De-Extinction
Abstract
1. Introduction
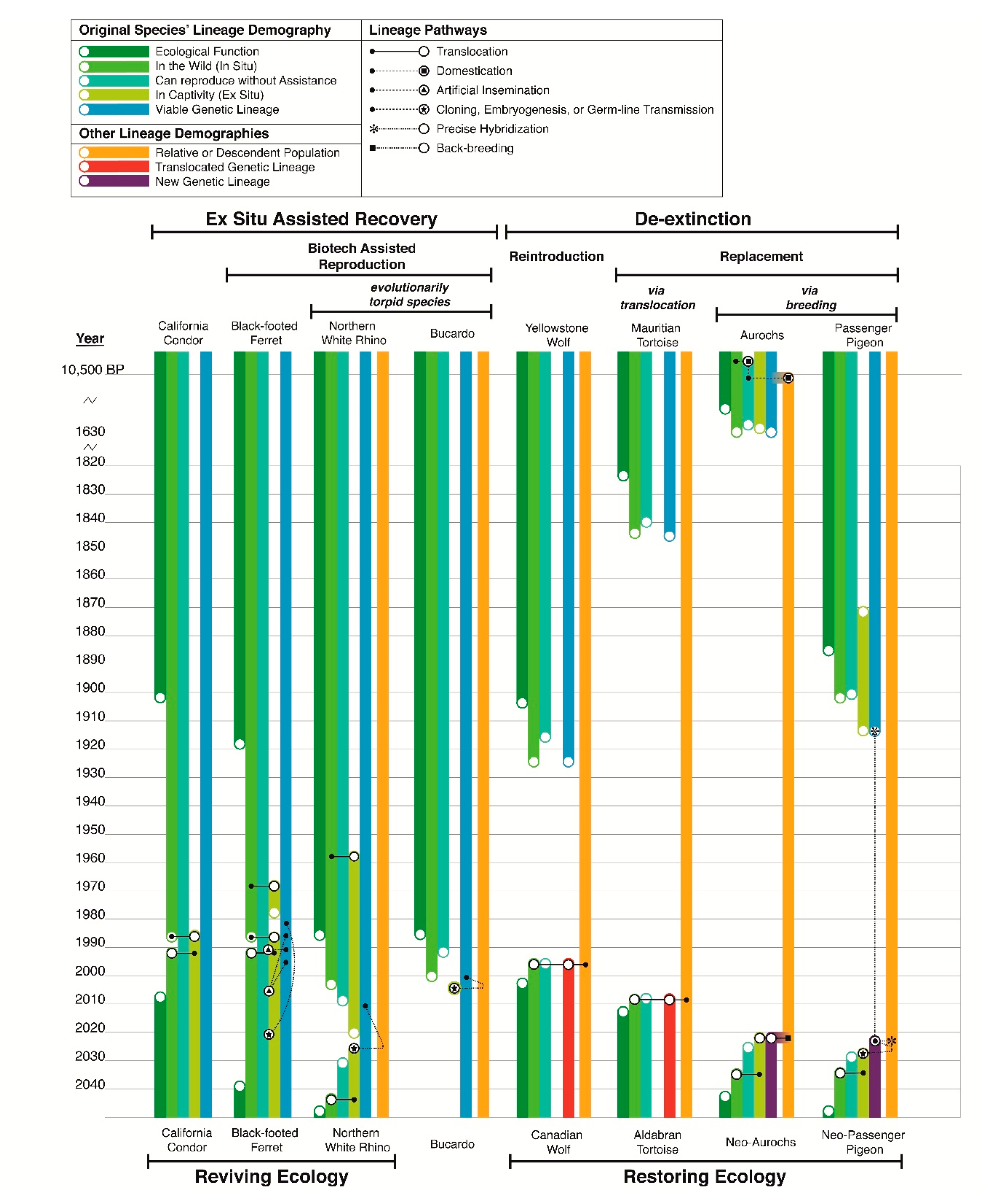
2. Defining De-Extinction: Replacement by Proxy versus Assisted Recovery
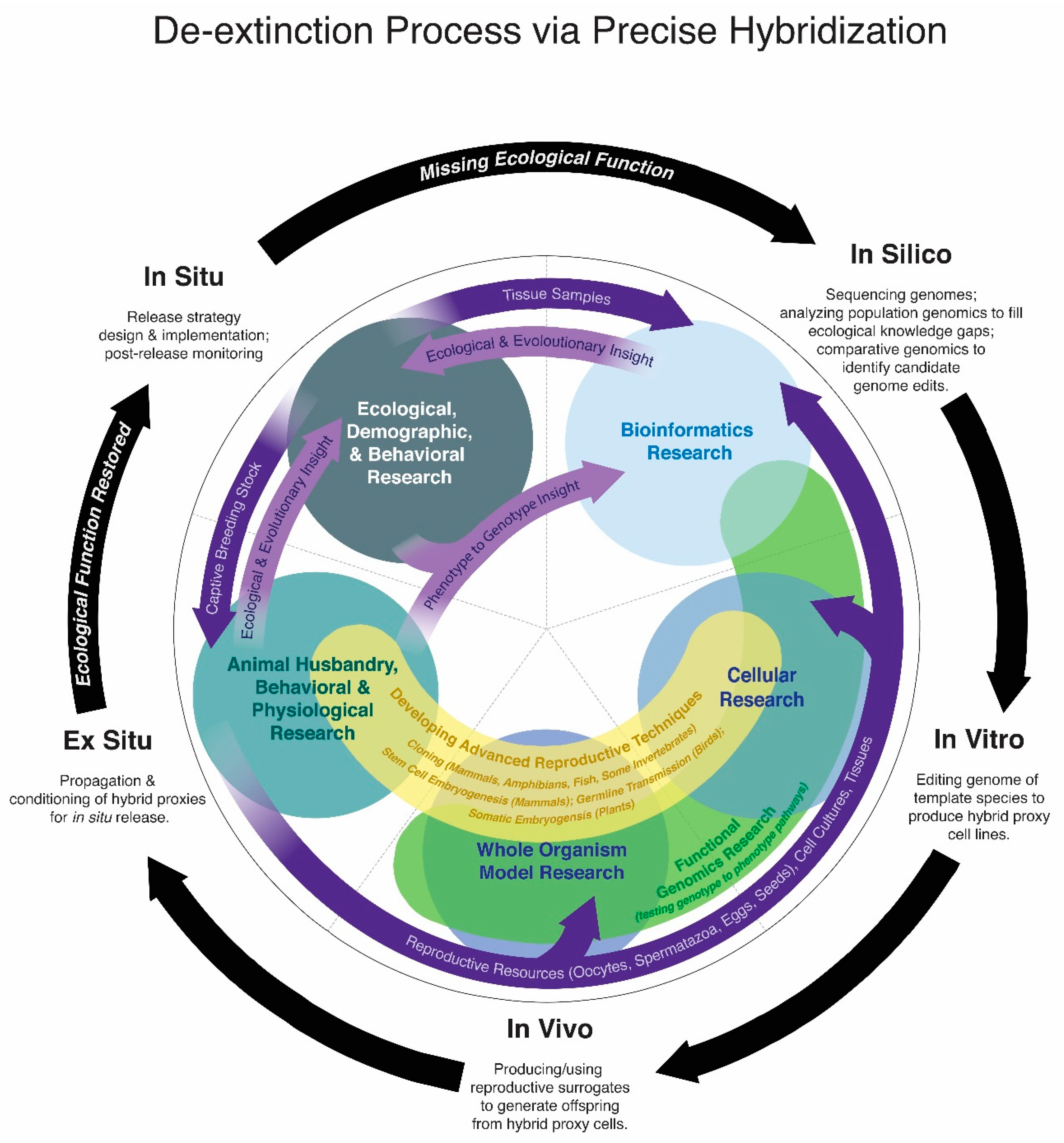
3. Biotechnology Changes the Concept of Extinction
4. Evolutionarily Torpid Species
5. Limitations of De-Extinction via Breeding
6. What Is a Proxy?
7. Prioritizing De-Extinction Candidates
8. Active De-Extinction Breeding Initiatives
9. Criticisms of De-Extinction via Breeding
9.1. De-Extinction Is “New”
9.2. Extant Species Should Be Given Priority
9.3. Recently Extinct Species Are Better De-Extinction Candidates
9.4. De-Extinction Detracts Funds from “Proven” Conservation and Endangered Species
10. Coming to a Consensus: Restoring Centricity to Constructive Dialogue
Funding
Acknowledgments
Conflicts of Interest
References
- TEDxDeExtinction|Revive & Restore. Available online: http://reviverestore.org/events/tedxdeextinction/ (accessed on 16 May 2018).
- Revive & Restore. The Great Passenger Pigeon Comeback. Available online: http://reviverestore.org/about-the-passenger-pigeon/ (accessed on 9 July 2018).
- Church, G.M. Regenesis: How Synthetic Biology Will Reinvent Nature and Ourselves; Basic Books: New York, NY, USA, 2014; ISBN 0465075703. [Google Scholar]
- O’Connor, M.R. Resurrection Science: Conservation, De-Extinction and the Precarious Future of Wild Things; St. Martin’s Press: New York, NY, USA, 2015; ISBN 113727929X. [Google Scholar]
- Biello, D. The Unnatural World: The Race to Remake Civilization in Earth’s Newest Age, 1st ed.; Scribner: New York, NY, USA, 2016; ISBN 1476743908. [Google Scholar]
- Minteer, B.A. The Fall of the Wild: Extinction, De-Extinction, and the Ethics of Conservation; Columbia University Press: New York, NY, USA, 2018; ISBN 023117778X. [Google Scholar]
- Shapiro, B. How to Clone a Mammoth: The Science of De-extinction, 1st ed.; Princeton University Press: Princeton, NJ, USA, 2015; ISBN 9781400865482. [Google Scholar]
- Hirsch, R.E. De-Extinction: The Science of Bringing Lost Species Back to Life; Twenty-First Century Books: Minneapolis, MN, USA, 2017; ISBN 1467794902. [Google Scholar]
- Wray, B. Rise of the Necrofauna: The Science, Ethics, and Risks of De-Extinction; Greystone Books: Vancouver, BC, Canada, 2017; ISBN 1771641649. [Google Scholar]
- Kornfeldt, T. The Re-Origin of Species: A Second Chance for Extinct Animals; English; Scribe US: Melbourne, Australia, 2018; ISBN 194753436X. [Google Scholar]
- Pilcher, H. Bring Back the King: The New Science of De-Extinction; Bloomsbury Sigma: New York, NY, USA, 2017; ISBN 147291225X. [Google Scholar]
- Mezrich, B. Woolly: The True Story of the Quest to Revive One of History’s Most Iconic Extinct Creatures; Atria Books: New York, NY, USA, 2017; ISBN 1501135554. [Google Scholar]
- Lewis, R.P. Passengers Back? Passenger Pigeons; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2015; ISBN 1512036331. [Google Scholar]
- Oksanen, M.; Siipi, H. The Ethics of Animal Re-Creation and Modification: Reviving, Rewilding, Restoring (Palgrave MacMillan Animal Ethics), 2014th ed.; Palgrave Macmillan: Basingstoke, UK, 2014; ISBN 113733763X. [Google Scholar]
- Fletcher, A.L. Mendel’s Ark: Biotechnology and the Future of Extinction, 1st ed.; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-017-9120-5. [Google Scholar]
- Campbell, D.I.; Whittle, P.M. Resurrecting Extinct Species: Ethics and Authenticity, 1st ed.; Palgrave Macmillan: Cham, Switzerland, 2017; ISBN 9783319695778. [Google Scholar]
- Siipi, H.; Finkelman, L. The Extinction and De-Extinction of Species. Philos. Technol. 2017, 30, 427–441. [Google Scholar] [CrossRef]
- Delord, J. The nature of extinction. Stud. Hist. Philos. Sci. Part C Stud. Hist. Philos. Biol. Biomed. Sci. 2007, 38, 656–667. [Google Scholar] [CrossRef] [PubMed]
- IUCN. SSC IUCN SSC Guiding Principles on Creating Proxies of Extinct Species for Conservation Benefit. Version 1.0; IUCN: Gland, Switzerland, 2016. [Google Scholar]
- Kasperbauer, T.J. Should we bring back the passenger pigeon? The ethics of de-extinction. Ethics Policy Environ. 2017, 20, 1–14. [Google Scholar] [CrossRef]
- Evans Ogden, L. Extinction is forever... Or is it? Bioscience 2014, 64, 469–475. [Google Scholar] [CrossRef]
- Minteer, B. The Perils of De-extinction. Minding Nat. 2015, 8, 11–17. [Google Scholar] [CrossRef]
- Shapiro, B. Pathways to de-extinction: How close can we get to resurrection of an extinct species? Funct. Ecol. 2016, 31, 996–1002. [Google Scholar] [CrossRef]
- Folch, J.; Cocero, M.J.; Chesné, P.; Alabart, J.L.; Domínguez, V.; Cognié, Y.; Roche, A.; Fernández-Árias, A.; Martí, J.I.; Sánchez, P.; et al. First birth of an animal from an extinct subspecies (Capra pyrenaica pyrenaica) by cloning. Theriogenology 2009, 71, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, D. Reintroduction and de-extinction. Bioscience 2013, 63, 719–720. [Google Scholar] [CrossRef]
- Seddon, P.J.; Griffiths, C.J.; Soorae, P.S.; Armstrong, D.P. Reversing defaunation: Restoring species in a changing world. Science 2014, 345, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Fritts, S.H.; Bangs, E.E.; Fontaine, J.A.; Johnson, M.R.; Phillips, M.K.; Koch, E.D.; Gunson, J.R. Planning and implementing a reintroduction of wolves to Yellowstone National Park and Central Idaho. Restor. Ecol. 1997, 5, 7–27. [Google Scholar] [CrossRef]
- Seddon, P.J.; Armstrong, D.P.; Maloney, R.F. Developing the science of reintroduction biology. Conserv. Biol. 2007, 21, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.G.; Lynch, C.; Santymire, R.M.; Marinari, P.E.; Wildt, D.E. Recovery of gene diversity using long-term cryopreserved spermatozoa and artificial insemination in the endangered black-footed ferret. Anim. Conserv. 2016, 19, 102–111. [Google Scholar] [CrossRef]
- Wisely, S.M.; Ryder, O.A.; Santymire, R.M.; Engelhardt, J.F.; Novak, B.J. A Road Map for 21st century genetic restoration: Gene pool enrichment of the black-footed ferret. J. Hered. 2015, 106, 581–592. [Google Scholar] [CrossRef] [PubMed]
- White, C.M.; Cade, T.J.; Enderson, J.H. Peregrine Falcons of the World, 1st ed.; Lynx Edicions: Barcelona, Spain, 2013. [Google Scholar]
- Tordoff, H.B.; Redig, P.T. Role of genetic background in the success of reintroduced peregrine falcons. Conserv. Biol. 2001, 15, 528–532. [Google Scholar] [CrossRef]
- Cahill, J.A.; Green, R.E.; Fulton, T.L.; Stiller, M.; Jay, F.; Ovsyanikov, N.; Salamzade, R.; St. John, J.; Stirling, I.; Slatkin, M.; et al. Genomic evidence for island population conversion resolves conflicting theories of polar bear evolution. PLoS Genet. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Tunstall, T.; Kock, R.; Vahala, J.; Diekhans, M.; Fiddes, I.; Armstrong, J.; Paten, B.; Ryder, O.A.; Steiner, C.C. Evaluating recovery potential of the northern white rhinoceros from cryopreserved somatic cells. Genome Res. 2018, 28, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Saragusty, J.; Diecke, S.; Drukker, M.; Durrant, B.; Friedrich Ben-Nun, I.; Galli, C.; Göritz, F.; Hayashi, K.; Hermes, R.; Holtze, S.; et al. Rewinding the process of mammalian extinction. Zoo Biol. 2016, 35, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Korody, M.L.; Pivaroff, C.; Nguyen, T.D.; Peterson, S.E.; Ryder, O.A.; Loring, J.F. Four new induced pluripotent stem cell lines produced from northern white rhinoceros with non-integrating reprogramming factors. bioRxiv 2017, 1–3. [Google Scholar] [CrossRef]
- Archer, M. (University of New South Wales, Sydney, NSW, Australia). Personal communication regarding gastric brooding frog cloning efforts and preserved tissues/cells, 2018.
- Zhou, X.; Sun, F.; Xu, S.; Fan, G.; Zhu, K.; Liu, X.; Chen, Y.; Shi, C.; Yang, Y.; Huang, Z.; et al. Baiji genomes reveal low genetic variability and new insights into secondary aquatic adaptations. Nat. Commun. 2013, 4, 2708. [Google Scholar] [CrossRef] [PubMed]
- Ryder, O.A. (San Diego Zoo Institution for Conservation Research, San Diego, CA, USA). Personal communication regarding preserved cell lines at the San Diego Zoo Global’s Frozen Zoo, 2018.
- Bushell, M. (Bristol Zoological Society, Bristol, UK). Personal communication regarding preserved tissues/cells of Partula faba, 2018.
- Pearce-Kelly, P. (Zoological Society of London, London, UK). Personal communication regarding preserved tissues/cells of Partula turgida, 2018.
- Orlando, L.; Ginolhac, A.; Zhang, G.; Froese, D.; Albrechtsen, A.; Stiller, M.; Schubert, M.; Cappellini, E.; Petersen, B.; Moltke, I.; et al. Recalibrating Equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature 2013, 499, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Arsuaga, J.-L.; de Filippo, C.; Nagel, S.; Aximu-Petri, A.; Nickel, B.; Martínez, I.; Gracia, A.; de Castro, J.M.B.; Carbonell, E.; et al. Nuclear DNA sequences from the Middle Pleistocene Sima de los Huesos hominins. Nature 2016, 531, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Kircher, M.; Gansauge, M.-T.; Li, H.; Racimo, F.; Mallick, S.; Schraiber, J.G.; Jay, F.; Prüfer, K.; de Filippo, C.; et al. A high-coverage genome sequence from an archaic Denisovan individual. Science 2012, 338, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Palkopoulou, E.; Lipson, M.; Mallick, S.; Nielsen, S.; Rohland, N.; Baleka, S.; Karpinski, E.; Ivancevic, A.M.; To, T.-H.; Kortschak, R.D.; et al. A comprehensive genomic history of extinct and living elephants. Proc. Natl. Acad. Sci. USA 2018, 201720554. [Google Scholar] [CrossRef] [PubMed]
- Green, R.E.; Krause, J.; Briggs, A.W.; Maricic, T.; Stenzel, U.; Kircher, M.; Patterson, N.; Li, H.; Zhai, W.; Fritz, M.H.-Y.; et al. A draft sequence of the Neandertal genome. Science 2010, 328, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Barlow, A.; Cahill, J.A.; Hartmann, S.; Theunert, C.; Xenikoudakis, G.; Fortes, G.G.; Paijmans, J.L.A.; Rabeder, G.; Frischauf, C.; Grandal-d’Anglade, A.; et al. Partial genomic survival of cave bears in living brown bears. Nat. Ecol. Evol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Heintzman, P.D.; Zazula, G.D.; Cahill, J.A.; Reyes, A.V.; MacPhee, R.D.E.; Shapiro, B. Genomic Data from Extinct North American Camelops Revise Camel Evolutionary History. Mol. Biol. Evol. 2015, 32, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Heintzman, P.D.; Zazula, G.D.; MacPhee, R.D.E.; Scott, E.; Cahill, J.A.; McHorse, B.K.; Kapp, J.D.; Stiller, M.; Wooller, M.J.; Orlando, L.; et al. A new genus of horse from Pleistocene North America. Elife 2017, 6, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Palkopoulou, E.; Mallick, S.; Skoglund, P.; Enk, J.; Rohland, N.; Li, H.; Omrak, A.; Vartanyan, S.; Poinar, H.; Götherström, A.; et al. Complete genomes reveal signatures of demographic and genetic declines in the woolly mammoth. Curr. Biol. 2015, 25, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Mohandesan, E.; Speller, C.F.; Peters, J.; Uerpmann, H.-P.; Uerpmann, M.; De Cupere, B.; Hofreiter, M.; Burger, P.A. Combined hybridization capture and shotgun sequencing for ancient DNA analysis of extinct wild and domestic dromedary camel. Mol. Ecol. Res. 2017, 17, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, A.; Sackton, T.B.; Grayson, P.; Edwards, S.V.; Baker, A.J. First nuclear genome assembly of an extinct moa species, the little bush moa (Anomalopteryx didiformis). bioRxiv 2018. [Google Scholar] [CrossRef]
- Sinding, M.S.; Gilbert, M.T.P. The draft genome of extinct european Aurochs and its implications for de-extinction. Open Quat. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Shapiro, B. The curious case of the Dodo: Leveraging the Nicobar pigeon genome to resurrect this long-extinct bird. In Proceedings of the Plant and Animal Genomes Conference XXIV, San Diego, CA, USA, 9–13 January 2016. [Google Scholar]
- Gilbert, T.P.M. (University of Copehnhagen, Copenhagen, Denmark). Personal communication regarding sequencing of the great auk genome, 2015.
- Jónsson, H.; Schubert, M.; Seguin-Orlando, A.; Ginolhac, A.; Petersen, L.; Fumagalli, M.; Albrechtsen, A.; Petersen, B.; Korneliussen, T.S.; Vilstrup, J.T.; et al. Speciation with gene flow in equids despite extensive chromosomal plasticity. Proc. Natl. Acad. Sci. USA 2014, 111, 18655–18660. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-M.; Shaner, P.-J. L.; Zink, R.M.; Liu, W.-C.; Chu, T.-C.; Huang, W.-S.; Li, S.-H. Drastic population fluctuations explain the rapid extinction of the passenger pigeon. Proc. Natl. Acad. Sci. USA 2014, 111, 10636–10641. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.G.R.; Soares, A.E.R.; Novak, B.J.; Schaefer, N.K.; Cahill, J.A.; Baker, A.J.; Demboski, J.R.; Doll, A.; Da Fonseca, R.R.; Fulton, T.L.; et al. Natural selection shaped the rise and fall of passenger pigeon genomic diversity. Science 2017, 358, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Novak, B.; Athrey, G.; Shapiro, B.; Phelan, R.; Brand, S. Whole genome sequence analysis reveals evolutionary history of extinct Heath Hen. In Proceedings of the Joint Meeting of the American Ornithologist’s Union & Cooper Ornithological Society, Norman, OK, USA, 28 July–1 August 2015. [Google Scholar]
- Feigin, C.Y.; Newton, A.H.; Doronina, L.; Schmitz, J.; Hipsley, C.A.; Mitchell, K.J.; Gower, G.; Llamas, B.; Soubrier, J.; Heider, T.N.; et al. Genome of the Tasmanian tiger provides insights into the evolution and demography of an extinct marsupial carnivore. Nat. Ecol. Evol. 2018, 2, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Allentoft, M.E.; Collins, M.; Harker, D.; Haile, J.; Oskam, C.L.; Hale, M.L.; Campos, P.F.; Samaniego, J.A.; Gilbert, T.P.M.; Willerslev, E.; et al. The half-life of DNA in bone: Measuring decay kinetics in 158 dated fossils. Proc. R. Soc. B Biol. Sci. 2012, 279, 4724–4733. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, Y.; Chen, T.; Gao, F.; Gong, J.; Abramczyk, D.; Walker, R.; Zhao, H.; Chen, S.; Liu, W.; et al. Deep functional analysis of synII, a 770-kilobase synthetic yeast chromosome. Science 2017, 355. [Google Scholar] [CrossRef] [PubMed]
- Lynch, V.J.; Bedoya-Reina, O.C.; Ratan, A.; Sulak, M.; Drautz-Moses, D.I.; Perry, G.H.; Miller, W.; Schuster, S.C. Elephantid genomes reveal the molecular bases of woolly mammoth adaptations to the Arctic. Cell Rep. 2015, 12, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Lerner, S. Scientists Might Create Mammoth-Elephant Hybrid After “Resurrecting” 44 Genes, Will Start with Mice First. Available online: https://www.techtimes.com/articles/226529/20180430/scientists-might-create-mammoth-elephant-hybrid-after-resurrecting-44-genes-will-start-with-mice-first.htm (accessed on 3 September 2018).
- Camacho, A.E. Going the way of the Dodo: De-extinction, dualisms, and reframing conservation. Wash. Univ. Law Rev. 2015, 92, 849–906. [Google Scholar]
- Wagner, N.; Hockkirch, A.; Rohde, K.; Wacht, F.; Wesch, C.; Wirtz, S.; Klein, R.; Veith, M. De-extinction, nomenclature, and the law. Science 2017, 356, 1016–1017. [Google Scholar] [CrossRef] [PubMed]
- Young, N.M.; Linde-Medina, M.; Fondon Iii, J.W.; Hallgrímsson, B.; Marcucio, R.S. Craniofacial diversification in the domestic pigeon and the evolution of the avian skull. Nat. Ecol. Evol. 2017, 1, 95. [Google Scholar] [CrossRef] [PubMed]
- Sankararaman, S.; Mallick, S.; Dannemann, M.; Prüfer, K.; Kelso, J.; Svante, P.; Patterson, N.; Reich, D. The landscape of Neandertal ancestry in present-day humans. Nature 2014, 507, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Sherkow, J.S.; Greely, H.T. What if extinction is not forever? Science 2013, 340, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Greely, H.T. Is de-extinction special? Hastings Cent. Rep. 2017, 47, S30–S36. [Google Scholar] [CrossRef] [PubMed]
- Seddon, P.J.; Moehrenschlager, A.; Ewen, J. Reintroducing resurrected species: Selecting DeExtinction candidates. Trends Ecol. Evol. 2014, 29, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E. From dinosaurs to dodos: Who and what should we: de-extinct. Front. Biogeogr. 2014, 6, 20–24. [Google Scholar] [CrossRef]
- Mondry, H. Selecting candidates for de-extinction and resurrection: Mammoths, Lenin’s Tomb and Neo-Eurasianism. Anim. Stud. J. 2017, 6, 12–39. [Google Scholar]
- Turner, D.D. Biases in the selection of candidate species for de-extinction. Ethics Policy Environ. 2017, 20, 21–24. [Google Scholar] [CrossRef]
- McCauley, D.J.; Hardesty-Moore, M.; Halpern, B.S.; Young, H.S. A mammoth undertaking: Harnessing insight from functional ecology to shape de-extinction priority setting. Funct. Ecol. 2017, 31, 1003–1011. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Wright, J.P.; Jones, C.G.; Flecker, A.S. An ecosystem engineer, the beaver, increases species richness at the landscape scale. Oecologia 2002, 132, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Power, M.E.; Tilman, D.; Estes, J.A.; Menge, B.A.; Bond, W.J.; Mills, L.S.; Daily, G.; Castilla, J.C.; Lubchenco, J.; Paine, R.T. Challenges in the quest for keystones. Bioscience 1996, 46, 609–620. [Google Scholar] [CrossRef]
- Wilmers, C.C.; Crabtree, R.L.; Smith, D.W.; Murphy, K.M.; Getz, W.M. Trophic facilitation by introduced top predators: Grey wolf subsidies to scavengers in Yellowstone National Park. J. Anim. Ecol. 2003, 72, 909–916. [Google Scholar] [CrossRef]
- Beschta, R.L.; Ripple, W.J. Riparian vegetation recovery in Yellowstone: The first two decades after wolf reintroduction. Biol. Conserv. 2016, 198, 93–103. [Google Scholar] [CrossRef]
- Ripple, W.J.; Beschta, R.L. Trophic cascades in Yellowstone: The first 15years after wolf reintroduction. Biol. Conserv. 2012, 145, 205–213. [Google Scholar] [CrossRef]
- Roberge, J.M.; Angelstam, P. Usefulness of the umbrella species concepts as a conservation tool. Conserv. Biol. 2004, 18, 76–85. [Google Scholar] [CrossRef]
- Li, B.V.; Pimm, S.L. China’s endemic vertebrates sheltering under the protective umbrella of the giant panda. Conserv. Biol. 2016, 30, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C. Assessing the indicator properties of species assemblages for natural areas monitoring author. Ecol. Appl. 1992, 2, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Landres, P.B.; Verner, J.; Thomas, J.W. Ecological uses of vertebrate indicator species: A critique. Conserv. Biol. 1988, 2, 316–328. [Google Scholar] [CrossRef]
- Kyne, P.M.; Adams, V.M. Extinct flagships: Linking extinct and threatened species. Oryx 2017, 51, 471–476. [Google Scholar] [CrossRef]
- Greenberg, J. A Feathered River across the Sky, 1st ed.; Bloomsbury: New York, NY, USA, 2014. [Google Scholar]
- Higuchi, R.; Bowman, B.; Freiberger, M.; Ryder, O.A.; Wilson, A.C. DNA sequences from the quagga, an extinct member of the horse family. Nature 1984, 312, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Harley, E.H.; Knight, M.H.; Lardner, C.; Wooding, B.; Gregor, M. The Quagga project: Progress over 20 years of selective breeding. S. Afr. J. Wildl. Res. 2009, 39, 155–163. [Google Scholar] [CrossRef]
- Stokstad, E. Bringing Back the Aurochs. Science 2015, 350, 1144–1147. [Google Scholar] [CrossRef]
- Kirkdijk-Otten, H. (Former chair of the True Nature Foundation, Nijmegen, The Netherlands). Personal communication regarding present status of aurochs backbreeding programs, 2018.
- Miller, J.M.; Quinzin, M.C.; Poulakakis, N.; Gibbs, J.P.; Beheregaray, L.B.; Garrick, R.C.; Russello, M.A.; Ciofi, C.; Edwards, D.L.; Hunter, E.A.; et al. Identification of genetically important individuals of the rediscovered Floreana Galápagos giant tortoise (Chelonoidis elephantopus) provide founders for species restoration program. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.J.; Hansen, D.M.; Jones, C.G.; Zuël, N.; Harris, S. Resurrecting extinct interactions with extant substitutes. Curr. Biol. 2011, 21, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A. A Programmable dual-RNA—Guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.P.; Sterling, E.J.; Zabala, F.J. Giant Tortoises as Ecological Engineers: A Long-term Quasi-experiment in the Galápagos Islands. Biotropica 2010, 42, 208–214. [Google Scholar] [CrossRef]
- Boast, A.P.; Weyrich, L.S.; Wood, J.R.; Metcalf, J.L.; Knight, R.; Cooper, A. Coprolites reveal ecological interactions lost with the extinction of New Zealand birds. Proc. Natl. Acad. Sci. USA 2018, 115, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- McQueen, D.R. Divaricating shrubs in Patagonia and New Zealand. N. Z. J. Ecol. 2000, 24, 69–80. [Google Scholar]
- Putshkov, P.V. The impact of mammoths on their biome: Clash of two paradigms. In Advances in Mammoth Research, Proceedings of the Second International Mamoth Conference, Rotterdam, The Netherlands, 16–20 May 1999; Reumer, J.W.F., de vos, J., Mol, D., Eds.; DEINSEA: Rotterdam, The Netherlands, 2003; pp. 365–379. [Google Scholar]
- Zimov, S.A.; Zimov, N.S.; Tikhonov, A.N.; Chapin III, F.S. Mammoth Steppe: A high-productivity phenomenon. Quat. Sci. Rev. 2012, 57, 26–45. [Google Scholar] [CrossRef]
- Novak, B.J. Deciphering the Ecological Impact of the Passenger Pigeon: A Synthesis of Paleogenetics, Paleoecology, Morphology, and Physiology. Master’s Thesis, University of California Santa Cruz, Santa Cruz, CA, USA, 2016. [Google Scholar]
- DeGraaf, R.M.; Yamasaki, M. Options for managing early-successional forest and shrubland bird habitats in the northeastern United States. For. Ecol. Manag. 2003, 185, 179–191. [Google Scholar] [CrossRef]
- Swanson, M.E.; Franklin, J.F.; Beschta, R.L.; Crisafulli, C.M.; DellaSala, D.A.; Hutto, R.L.; Lindenmayer, D.B.; Swanson, F.J. The forgotten stage of forest succession: Early-successional ecosystems on forest sites. Front. Ecol. Environ. 2011, 9, 117–125. [Google Scholar] [CrossRef]
- Askins, R. A Sustaining Biological Diversity in Early Successional communities: The Challenge of Managing Unpopular Habitats. Wildl. Soc. Bull. 2001, 29, 407–412. [Google Scholar]
- Trani, M.K.; Brooks, R.T.; Schmidt, T.L.; Rudis, V.A.; Gabbard, C.M. Patterns and trends of early successional forests in the eastern United States. Wildl. Soc. Bull. 2001, 29, 413–424. [Google Scholar]
- Brooks, R.T. Abundance, distribution, trends, and ownership patterns of early-successional forests in the northeastern United States. For. Ecol. Manag. 2003, 185, 65–74. [Google Scholar] [CrossRef]
- King, D.I.; Schlossberg, S. Synthesis of the conservation value of the early-successional stage in forests of eastern North America. For. Ecol. Manag. 2014, 324, 186–195. [Google Scholar] [CrossRef]
- McEwan, R.W.; Dyer, J.M.; Pederson, N. Multiple interacting ecosystem drivers: Toward an encompassing hypothesis of oak forest dynamics across eastern North America. Ecography 2011, 34, 244–256. [Google Scholar] [CrossRef]
- Dey, D.C. Sustaining Oak Forests in Eastern North America: Regeneration and Recruitment, the Pillars of Sustainability. For. Sci. 2014, 60, 926–942. [Google Scholar] [CrossRef]
- Hutchinson, T.F.; Sutherland, E.K.; Yaussy, D.A. Effects of repeated prescribed fires on the structure, composition, and regeneration of mixed-oak forests in Ohio. For. Ecol. Manag. 2005, 218, 210–228. [Google Scholar] [CrossRef]
- Amthor, J.; Cushman, J.H.; Dale, V.; Edwards, N.T.; Farrell, M.P.; Garten, C.T.; Gunderson, C.A.; Hanson, P.; Hildebrand, S.G.; Huston, M.; et al. Terrestrial ecosystem responses to global change: A research strategy. ORNL Tech. Memo. 1998, 27, 37. [Google Scholar]
- Rane, R.V.; Oakeshott, J.G.; Nguyen, T.; Hoffmann, A.A.; Lee, S.F. Orthonome—A new pipeline for predicting high quality orthologue gene sets applicable to complete and draft genomes. BMC Genom. 2017, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Novak, B.J.; Estes, J.A.; Shaw, H.E.; Novak, E.V.; Shapiro, B. Experimental Investigation of the Dietary Ecology of the Extinct Passenger Pigeon, Ectopistes migratorius. Front. Ecol. Evol. 2018, 6, 20. [Google Scholar] [CrossRef]
- Oehler, D.A.; Novak, B.J.; Schmid, S.C.; Huth, K.J.; Totha, A.I.; Audhya, T. Husbandry protocols for the Band-tailed pigeon, Patagioenas fasciata albilinea, at the WCS, Bronx Zoo for future conservation management programs. Zoo Biol. 2018, 37, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Marcus, A. Meet the scientists bringing extinct species back from the dead. The Future of Everything: A look ahead from the Wall Street Journal, November/December 2018. [Google Scholar]
- Progress to Date|The Great Passenger Pigeon Comeback. Available online: http://reviverestore.org/projects/the-great-passenger-pigeon-comeback/progress-to-date/ (accessed on 3 September 2018).
- Andrews-Cookson, M. (The Genetic Rescue Foundation, Wellington, New Zealand). Personal communication regarding details about the Genetic Rescue Foundation’s moa de-extinction program, 2018.
- Pilcher, H. Reviving Woolly Mammoths Will Take More Than Two Years. Available online: http://www.bbc.com/earth/story/20170221-reviving-woolly-mammoths-will-take-more-than-two-years (accessed on 9 March 2018).
- Marini, P.; Novak, B.J. Effect of Controlled Lighting on Band-Tailed Pigeon (Patagioenas fasciata) Breeding. Available online: http://reviverestore.org/wp-content/uploads/2015/09/Effect_of_controlled_lighting_on_BTP_breeding_6.18.15.pdf (accessed on 26 August 2018).
- Novak, B.J. The Great Comeback down under. Available online: http://reviverestore.org/the-great-comeback-down-under/ (accessed on 30 May 2018).
- Novak, B.J. De-extinction and Animal Welfare; Fischer, R., Ed.; Routledge: Abingdon, UK, 2019. [Google Scholar]
- Turner, D.D. The Restorationist Argument for Extinction Reversal. In The Ethics of Animal Re-creation and Modification; Siipi, H., Oksanen, M., Eds.; Palgrave Macmillan: London, UK, 2014. [Google Scholar]
- Foster, D.R.; Motzkin, G.; Bernardos, D.; Cardoza, J. Wildlife dynamics in the changing New England landscape. J. Biogeogr. 2002, 29, 1337–1357. [Google Scholar] [CrossRef]
- Applebaum, Y. Why Wild Turkeys Hate the Wild. Available online: https://www.theatlantic.com/science/archive/2015/11/return-of-the-turkey/417648/ (accessed on 26 August 2018).
- Bibikova, M.; Beumer, K.; Trautman, J.K.; Carroll, D. Enhancing Gene Targeting with Designed Zinc Finger Nucleases. Science 2003, 300, 764. [Google Scholar] [CrossRef] [PubMed]
- Christian, M.; Cermak, T.; Doyle, E.L.; Schmidt, C.; Zhang, F.; Hummel, A.; Bogdanove, A.J.; Voytas, D.F. Targeting DNA Double-Strand Breaks with TAL Effector Nucleases. Genetics 2010, 186, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.F.; Cohent, S.N.; Changt, A.C.Y.; Boyer, H.W.; Goodman, H.M.; Helling, R.B. Replication and Transcription of Eukaryotic DNA in Escherichia coli (restriction/plansmid/transformation/recombination/ribosomal DNA). Proc. Natl. Acad. Sci. USA 1974, 71, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Elmqvist, T. Pollinator Extinction in the Pacific Islands. Conserv. Biol. 2000, 14, 1237–1239. [Google Scholar] [CrossRef]
- Caves, E.M.; Jennings, S.B.; HilleRisLambers, J.; Tewksbury, J.J.; Rogers, H.S. Natural Experiment Demonstrates That Bird Loss Leads to Cessation of Dispersal of Native Seeds from Intact to Degraded Forests. PLoS ONE 2013, 8, e65618. [Google Scholar] [CrossRef] [PubMed]
- Traveset, A.; González-Varo, J.P.; Valido, A. Long-term demographic consequences of a seed dispersal disruption. Proc. Biol. Sci. 2012, 279, 3298–3303. [Google Scholar] [CrossRef] [PubMed]
- Sekercioglu, C.H. Functional Extinctions of Bird Pollinators Cause Plant Declines. Science 2011, 331, 1019–1020. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.M.; Kaiser, C.N.; Müller, C.B. Seed Dispersal and Establishment of Endangered Plants on Oceanic Islands: The Janzen-Connell Model, and the Use of Ecological Analogues. PLoS ONE 2008, 3, e2111. [Google Scholar] [CrossRef] [PubMed]
- Van de Lavoir, M.-C.; Diamond, J.H.; Leighton, P.A.; Mather-Love, C.; Heyer, B.S.; Bradshaw, R.; Kerchner, A.; Hooi, L.T.; Gessaro, T.M.; Swanberg, S.E.; et al. Germline transmission of genetically modified primordial germ cells. Nature 2006, 441, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Van de Lavoir, M.C.; Collarini, E.J.; Leighton, P.A.; Fesler, J.; Lu, D.R.; Harriman, W.D.; Thiyagasundaram, T.S.; Etches, R.J. Interspecific germline transmission of cultured primordial germ cells. PLoS ONE 2012, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- New Zealand Department of Conservation. Kākāpō Recovery. Available online: https://www.doc.govt.nz/kakapo-recovery (accessed on 26 August 2018).
- Elphick, C.S.; Roberts, D.L.; Michael Reed, J. Estimated dates of recent extinctions for North American and Hawaiian birds. Biol. Conserv. 2010, 143, 617–624. [Google Scholar] [CrossRef]
- Butchart, S.H.M.; Lowe, S.; Martin, R.W.; Symes, A.; Westrip, J.R.S.; Wheatley, H. Which bird species have gone extinct? A novel quantitative classification approach. Biol. Conserv. 2018, 227, 9–18. [Google Scholar] [CrossRef]
- Stringer, A.P.; Gaywood, M.J. The impacts of beavers Castor spp. on biodiversity and the ecological basis for their reintroduction to Scotland, UK. Mammal Review. 2016, 46, 270–283. [Google Scholar] [CrossRef]
- University of Groningen. Predecessor of Cows, the Aurochs, Were Still Living in The Netherlands Around AD 600. ScienceDaily. 2008. Available online: https://www.sciencedaily.com/releases/2008/12/081212081544.htm (accessed on 26 August 2018).
- Řičánková, V.P.; Robovský, J.; Riegert, J.; Zrzavý, J. Regional patterns of postglacial changes in the Palearctic mammalian diversity indicate retreat to Siberian steppes rather than extinction. Sci. Rep. 2015, 5, 12682. [Google Scholar] [CrossRef] [PubMed]
- Condor Reintroduction and Recovery. Available online: https://www.nps.gov/grca/learn/nature/condor-reintroduction-and-recovery.htm (accessed on 26 August 2018).
- Bennett, J.R.; Maloney, R.F.; Steeves, T.E.; Brazill-Boast, J.; Possingham, H.P.; Seddon, P.J. Spending limited resources on de-extinction could lead to net biodiversity loss. Nat. Ecol. Evol. 2017, 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Masterson, A. De-Extinction May Cause Extinction. Available online: https://cosmosmagazine.com/biology/de-extinction-may-cause-extinction (accessed on 30 August 2018).
- Zielinski, S. De-Extinction Probably Isn’t Worth It. Available online: https://www.sciencenews.org/blog/wild-things/de-extinction-probably-isnt-worth-it (accessed on 30 August 2018).
- Shultz, D. Bringing Extinct species back from the dead could hurt—not help—Conservation Efforts. Available online: http://www.sciencemag.org/news/2017/02/bringing-extinct-species-back-dead-could-hurt-not-help-conservation-efforts (accessed on 30 August 2018).
- Williams, T. The Tech Donors Backing the De-Extinction Movement. Available online: https://www.insidephilanthropy.com/home/2018/1/12/the-tech-donors-backing-the-de-extinction-movement (accessed on 25 August 2018).
- U.S. Fish & Wildlife Service. Federal and State Endangered and Threatened Species Expenditures; U.S. Fish & Wildlife Service: Washington, DC, USA, 2016. [Google Scholar]
- Bateson, Z.W.; Dunn, P.O.; Hull, S.D.; Henschen, A.E.; Johnson, J.A. Genetic restoration of a threatened population of greater prairie-chickens. Biol. Conserv. 2014, 174, 12–19. [Google Scholar] [CrossRef]
- Bouzat, J.L.; Johnson, J.A.; Toepfer, J.E.; Simpson, S.A.; Esker, T.L.; Westemeier, R.L. Beyond the beneficial effects of translocations as an effective tool for the genetic restoration of isolated populations. Conserv. Genet. 2009, 10, 191–201. [Google Scholar] [CrossRef]
- Bouzat, J.L.; Cheng, H.H.; Lewin, H.A.; Westemeier, R.L.; Brawn, J.D.; Paige, K.N. Genetic Evaluation of a Demographic Bottleneck in the Greater Prairie Chicken. Conserv. Biol. 1998, 12, 836–843. [Google Scholar] [CrossRef]
- Westemeier, R.L.; Brawn, J.D.; Simpson, S.A.; Esker, T.L.; Jansen, R.W.; Walk, J.W.; Kershner, E.L.; Bouzat, J.L.; Paige, K.N. Tracking the long-term decline and recovery of an isolated population. Science 1998, 282, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- Wray, B. Bringing Back the Woolly Mammoth Has Already Had an Unintended Consequence. Available online: https://medium.com/neodotlife/de-extinction-woolly-mammoth-16c31a2dc3b3 (accessed on 3 September 2018).
- Hayward, G.S. Conservation: Clarifying the risk from herpesvirus to captive Asian elephants. Vet. Rec. 2012, 170, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Long, S.Y.; Latimer, E.M.; Hayward, G.S. Review of elephant endotheliotropic herpesviruses and acute hemorrhagic disease. ILAR J. 2016, 56, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Sandler, R. The ethics of reviving long extinct species. Conserv. Biol. 2014, 28, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Sandler, R. De-extinction and Conservation Genetics in the Anthropocene. Hastings Cent. Rep. 2017, 47, S43–S47. [Google Scholar] [CrossRef] [PubMed]
- Sandler, R. Costs, benefits and ethics. Nat. Ecol. Evol. 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S. The Ethics of De-Extinction. Nanoethics 2014, 8, 165–178. [Google Scholar] [CrossRef]
- Turner, D.D. De-extinction as Artificial Species Selection. Philos. Technol. 2017, 30, 395–411. [Google Scholar] [CrossRef]
- Friese, C.; Marris, C. Making De-Extinction Mundane? PLoS Biol. 2014, 12, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D. On the Authenticity of De-Extinct Organisms, and the Genesis Argument. Anim. Stud. J. 2017, 6, 1–15. [Google Scholar]
- Mason, C. The Unnaturalness Objection to De-Extinction: A Critical Evaluation. Anim. Stud. J. 2017, 6, 40–60. [Google Scholar]
- Welchman, J. How Much Is That Mammoth in the Window? Ethics Policy Environ. 2017, 20, 41–43. [Google Scholar] [CrossRef]
- Jennings, B. The Moral Imagination of De-extinction. Hastings Cent. Rep. 2017, 47, S54–S59. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.A. De-extinction and the Flourishing of Species. Ethics Policy Environ. 2017, 20, 38–40. [Google Scholar] [CrossRef]
- Kaebnick, G.E. The Spectacular Garden: Where Might De-extinction Lead? Hastings Cent. Rep. 2017, 47, S60–S64. [Google Scholar] [CrossRef] [PubMed]
- Kaebnick, G.E.; Jennings, B. De-extinction and Conservation. Hastings Cent. Rep. 2017, 47, S2–S4. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, M.; Vuorisalo, T. De-Extinct Species as Wildlife. Finn. J. Hum.-Anim. Stud. 2017, 3, 4–27. [Google Scholar] [CrossRef]
- Kohl, P. Reclaiming Hope in Extinction Storytelling. Hastings Cent. Rep. 2017, 47, S24–S29. [Google Scholar] [CrossRef] [PubMed]
- Kohl, P. Using De-extinction to Create Extinct Species Proxies; Natural History not Included. Ethics Policy Environ. 2017, 20, 15–17. [Google Scholar] [CrossRef]
- Beever, J. The Ontology of Species: Commentary on Kasperbauer’s “Should We Bring Back the Passenger Pigeon? The Ethics of De-Extinction”. Ethics Policy Environ. 2017, 20, 18–20. [Google Scholar] [CrossRef]
- Campagna, C.; Guevara, D.; Le Boeuf, B. De-scenting Extinction: The Promise of De-extinction May Hasten Continuing Extinctions. Hastings Cent. Rep. 2017, 47, S48–S53. [Google Scholar] [CrossRef] [PubMed]
- Diehm, C. De-extinction and Deep Questions About Species Conservation. Ethics Policy Environ. 2017, 20, 25–28. [Google Scholar] [CrossRef]
- Preston, C.J. De-extinction and Taking Control of Earth’s “Metabolism”. Hastings Cent. Rep. 2017, 47, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Okuno, E. Frankenstein’s Mammoth: Anticipating The Global Legal Framework For De-extinction. Ecol. Law Q. 2016, 43, 581–634. [Google Scholar] [CrossRef]
- Haught, P. Integral Value and the Virtue of Hospitality: A Response to Kasperbauer. Ethics Policy Environ. 2017, 20, 29–32. [Google Scholar] [CrossRef]
- Ibbotson, R. Making sense? Visual Cultures of De-extinction and the Anthropocentric Archive. Anim. Stud. J. 2017, 6, 80–103. [Google Scholar]
- Piotrowska, M. Meet the new mammoth, same as the old? Resurrecting the Mammuthus primigenius. Biol. Philos. 2018, 33, 5. [Google Scholar] [CrossRef]
- Rohwer, Y.; Marris, E. An Analysis of Potential Ethical Justifications for Mammoth De-extinction and a Call for Empirical Research. Ethics Policy Environ. 2018, 21, 127–142. [Google Scholar] [CrossRef]
- Slater, M.H.; Clatterbuck, H. A pragmatic approach to the possibility of de-extinction. Biol. Philos. 2018, 33, 1–21. [Google Scholar] [CrossRef]
- Richmond, D.J.; Sinding, M.H.S.; Gilbert, M.T.P. The potential and pitfalls of de-extinction. Zool. Scr. 2016, 45, 22–36. [Google Scholar] [CrossRef]
- Seddon, P.J. The ecology of de-extinction. Funct. Ecol. 2017, 31, 992–995. [Google Scholar] [CrossRef]
- Selbach, C.; Seddon, P.J.; Poulin, R. Parasites Lost: Neglecting a Crucial Element in De-Extinction. Trends Parasitol. 2018, 34, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; Dussex, N.; Van Heezik, Y. De-extinction needs consultation. Nat. Ecol. Evol. 2017, 1, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.R.; Perry, G.L.W.; Wilmshurst, J.M. Using palaeoecology to determine baseline ecological requirements and interaction networks for de-extinction candidate species. Funct. Ecol. 2017, 31, 1012–1020. [Google Scholar] [CrossRef]
- Peers, M.J.L.; Thornton, D.H.; Majchrzak, Y.N.; Bastille-Rousseau, G.; Murray, D.L. De-extinction potential under climate change: Extensive mismatch between historic and future habitat suitability for three candidate birds. Biol. Conserv. 2016, 197, 164–170. [Google Scholar] [CrossRef]
- Steeves, T.E.; Johnson, J.A.; Hale, M.L. Maximising evolutionary potential in functional proxies for extinct species: A conservation genetic perspective on de-extinction. Funct. Ecol. 2017, 31, 1032–1040. [Google Scholar] [CrossRef]
- Corlett, R.T. Restoration, Reintroduction, and Rewilding in a Changing World. Trends Ecol. Evol. 2016, 31, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Donlan, A. De-extinction in a crisis discipline. Front. Biogeogr. 2014, 6, 25–28. [Google Scholar] [CrossRef]
- Shapiro, B. Mammoth 2.0: Will genome engineering resurrect extinct species? Genome Biol. 2015, 16, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Iacona, G.; Maloney, R.F.; Chadès, I.; Bennett, J.R.; Seddon, P.J.; Possingham, H.P. Prioritizing revived species: What are the conservation management implications of de-extinction? Funct. Ecol. 2017, 31, 1041–1048. [Google Scholar] [CrossRef]
- Heard, M.J. De-extinction: Raising the dead and a number of important questions. Front. Biogeogr. 2014, 6, 4–6. [Google Scholar] [CrossRef]
- Desalle, R.; Amato, G. Conservation Genetics, Precision Conservation, and De-extinction. Hastings Cent. Rep. 2017, 47, S18–S23. [Google Scholar] [CrossRef] [PubMed]
- Meine, C. De-extinction and the Community of Being. Hastings Cent. Rep. 2017, 47, S9–S17. [Google Scholar] [CrossRef] [PubMed]
- Blockstein, D.E. We Can’t Bring Back the Passenger Pigeon: The Ethics of Deception Around De-extinction. Ethics Policy Environ. 2017, 20, 33–37. [Google Scholar] [CrossRef]
- Banks, P.B.; Hochuli, D.F. Extinction, de-extinction & conservation: A dangerous mix of ideas. Aust. Zool. 2017, 38, 390–394. [Google Scholar] [CrossRef]
- Martinelli, L.; Oksanen, M.; Siipi, H. De-extinction: A novel and remarkable case of bio-objectification. Croat. Med. J. 2014, 55, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.; Thévenin, C.; Princé, K.; Sarrazin, F.; Clavel, J. De-extinction and evolution. Funct. Ecol. 2017, 31, 1021–1031. [Google Scholar] [CrossRef]
- Davis, C.N.; Moran, M.D. An argument supporting de-extinction and a call for field research. Front. Biogeogr. 2016, 8, 1–9. [Google Scholar] [CrossRef]
- Minteer, B. Is it right to reverse extinction? Nature 2014, 509, 261. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Carlson, D.F.; Nandi, S.; Sherman, A.; Fahrenkrug, S.C.; McGrew, M.J. Efficient TALEN-mediated gene targeting of chicken primordial germ cells. Development 2017, 144, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Fulton, T.L.; Wagner, S.M.; Fisher, C.; Shapiro, B. Nuclear DNA from the extinct Passenger Pigeon (Ectopistes migratorius) confirms a single origin of New World pigeons. Ann. Anat. 2012, 194, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Seddon, P.J. De-extinction and barriers to the application of new conservation tools. Hastings Cent. Rep. 2017, 47, S5–S8. [Google Scholar] [CrossRef] [PubMed]
| Species | Common Name | Extinction Date | De-Extinction Technique | Conservation Category | Template Species | Template Species Conservation Status (IUCN/U.S. ESA) | Organization |
|---|---|---|---|---|---|---|---|
| Equus quagga quagga | Quagga Zebra | 1883 AD | Artificial Selection | Biodiversity | Equus quagga burchelli | Near Threatened/NA | The Quagga Project |
| Bos taurus primigenius | Aurochs | 1627 AD | Backbreeding of Domestic Descendants | Ecosystem engineer (grassland maintenance) | Bos taurus taurus | Not Assessed/NA | Operation Taurus; Taurus Project |
| Chelonoidis elephantopus | Floreana Island Giant Tortoise | Circa 1850 AD | Hybrid-backbreeding | Ecosystem engineer (megaherbivore) | C. elephantopus × Chelinoidis becki; C. elephantopus × Chelinoidis hoodensis | becki = vulnerable, hoodensis = critically endangered; U.S. ESA NA to both | Galápagos National Park Service |
| Ectopistes migratorius | Passenger Pigeon | 1914 AD | Precise Hybridization | Ecosystem engineer (forest disturbance/regeneration cycle) | Patagioenas fasciata | Least Concern/Not Listed | Revive & Restore |
| Mammuthus primigenius | Woolly Mammoth | ~3900 yr BP | Precise Hybridization | Ecosystem engineer (megaherbivore, grassland maintenance) | Elephas maximus | Endangered/Endangered | Revive & Restore |
| Tympanuchus cupido | Heath Hen | 1932 AD | Precise Hybridization | Indicator species | Tympanuchus pinnatus | Vulnerable/NA | Revive & Restore |
| Anamalopteryx didiformis * | Little Bush Moa | 15th Century AD | Precise Hybridization | Megaherbivore | Dromaius novaehollandiae | Least Concern/NA | Genetic Rescue Foundation |
| Species | Common Name | Multi-Celled Individuals | Cryopreserved Cultured Cells and/or Tissues | Active Recovery Program | Reference |
|---|---|---|---|---|---|
| Ceratotherium cottoni | Northern White Rhinoceros | 2 | Yes | Yes | [34,35] |
| Capra pyrenaica pyrenaica | Bucardo | 0 | Yes | No | [24] |
| Lipotes vexillifer | Yangtze River Dolphin | 0 | Yes | No | [38] |
| Melamprosops phaeosoma | Po’ouli Honeycreeper | >2 | Yes | No | [39] |
| Chelonoidis abingdoni | Pinta Island Giant Tortoise | 0 * | Yes | No | [39] |
| Rheobatrachus silus | Gastric Brooding Frog | 0 | Yes | Yes | [37] |
| Ecnomiohyla rabborum | Rabb’s Fringe-limbed Tree Frog | 0 | Yes | No | [39] |
| Achitinella apexfulva | Oahu Tree Snail Sp. | 1 | Yes | No | [39] |
| Partula faba | Captain Cook’s Bean Snail | 0 | Yes | No | [40] |
| Partula turgida | Raiatean Thin-shelled Polynesian Snail | 0 | Yes | No | [41] |
| Species | Common Name | Extinction | Reference |
|---|---|---|---|
| Homo sp.‡ | Sima de los Huesos hominins | Pleistocene | [43] |
| Homo sp. Altai | Denisovan Man | Pleistocene | [44] |
| Palaeoloxodon antiquus | Straight-tusked Elephant | ~35,000 yr BP | [45] |
| Homo neanderthalensis | Neanderthal | ~28,000 yr BP | [46] |
| Ursus spelaeus * | Cave Bear | ~24,000 yr BP | [47] |
| Camelops hesternus‡ | Camelops | ~13,000 yr BP | [48] |
| Haringtonhipppus francisci‡ | New World Stilt Legged Horse | ~12,000 yr BP | [49] |
| Mammuthus columbi * | Columbian Mammoth | ~10,900 yr BP | [45] |
| Mammut americanum * | Mastodon | ~10,000 yr BP | [45] |
| Mammuthus primigenius | Woolly Mammoth | ~3900 yr BP | [50] |
| Camelus dromedaries‡ | Wild Dromedary Camel * | ~2000 yr BP | [51] |
| Anomalopteryx didiformis * | Little Bush Moa | 15th Century AD | [52] |
| Bos taurus primigenius | Aurochs | 1627 AD ** | [53] |
| Raphus cucullatus * | Dodo | Late 17th Century AD | [54] |
| Pinguinus impennis | Great Auk | 1844 AD | [55] |
| Equus quagga quagga | Quagga Zebra | 1883 AD | [56] |
| Ectopistes migratorius | Passenger Pigeon | 1914 AD | [57,58] |
| Tympanuchus cupido * | Heath Hen | 1932 AD | [59] |
| Thylacinus cynocephalus | Thylacine | 1936 AD | [60] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novak, B.J. De-Extinction. Genes 2018, 9, 548. https://doi.org/10.3390/genes9110548
Novak BJ. De-Extinction. Genes. 2018; 9(11):548. https://doi.org/10.3390/genes9110548
Chicago/Turabian StyleNovak, Ben Jacob. 2018. "De-Extinction" Genes 9, no. 11: 548. https://doi.org/10.3390/genes9110548
APA StyleNovak, B. J. (2018). De-Extinction. Genes, 9(11), 548. https://doi.org/10.3390/genes9110548




