar] [CrossRef] [PubMed]
- Mawlood, S.K.; Dennany, L.; Watson, N.; Dempster, J.; Pickard, B.S. Quantification of global mitochondrial DNA methylation levels and inverse correlation with age at two CpG sites. Aging 2016, 8, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V.L.; Smith, R.A.; Ma, S.; Cutler, R.G. Genomic 5-methyldeoxycytidine decreases with age. J. Boil. Chem. 1987, 262, 9948–9951. [Google Scholar]
- Donkin, I.; Versteyhe, S.; Ingerslev, L.R.; Qian, K.; Mechta, M.; Nordkap, L.; Mortensen, B.; Appel, E.V.R.; Jørgensen, N.; Kristiansen, V.B.; et al. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab. 2016, 23, 369–378. [Google Scholar] [CrossRef] [PubMed]
| Overweight (n = 23) | Class 1/2 (n = 20) | Class 3 (n = 17) | |
|---|---|---|---|
| Age (years) | 55.70 ± 11.71 a | 56.70 ± 15.24 a | 41.53 ± 9.78 b |
| Gender (men/women) | 10/13 | 7/13 | 6/11 |
| BMI (kg/m2) | 27.41 ± 1.29 a | 33.23 ± 2.76 b | 49.78 ± 6.49 c |
| Waist (cm) | 93.96 ± 5.48 a | 106.75 ± 9.23 b | 133.69 ± 17.77 c |
| Glucose (mmol/L) | 6.12 ± 1.20 | 5.99 ± 1.47 | 5.79 ± 1.15 |
| HOMA-IR | 2.318 ± 0.98 a | 3.124 ± 1.59 a | 5.30 ± 4.91 b |
| Tg (mmol/L) | 1.42 ± 0.60 | 1.47 ± 0.54 | 1.37 ± 0.50 |
| Cholesterol (mmol/L) | 5.58 ± 1.00 a | 5.48 ± 1.28 a | 4.67 ± 0.94 b |
| HDL-cho (mmol/L) | 1.37 ± 0.34 a,b | 1.49 ± 0.32 a | 1.26 ± 0.32 b |
| SBP (mmHg) | 131.43 ± 22.37 | 135.65 ± 24.83 | 136.07 ± 19.39 |
| DBP (mmHg) | 79.52 ± 12.62 | 79.75 ± 12.78 | 83.79 ± 10.03 |
| ApoA1 (mmol/L) | 1.73 ± 0.20 | 1.81 ± 0.27 | 1.58 ± 0.15 |
| ApB (mmol/L) | 1.10 ± 0.27 | 1.01 ± 0.21 | 0.89 ± 0.28 |
| GOT (µkat/L) | 0.25 ± 0.12 a | 0.34 ± 0.18 a,b | 0.39 ± 0.16 b |
| GPT (µkat/L) | 0.59 ± 0.24 | 0.75 ± 0.37 | 0.75 ± 0.32 |
| GGT (µkat/L) | 0.63 ± 0.50 | 0.61 ± 0.29 | 0.55 ± 0.38 |
| Leptin (ng/mL) | 14.31 ± 7.13 a | 21.83 ± 11.12 a | 68.21 ± 30.09 b |
| Adiponectin (ng/mL) | 9.21 ± 3.92 | 10.49 ± 4.64 | 6.90 ± 3.83 |
| C3 mRNA | C3 Methylation | ASP | |
|---|---|---|---|
| Age | 0.076 | 0.226 | 0.035 |
| BMI | 0.177 | −0.411 ** | −0.04 |
| Waist | 0.192 | −0.26 | 0.062 |
| Insulin | 0.364 ** | −0.079 | 0.157 |
| Glucose | 0.324 * | 0.09 | −0.102 |
| HOMA-IR | 0.417 ** | −0.079 | 0.124 |
| HDL-cho | 0.073 | −0.176 | −0.370 ** |
| Leptin | 0.289 | −0.528 ** | −0.077 |
| Adiponectin | −0.316 * | 0.005 | −0.071 |
| A | BMI (R = 0.69, R2 = 0.48) | ||
| β | p | CI (95%) | |
| Age | −0.28 | 0.00 | −0.43–(−0.13) |
| Gender | 0.48 | 0.81 | −3.76–4.73 |
| HOMA-IR | 1.12 | 0.00 | 0.38–1.87 |
| C3 mRNA | 0.34 | 0.65 | −1.17–1.86 |
| C3 methylation | −0.79 | 0.00 | −1.35–(−0.23) |
| B | HOMA-IR (R = 0.59, R2 = 0.35) | ||
| β | p | CI (95%) | |
| Age | −0.00 | 0.96 | −0.06–0.05 |
| Gender | −0.71 | 0.32 | −2.14–0.72 |
| BMI | 0.13 | 0.00 | 0.04–0.21 |
| C3 mRNA | 0.74 | 0.00 | 0.26–1.22 |
| C3 methylation | 0.07 | 0.44 | −0.12–0.28 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellano-Castillo, D.; Moreno-Indias, I.; Fernandez-Garcia, J.C.; Clemente-Postigo, M.; Castro-Cabezas, M.; Tinahones, F.J.; Queipo-Ortuño, M.I.; Cardona, F. Complement Factor C3 Methylation and mRNA Expression Is Associated to BMI and Insulin Resistance in Obesity. Genes 2018, 9, 410. https://doi.org/10.3390/genes9080410
Castellano-Castillo D, Moreno-Indias I, Fernandez-Garcia JC, Clemente-Postigo M, Castro-Cabezas M, Tinahones FJ, Queipo-Ortuño MI, Cardona F. Complement Factor C3 Methylation and mRNA Expression Is Associated to BMI and Insulin Resistance in Obesity. Genes. 2018; 9(8):410. https://doi.org/10.3390/genes9080410
Chicago/Turabian StyleCastellano-Castillo, Daniel, Isabel Moreno-Indias, Jose Carlos Fernandez-Garcia, Mercedes Clemente-Postigo, Manuel Castro-Cabezas, Francisco José Tinahones, María Isabel Queipo-Ortuño, and Fernando Cardona. 2018. "Complement Factor C3 Methylation and mRNA Expression Is Associated to BMI and Insulin Resistance in Obesity" Genes 9, no. 8: 410. https://doi.org/10.3390/genes9080410
APA StyleCastellano-Castillo, D., Moreno-Indias, I., Fernandez-Garcia, J. C., Clemente-Postigo, M., Castro-Cabezas, M., Tinahones, F. J., Queipo-Ortuño, M. I., & Cardona, F. (2018). Complement Factor C3 Methylation and mRNA Expression Is Associated to BMI and Insulin Resistance in Obesity. Genes, 9(8), 410. https://doi.org/10.3390/genes9080410






