Coping with Reactive Oxygen Species to Ensure Genome Stability in Escherichia coli
Abstract
1. Introduction
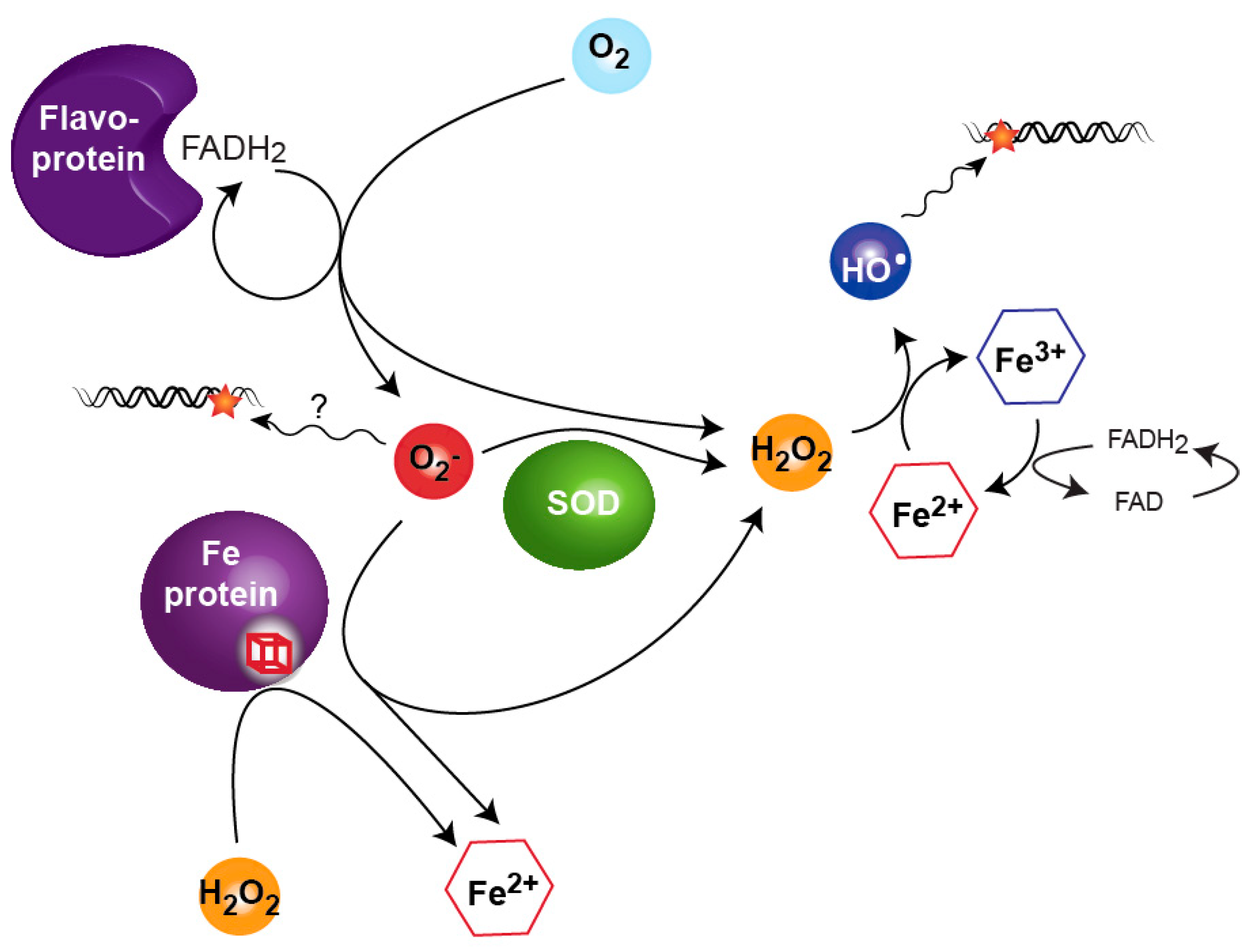
2. Sources of Reactive Oxygen Species
3. Sensing Reactive Oxygen Species
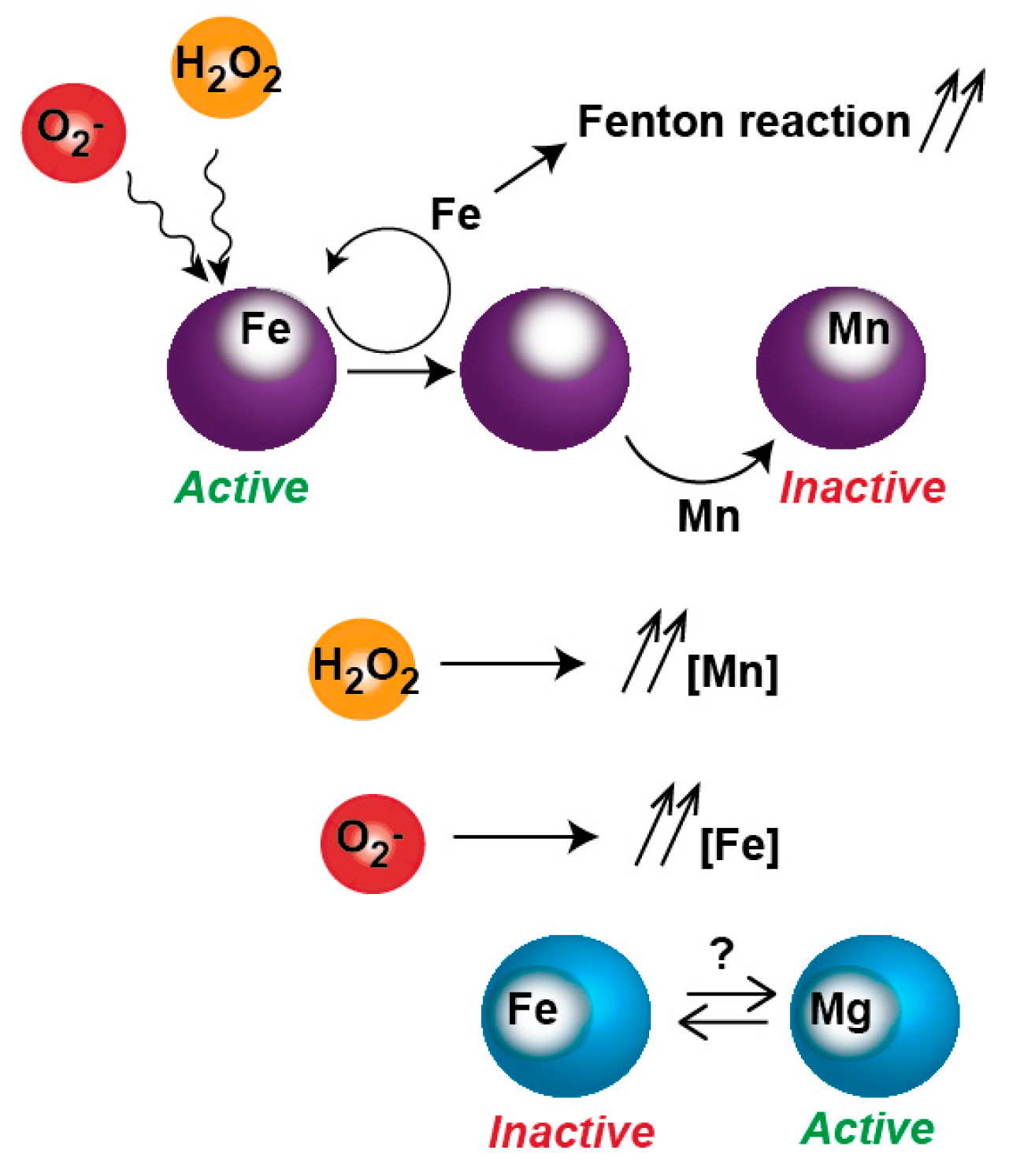
4. A Metal-Poisoning Disease
5. Responding to Oxygen
6. Repair of DNA Lesions by Base Excision Repair Pathway
7. Toxic Repair
8. Future Prospect
Funding
Acknowledgments
Conflicts of Interest
References
- Cooper, S.; Helmstetter, C.E. Chromosome replication and the division cycle of Escherichia coli B/r. J. Mol. Biol. 1968, 31, 519–540. [Google Scholar] [CrossRef]
- Michelsen, O.; Teixeira de Mattos, M.J.; Jensen, P.R.; Hansen, F.G. Precise determinations of C and D periods by flow cytometry in Escherichia coli K-12 and B/r. Microbiology 2003, 149, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Donachie, W.D. Relationship between cell size and time of initiation of DNA replication. Nature 1968, 219, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Lobner-Olesen, A.; Skarstad, K.; Hansen, F.G.; von Meyenburg, K.; Boye, E. The DnaA protein determines the initiation mass of Escherichia coli K-12. Cell 1989, 57, 881–889. [Google Scholar] [CrossRef]
- Katayama, T.; Kasho, K.; Kawakami, H. The DnaA cycle in Escherichia coli: Activation, function and inactivation of the initiator protein. Front. Microbiol. 2017, 8, 2496. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.C.; Grimwade, J.E. The orisome: Structure and function. Front. Microbiol. 2015, 6, 545. [Google Scholar] [CrossRef] [PubMed]
- Riber, L.; Frimodt-Møller, J.; Charbon, G.; Løbner-Olesen, A. Multiple DNA binding proteins contribute to timing of chromosome replication in E. coli. Front. Mol. Biosci. 2016, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Skarstad, K.; Katayama, T. Regulating DNA replication in bacteria. Cold Spring Harb. Perspect. Biol. 2013, 5, a012922. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.A.; Foti, J.J.; Cohen, S.E.; Walker, G.C. The SOS Regulatory Network. EcoSal. Plus 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Charbon, G.; Bjørn, L.; Mendoza-Chamizo, B.; Frimodt-Møller, J.; Løbner-Olesen, A. Oxidative DNA damage is instrumental in hyperreplication stress-induced inviability of Escherichia coli. Nucleic Acids Res. 2014, 42, 13228–13241. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.A.; Breier, A.M.; Cozzarelli, N.R.; Kaguni, J.M. Hyperinitiation of DNA replication in Escherichia coli leads to replication fork collapse and inviability. Mol. Microbiol. 2004, 51, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Bidnenko, V.; Ehrlich, S.D.; Michel, B. Replication fork collapse at replication terminator sequences. EMBO J. 2002, 21, 3898–3907. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Hill, T.M. Insertion of inverted Ter sites into the terminus region of the Escherichia coli chromosome delays completion of DNA replication and disrupts the cell cycle. Mol. Microbiol. 1995, 18, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K.; Durand, A.; Desfontaines, J.M.; Iurchenko, I.; Auger, H.; Leach, D.R.F.; Barre, F.X.; Michel, B. Division-induced DNA double strand breaks in the chromosome terminus region of Escherichia coli lacking RecBCD DNA repair enzyme. PLoS Genet. 2017, 13, e1006895. [Google Scholar] [CrossRef] [PubMed]
- Atlung, T.; Hansen, F.G. Three distinct chromosome replication states are induced by increasing concentrations of DnaA protein in Escherichia coli. J. Bacteriol. 1993, 175, 6537–6545. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, K.M.; Gomez, R.F.; Sinskey, A.J. Ionizing radiation damage to the folded chromosome of Escherichia coli K-12: Repair of double-strand breaks in deoxyribonucleic acid. J. Bacteriol. 1979, 138, 486–491. [Google Scholar] [PubMed]
- Krisch, R.E.; Krasin, F.; Sauri, C.J. DNA breakage, repair and lethality after 125I decay in rec+ and recA strains of Escherichia coli. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1976, 29, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Boveris, A.; Valdez, L.B.; Zaobornyj, T.; Bustamante, J. Mitochondrial metabolic states regulate nitric oxide and hydrogen peroxide diffusion to the cytosol. Biochim. Biophys. Acta 2006, 1757, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. The biological chemistry of hydrogen peroxide. Methods Enzymol. 2013, 528, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Pridmore, R.D.; Pittet, A.C.; Praplan, F.; Cavadini, C. Hydrogen peroxide production by Lactobacillus johnsonii NCC 533 and its role in anti-Salmonella activity. FEMS Microbiol. Lett. 2008, 283, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Whittenbury, R. Hydrogen peroxide formation and catalase activity in the lactic acid bacteria. J. Gen. Microbiol. 1964, 35, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005, 23, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Mavrodi, D.V.; Blankenfeldt, W.; Thomashow, L.S. Phenazine compounds in fluorescent Pseudomonas spp. biosynthesis and regulation. Annu. Rev. Phytopathol. 2006, 44, 417–445. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Fridovich, I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch. Biochem. Biophys. 1979, 196, 385–395. [Google Scholar] [CrossRef]
- Bus, J.S.; Gibson, J.E. Paraquat: Model for oxidant-initiated toxicity. Environ. Health Perspect. 1984, 55, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Seaver, L.C.; Imlay, J.A. Are respiratory enzymes the primary sources of intracellular hydrogen peroxide? J. Biol. Chem. 2004, 279, 48742–48750. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; You, X.; Imlay, J.A. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx- mutants of Escherichia coli. Proc. Natl. Acad. Sci. USA 2005, 102, 9317–9322. [Google Scholar] [CrossRef] [PubMed]
- Touati, D.; Jacques, M.; Tardat, B.; Bouchard, L.; Despied, S. Lethal oxidative damage and mutagenesis are generated by iron in Δfur mutants of Escherichia coli: Protective role of superoxide dismutase. J. Bacteriol. 1995, 177, 2305–2314. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Transcription factors that defend bacteria against reactive oxygen species. Annu. Rev. Microbiol. 2015, 69, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Baez, A.; Shiloach, J. Escherichia coli avoids high dissolved oxygen stress by activation of SoxRS and manganese-superoxide dismutase. Microb. Cell Fact. 2013, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Pomposiello, P.J.; Bennik, M.H.; Demple, B. Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. J. Bacteriol. 2001, 183, 3890–3902. [Google Scholar] [CrossRef] [PubMed]
- Nunoshiba, T.; Obata, F.; Boss, A.C.; Oikawa, S.; Mori, T.; Kawanishi, S.; Yamamoto, K. Role of iron and superoxide for generation of hydroxyl radical, oxidative DNA lesions, and mutagenesis in Escherichia coli. J. Biol. Chem. 1999, 274, 34832–34837. [Google Scholar] [CrossRef] [PubMed]
- Maringanti, S.; Imlay, J.A. An intracellular iron chelator pleiotropically suppresses enzymatic and growth defects of superoxide dismutase-deficient Escherichia coli. J. Bacteriol. 1999, 181, 3792–3802. [Google Scholar] [PubMed]
- Varghese, S.; Wu, A.; Park, S.; Imlay, K.R.; Imlay, J.A. Submicromolar hydrogen peroxide disrupts the ability of Fur protein to control free-iron levels in Escherichia coli. Mol. Microbiol. 2007, 64, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Almirón, M.; Link, A.J.; Furlong, D.; Kolter, R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 1992, 6, 2646–2654. [Google Scholar] [CrossRef] [PubMed]
- Altuvia, S.; Almirón, M.; Huisman, G.; Kolter, R.; Storz, G. The dps promoter is activated by OxyR during growth and by IHF and σS in stationary phase. Mol. Microbiol. 1994, 13, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.A.; Filman, D.J.; Finkel, S.E.; Kolter, R.; Hogle, J.M. The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nat. Struct. Biol. 1998, 5, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Chodavarapu, S.; Gomez, R.; Vicente, M.; Kaguni, J.M. Escherichia coli Dps interacts with DnaA protein to impede initiation: A model of adaptive mutation. Mol. Microbiol. 2008, 67, 1331–1346. [Google Scholar] [CrossRef] [PubMed]
- Janissen, R.; Arens, M.M.A.; Vtyurina, N.N.; Rivai, Z.; Sunday, N.D.; Eslami-Mossallam, B.; Gritsenko, A.A.; Laan, L.; de Ridder, D.; Artsimovitch, I.; et al. Global DNA compaction in stationary-phase bacteria does not affect transcription. Cell 2018, 174, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. The mismetallation of enzymes during oxidative stress. J. Biol. Chem. 2014, 289, 28121–28128. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Imlay, J.A. Superoxide poisons mononuclear iron enzymes by causing mismetallation. Mol. Microbiol. 2013, 89, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Imlay, J.A. The alternative aerobic ribonucleotide reductase of Escherichia coli, NrdEF, is a manganese-dependent enzyme that enables cell replication during periods of iron starvation. Mol. Microbiol. 2011, 80, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Keyer, K.; Imlay, J.A. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. USA 1996, 93, 13635–13640. [Google Scholar] [CrossRef] [PubMed]
- Khademian, M.; Imlay, J.A. Escherichia coli cytochrome c peroxidase is a respiratory oxidase that enables the use of hydrogen peroxide as a terminal electron acceptor. Proc. Natl. Acad. Sci. USA 2017, 114, E6922–E6931. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, S.; Yu, Y.; Pinkse, M.; Hoeser, J.; Friedrich, T.; Bald, D.; de Vries, S. Cytochrome bd displays significant quinol peroxidase activity. Sci. Rep. 2016, 6, 27631. [Google Scholar] [CrossRef] [PubMed]
- Park, D.M.; Akhtar, M.S.; Ansari, A.Z.; Landick, R.; Kiley, P.J. The bacterial response regulator ArcA uses a diverse binding site architecture to regulate carbon oxidation globally. PLoS Genet. 2013, 9, e1003839. [Google Scholar] [CrossRef] [PubMed]
- Federowicz, S.; Kim, D.; Ebrahim, A.; Lerman, J.; Nagarajan, H.; Cho, B.K.; Zengler, K.; Palsson, B. Determining the control circuitry of redox metabolism at the genome-scale. PLoS Genet. 2014, 10, e1004264. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.W.; Kim, D.; Latif, H.; O’Brien, E.J.; Szubin, R.; Palsson, B.O. Deciphering Fur transcriptional regulatory network highlights its complex role beyond iron metabolism in Escherichia coli. Nat. Commun. 2014, 5, 4910. [Google Scholar] [CrossRef] [PubMed]
- Boiteux, S.; Coste, F.; Castaing, B. Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases. Free Radic. Biol. Med. 2017, 107, 179–201. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.L.; Jakob, B.; Taucher-Scholz, G.; Dianov, G.L.; Becherel, O.J.; Lavin, M.F. Aprataxin, poly-ADP ribose polymerase 1 (PARP-1) and apurinic endonuclease 1 (APE1) function together to protect the genome against oxidative damage. Hum. Mol. Genet. 2009, 18, 4102–4117. [Google Scholar] [CrossRef] [PubMed]
- Freudenthal, B.D.; Beard, W.A.; Perera, L.; Shock, D.D.; Kim, T.; Schlick, T.; Wilson, S.H. Uncovering the polymerase-induced cytotoxicity of an oxidized nucleotide. Nature 2015, 517, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.C.; Walker, G.C. Incomplete base excision repair contributes to cell death from antibiotics and other stresses. DNA Repair (Amst.) 2018, 71, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Hazra, T.K.; Izumi, T.; Venkataraman, R.; Kow, Y.W.; Dizdaroglu, M.; Mitra, S. Characterization of a novel 8-oxoguanine-DNA glycosylase activity in Escherichia coli and identification of the enzyme as endonuclease VIII. J. Biol. Chem. 2000, 275, 27762–27767. [Google Scholar] [CrossRef] [PubMed]
- Blaisdell, J.O.; Hatahet, Z.; Wallace, S.S. A novel role for Escherichia coli endonuclease VIII in prevention of spontaneous G-->T transversions. J. Bacteriol. 1999, 181, 6396–6402. [Google Scholar] [PubMed]
- Lee, A.J.; Wallace, S.S. Hide and seek: How do DNA glycosylases locate oxidatively damaged DNA bases amidst a sea of undamaged bases? Free Radic. Biol. Med. 2017, 107, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Michaels, M.L.; Miller, J.H. The GO system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine). J. Bacteriol. 1992, 174, 6321–6325. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.L.; Lee, H.; Popodi, E.; Townes, J.P.; Tang, H. Determinants of spontaneous mutation in the bacterium Escherichia coli as revealed by whole-genome sequencing. Proc. Natl. Acad. Sci. USA 2015, 112, E5990–E5999. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.H. Spontaneous mutators in bacteria: Insights into pathways of mutagenesis and repair. Annu. Rev. Microbiol. 1996, 50, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, T.; Maki, H.; Sekiguchi, M. Functional cooperation of MutT, MutM and MutY proteins in preventing mutations caused by spontaneous oxidation of guanine nucleotide in Escherichia coli. Mutat. Res. 1995, 336, 257–267. [Google Scholar] [CrossRef]
- Sakai, A.; Nakanishi, M.; Yoshiyama, K.; Maki, H. Impact of reactive oxygen species on spontaneous mutagenesis in Escherichia coli. Genes Cells 2006, 11, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Laguna, J.; Pueyo, C. Hydrogen peroxide and coffee induce G:C-->T:A transversions in the lacI gene of catalase-defective Escherichia coli. Mutagenesis 1999, 14, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Mol, C.D.; Hosfield, D.J.; Tainer, J.A. Abasic site recognition by two apurinic/apyrimidinic endonuclease families in DNA base excision repair: The 3’ ends justify the means. Mutat. Res. 2000, 460, 211–229. [Google Scholar] [CrossRef]
- Chan, E.; Weiss, B. Endonuclease IV of Escherichia coli is induced by paraquat. Proc. Natl. Acad. Sci. USA 1987, 84, 3189–3193. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G. Collaborative effects of Photobacterium CuZn superoxide dismutase (SODs) and human AP endonuclease in DNA repair and SOD-deficient Escherichia coli under oxidative stress. Free Radic. Biol. Med. 2004, 36, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Bodine, T.J.; Evangelista, M.A.; Chang, H.T.; Ayoub, C.A.; Samuel, B.S.; Sucgang, R.; Zechiedrich, L. Escherichia coli DNA ligase B may mitigate damage from oxidative stress. PLoS ONE 2017, 12, e0180800. [Google Scholar] [CrossRef] [PubMed]
- Ananthaswamy, H.N.; Eisenstark, A. Repair of hydrogen peroxide-induced single-strand breaks in Escherichia coli deoxyribonucleic acid. J. Bacteriol. 1977, 130, 187–191. [Google Scholar] [PubMed]
- Yonei, S.; Noda, A.; Tachibana, A.; Akasaka, S. Mutagenic and cytotoxic effects of oxygen free radicals generated by methylviologen (paraquat) on Escherichia coli with different DNA-repair capacities. Mutat. Res. 1986, 163, 15–22. [Google Scholar] [CrossRef]
- Posnick, L.M.; Samson, L.D. Imbalanced base excision repair increases spontaneous mutation and alkylation sensitivity in Escherichia coli. J. Bacteriol. 1999, 181, 6763–6771. [Google Scholar] [PubMed]
- Wyatt, M.D.; Allan, J.M.; Lau, A.Y.; Ellenberger, T.E.; Samson, L.D. 3-methyladenine DNA glycosylases: Structure, function, and biological importance. Bioessays 1999, 21, 668–676. [Google Scholar] [CrossRef]
- Nowosielska, A.; Smith, S.A.; Engelward, B.P.; Marinus, M.G. Homologous recombination prevents methylation-induced toxicity in Escherichia coli. Nucleic Acids Res. 2006, 34, 2258–2268. [Google Scholar] [CrossRef] [PubMed]
- Blaisdell, J.O.; Wallace, S.S. Abortive base-excision repair of radiation-induced clustered DNA lesions in Escherichia coli. Proc. Natl. Acad. Sci. USA 2001, 98, 7426–7430. [Google Scholar] [CrossRef] [PubMed]
- Shevell, D.E.; LeMotte, P.K.; Walker, G.C. Alteration of the carboxyl-terminal domain of Ada protein influences its inducibility, specificity, and strength as a transcriptional activator. J. Bacteriol. 1988, 170, 5263–5271. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Gruber, C.C.; Yang, J.H.; Liu, X.; Braff, D.; Yashaswini, C.N.; Bhubhanil, S.; Furuta, Y.; Andreescu, S.; Collins, J.J.; et al. Lethality of MalE-LacZ hybrid protein shares mechanistic attributes with oxidative component of antibiotic lethality. Proc. Natl. Acad. Sci. USA 2017, 114, 9164–9169. [Google Scholar] [CrossRef] [PubMed]
- Foti, J.J.; Devadoss, B.; Winkler, J.A.; Collins, J.J.; Walker, G.C. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science 2012, 336, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Galhardo, R.S.; Almeida, C.E.; Leitao, A.C.; Cabral-Neto, J.B. Repair of DNA lesions induced by hydrogen peroxide in the presence of iron chelators in Escherichia coli: Participation of endonuclease IV and Fpg. J. Bacteriol. 2000, 182, 1964–1968. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.P.; Weiss, B. Endonuclease III (nth) mutants of Escherichia coli. Proc. Natl. Acad. Sci. USA 1985, 82, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Kuzminov, A. Single-strand interruptions in replicating chromosomes cause double-strand breaks. Proc. Natl. Acad. Sci. USA 2001, 98, 8241–8246. [Google Scholar] [CrossRef] [PubMed]
- Sutera, V.A., Jr.; Lovett, S.T. The role of replication initiation control in promoting survival of replication fork damage. Mol. Microbiol. 2006, 60, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Giroux, X.; Su, W.L.; Bredeche, M.F.; Matic, I. Maladaptive DNA repair is the ultimate contributor to the death of trimethoprim-treated cells under aerobic and anaerobic conditions. Proc. Natl. Acad. Sci. USA 2017, 114, 11512–11517. [Google Scholar] [CrossRef] [PubMed]
- Charbon, G.; Campion, C.; Chan, S.H.; Bjørn, L.; Weimann, A.; da Silva, L.C.; Jensen, P.R.; Løbner-Olesen, A. Re-wiring of energy metabolism promotes viability during hyperreplication stress in E. coli. PLoS Genet. 2017, 13, e1006590. [Google Scholar] [CrossRef] [PubMed]
- Charbon, G.; Riber, L.; Løbner-Olesen, A. Countermeasures to survive excessive chromosome replication in Escherichia coli. Curr. Genet. 2018, 64, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Charbon, G.; Riber, L.; Cohen, M.; Skovgaard, O.; Fujimitsu, K.; Katayama, T.; Løbner-Olesen, A. Suppressors of DnaA(ATP) imposed overinitiation in Escherichia coli. Mol. Microbiol. 2011, 79, 914–928. [Google Scholar] [CrossRef] [PubMed]
- Babu, V.M.; Itsko, M.; Baxter, J.C.; Schaaper, R.M.; Sutton, M.D. Insufficient levels of the nrdAB-encoded ribonucleotide reductase underlie the severe growth defect of the Δhda E. coli strain. Mol. Microbiol. 2017, 104, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Grigorian, A.V.; Lustig, R.B.; Guzman, E.C.; Mahaffy, J.M.; Zyskind, J.W. Escherichia coli cells with increased levels of DnaA and deficient in recombinational repair have decreased viability. J. Bacteriol. 2003, 185, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Charbon, G.; Klitgaard, R.N.; Liboriussen, C.D.; Thulstrup, P.W.; Maffioli, S.I.; Donadio, S.; Løbner-Olesen, A. Iron chelation increases the tolerance of Escherichia coli to hyper-replication stress. Sci. Rep. 2018, 8, 10550. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A.; Chin, S.M.; Linn, S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 1988, 240, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bauer, S.C.; Imlay, J.A. The YaaA protein of the Escherichia coli OxyR regulon lessens hydrogen peroxide toxicity by diminishing the amount of intracellular unincorporated iron. J. Bacteriol. 2011, 193, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.L.; Buettner, G.R.; Britigan, B.E. Endogenous superoxide dismutase levels regulate iron-dependent hydroxyl radical formation in Escherichia coli exposed to hydrogen peroxide. J. Bacteriol. 1998, 180, 622–625. [Google Scholar] [PubMed]
- Woodmansee, A.N.; Imlay, J.A. Reduced flavins promote oxidative DNA damage in non-respiring Escherichia coli by delivering electrons to intracellular free iron. J. Biol. Chem. 2002, 277, 34055–34066. [Google Scholar] [CrossRef] [PubMed]
- Michel, B. After 30 years of study, the bacterial SOS response still surprises us. PLoS Biol. 2005, 3, e255. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, R.P.; Fujii, S. Translesion DNA synthesis and mutagenesis in prokaryotes. Cold Spring Harb. Perspect. Biol. 2013, 5, a012682. [Google Scholar] [CrossRef] [PubMed]
- Gon, S.; Napolitano, R.; Rocha, W.; Coulon, S.; Fuchs, R.P. Increase in dNTP pool size during the DNA damage response plays a key role in spontaneous and induced-mutagenesis in Escherichia coli. Proc. Natl. Acad. Sci. USA 2011, 108, 19311–19316. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.B.; Kogoma, T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 1991, 55, 561–585. [Google Scholar] [PubMed]
- Keyer, K.; Gort, A.S.; Imlay, J.A. Superoxide and the production of oxidative DNA damage. J. Bacteriol. 1995, 177, 6782–6790. [Google Scholar] [CrossRef] [PubMed]
- McNeill, D.R.; Narayana, A.; Wong, H.K.; Wilson, D.M., 3rd. Inhibition of Ape1 nuclease activity by lead, iron, and cadmium. Environ. Health Perspect. 2004, 112, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Seki, T.; Matsumoto, A.; Miura, H.; Sato, E.; Niwano, Y.; Kohno, M.; Omura, S.; Takahashi, Y. Generation of reactive oxygen species from conventional laboratory media. J. Biosci. Bioeng. 2010, 110, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Imlay, J.A. Improved measurements of scant hydrogen peroxide enable experiments that define its threshold of toxicity for Escherichia coli. Free Radic. Biol. Med. 2018, 120, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kawasaki, K.; Daimon, S.; Kitagawa, W.; Yamamoto, K.; Tamaki, H.; Tanaka, M.; Nakatsu, C.H.; Kamagata, Y. A hidden pitfall in the preparation of agar media undermines microorganism cultivability. Appl. Environ. Microbiol. 2014, 80, 7659–7666. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Henry, C.; Herisse, M.; Denamur, E.; Barras, F. Commercial Lysogeny Broth culture media and oxidative stress: A cautious tale. Free Radic. Biol. Med. 2014, 74, 245–251. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Chamizo, B.; Løbner-Olesen, A.; Charbon, G. Coping with Reactive Oxygen Species to Ensure Genome Stability in Escherichia coli. Genes 2018, 9, 565. https://doi.org/10.3390/genes9110565
Mendoza-Chamizo B, Løbner-Olesen A, Charbon G. Coping with Reactive Oxygen Species to Ensure Genome Stability in Escherichia coli. Genes. 2018; 9(11):565. https://doi.org/10.3390/genes9110565
Chicago/Turabian StyleMendoza-Chamizo, Belén, Anders Løbner-Olesen, and Godefroid Charbon. 2018. "Coping with Reactive Oxygen Species to Ensure Genome Stability in Escherichia coli" Genes 9, no. 11: 565. https://doi.org/10.3390/genes9110565
APA StyleMendoza-Chamizo, B., Løbner-Olesen, A., & Charbon, G. (2018). Coping with Reactive Oxygen Species to Ensure Genome Stability in Escherichia coli. Genes, 9(11), 565. https://doi.org/10.3390/genes9110565





