Whole-Genome Sequencing of 84 Japanese Eels Reveals Evidence against Panmixia and Support for Sympatric Speciation
Abstract
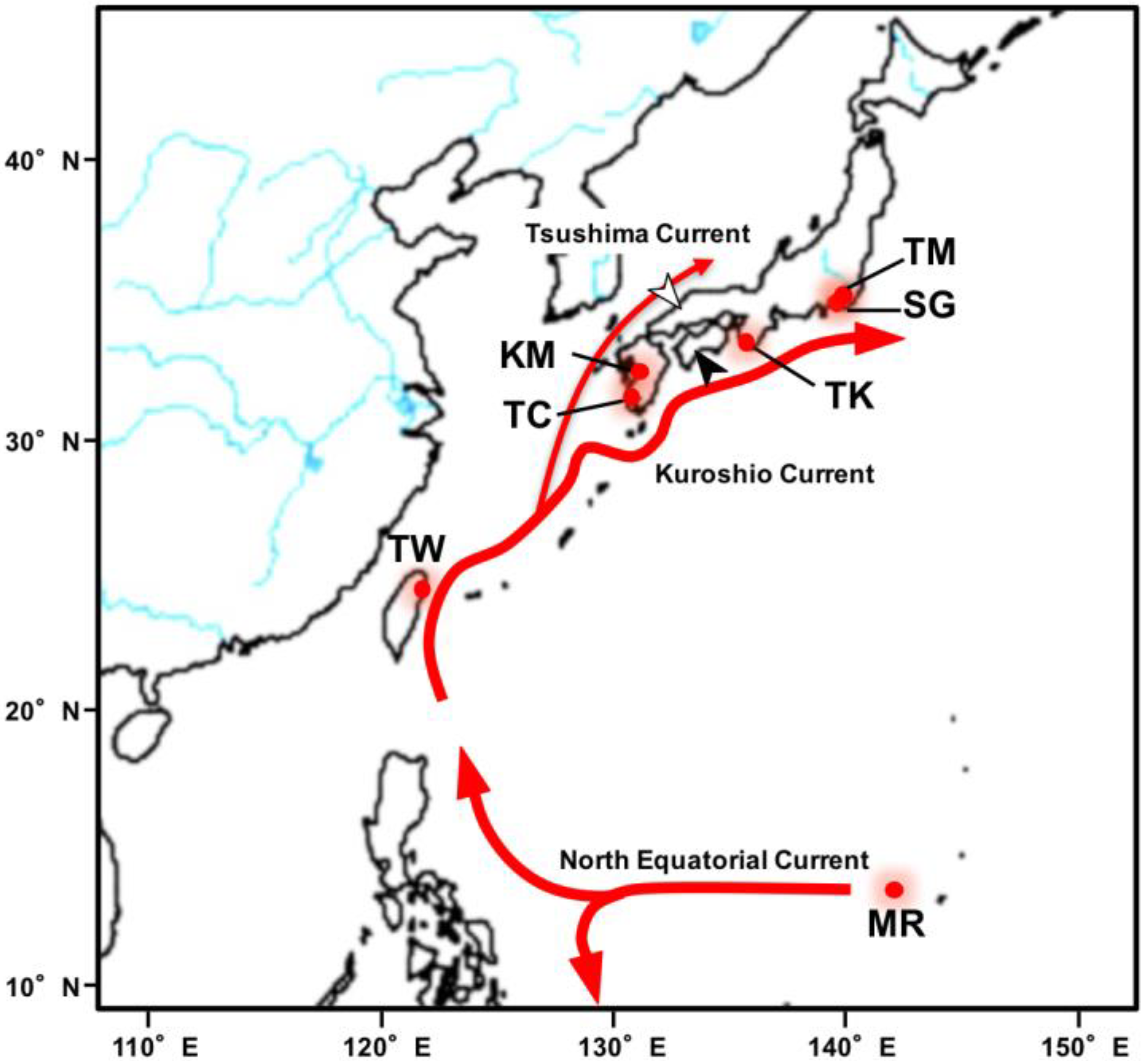
1. Introduction
2. Materials and Methods
2.1. DNA Extraction
2.2. Library Preparation and Sequencing
2.3. Analysis Based on Mitochondrial DNA
2.4. Mapping
2.5. Single Nucleotide Polymorphism (SNP) Calling and SNP Quality Control
2.6. Genetic Population Analysis Using Single Nucleotide Polymorphism Genotype Data
2.7. Analyses on Each Linkage Group
2.8. Fixation Index Values and Tests for Local Selection
3. Results
3.1. Eel Species Identification Based on Full-Length Mitochondrial DNA
3.2. Species Identification Based on the Eel Nuclear Genome
3.3. Single Nucleotide Polymorphism Calling
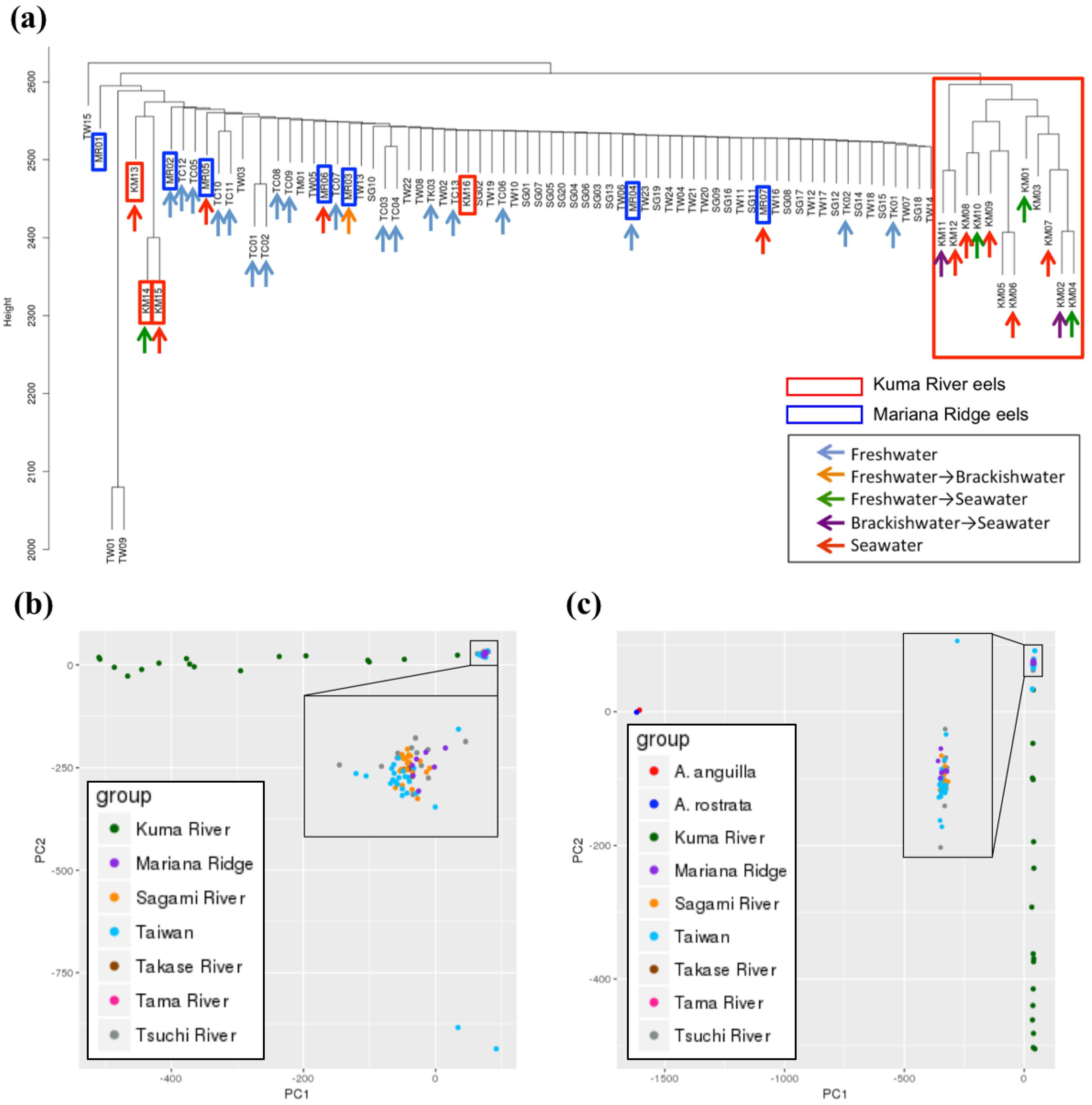
3.4. Genetic Population Analysis Using Single Nucleotide Polymorphism Genotype Data for the Whole Genome
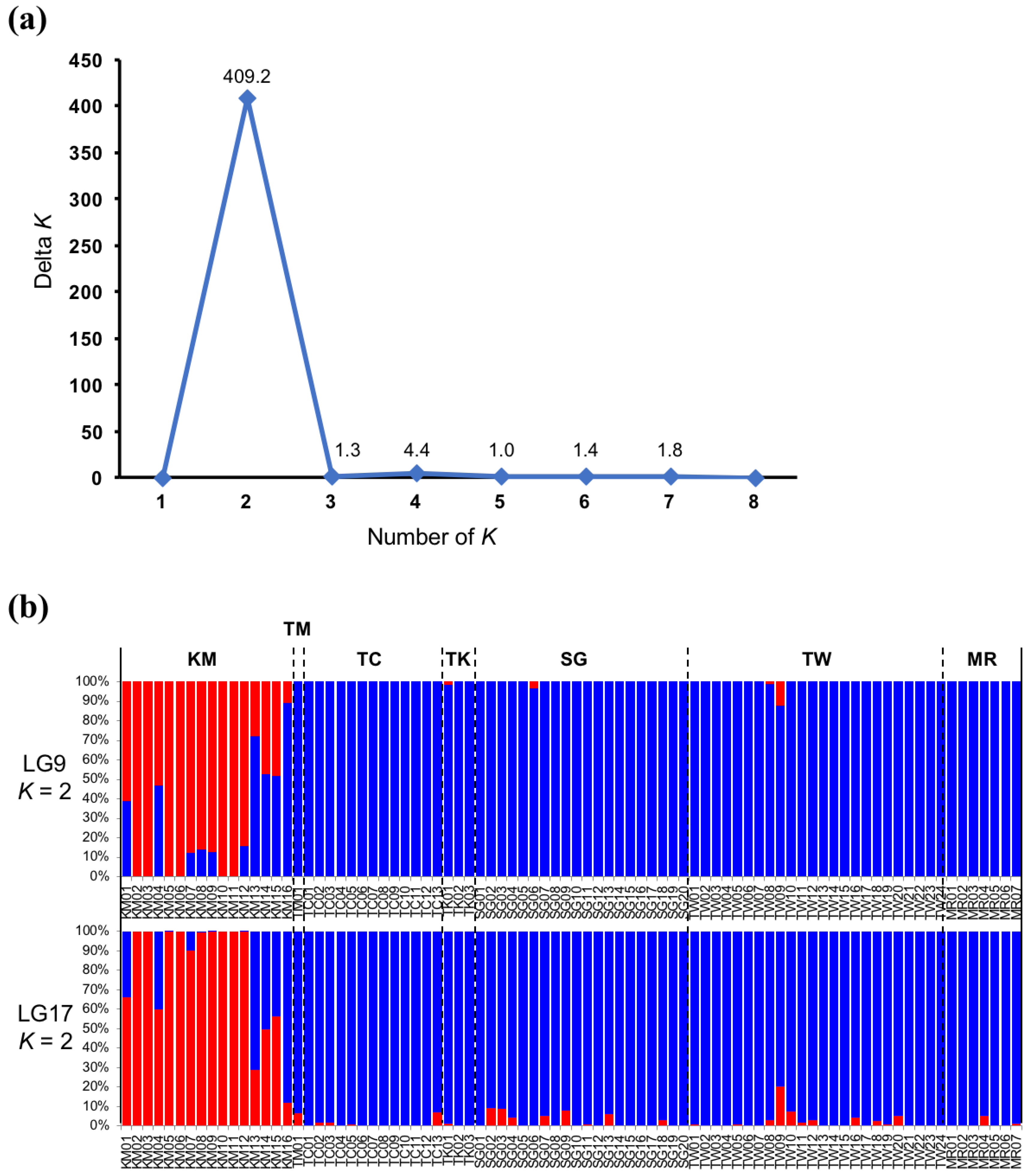
3.5. Genetic Population Analysis Using Single Nucleotide Polymorphisms Genotype Data for Each Linkage Group
3.6. Fixation Index Values for Pairwise Genetic Differentiation
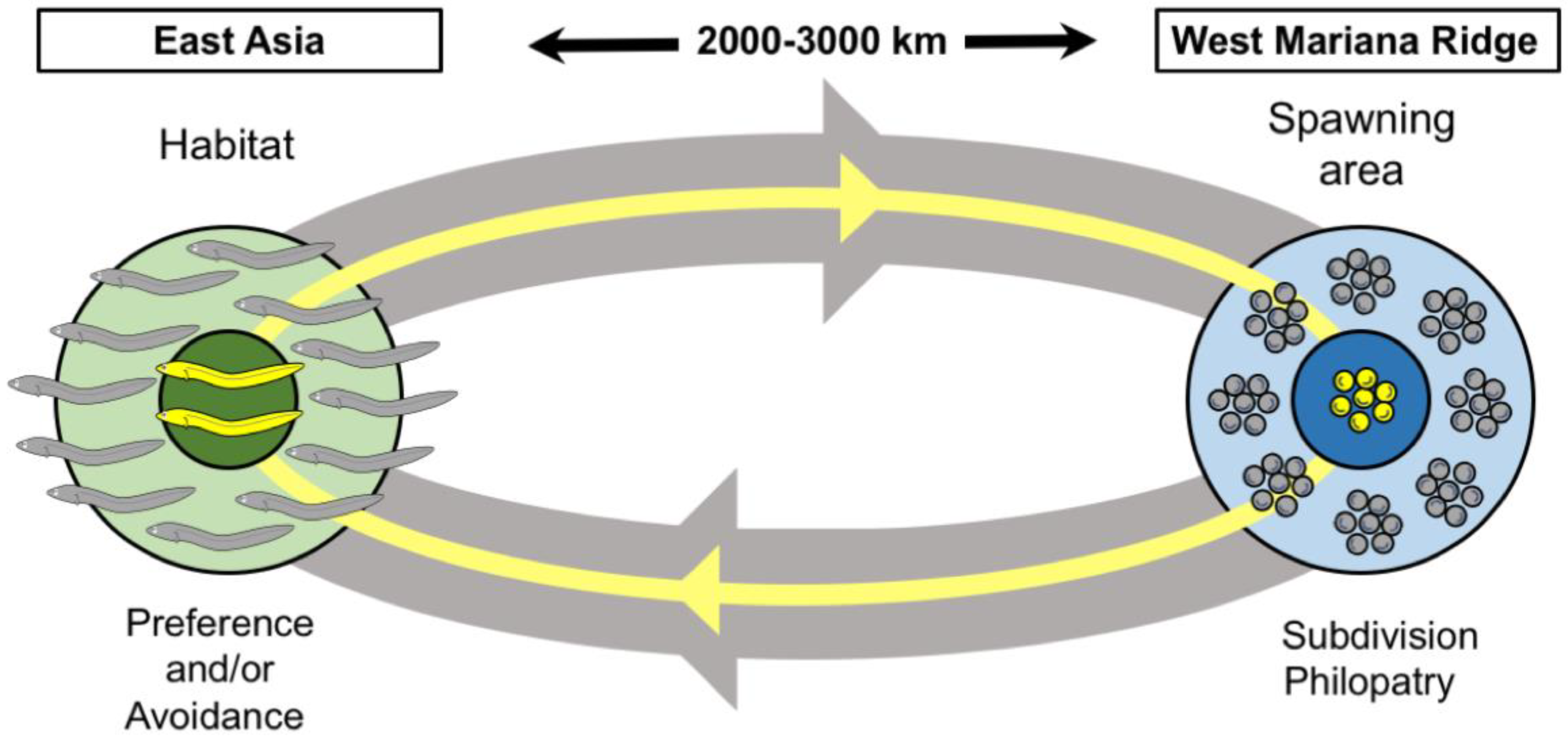
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aida, K.; Tsukamoto, K.; Yamauchi, K. (Eds.) Eel Biology; Springer: Tokyo, Japan, 2003. [Google Scholar]
- Aoyama, J. Life history and evolution of migration in Catadromous eels (Genus Anguilla). Aqua-Biosci. Monogr. 2009, 2, 1–42. [Google Scholar] [CrossRef]
- Als, T.D.; Hansen, M.M.; Maes, G.E.; Castonguay, M.; Riemann, L.; Aarestrup, K.; Munk, P.; Sparholt, H.; Hanel, R.; Bernatchez, L. All roads lead to home: Panmixia of European eel in the Sargasso Sea. Mol. Ecol. 2011, 20, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Béguer-Pon, M.; Castonguay, M.; Shan, S.; Benchetrit, J.; Dodson, J.J. Direct observations of American eels migrating across the continental shelf to the Sargasso Sea. Nat. Commun. 2015, 6, 8705. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Bernatchez, L. Genetic evidence against panmixia in the European eel. Nature 2001, 409, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Pujolar, J.M.; Jacobsen, M.W.; Als, T.D.; Frydenberg, J.; Munch, K.; Jónsson, B.; Jian, J.B.; Cheng, L.; Maes, G.E.; Bernatchez, L.; et al. Genome-wide single-generation signatures of local selection in the panmictic European eel. Mol. Ecol. 2014, 23, 2514–2528. [Google Scholar] [CrossRef] [PubMed]
- Côté, C.L.; Gagnaire, P.A.; Bourret, V.; Verreault, G.; Castonguay, M.; Bernatchez, L. Population genetics of the American eel (Anguilla rostrata): FST = 0 and North Atlantic Oscillation effects on demographic fluctuations of a panmictic species. Mol. Ecol. 2013, 22, 1763–1776. [Google Scholar] [CrossRef]
- Pujolar, J.M. Conclusive evidence for panmixia in the American eel. Mol. Ecol. 2013, 22, 1761–1762. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K. Oceanic biology: Spawning of eels near a seamount. Nature 2006, 439, 929. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Chow, S.; Otake, T.; Kurogi, H.; Mochioka, N.; Miller, M.J.; Aoyama, J.; Kimura, S.; Watanabe, S.; Yoshinaga, T.; et al. Oceanic spawning ecology of freshwater eels in the western North Pacific. Nat. Commun. 2011, 2, 179. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, J.; Watanabe, S.; Miller, M.J.; Mochioka, N.; Otake, T.; Yoshinaga, T.; Tsukamoto, K. Spawning sites of the Japanese eel in relation to oceanographic structure and the West Mariana Ridge. PLoS ONE 2014, 9, e88759. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Numachi, K. Genetic variation of 6-phosphogluconate dehydrogenase, isocitrate dehydrogenase, and glutamic-oxaloacetic transaminase in the liver of Japanese eel. Nippon Suisan Gakkaishi 1978, 44, 1351–1355. [Google Scholar] [CrossRef]
- Sang, T.K.; Chang, H.Y.; Chen, C.T.; Hui, C.F. Population structure of the Japanese eel Anguilla japonica. Mol. Biol. Evol. 1994, 11, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Aoyama, J.; Tsukamoto, K.; Nishida, M. Population structure of the Japanese eel Anguilla japonica as examined by mitochondrial DNA sequencing. Fish Sci. 2001, 67, 246–253. [Google Scholar] [CrossRef]
- Han, Y.S.; Hung, C.L.; Liao, Y.F.; Tzeng, W.N. Population genetic structure of the Japanese eel Anguilla japonica: Panmixia at spatial and temporal scales. Mar. Ecol. Prog. Ser. 2010, 401, 221–232. [Google Scholar] [CrossRef]
- Minegishi, Y.; Aoyama, J.; Inoue, J.G.; Miya, M.; Nishida, M.; Tsukamoto, K. Lack of genetic heterogeneity in the Japanese eel based on a spatiotemporal sampling. Coast. Mar. Sci. 2012, 35, 269–276. [Google Scholar]
- Gong, X.L.; Ren, S.J.; Cui, Z.K.; Yue, L.J. Genetic evidence for panmixia of Japanese eel (Anguilla japonica) populations in China. Genet. Mol. Res. 2014, 13, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Sekino, M.; Nakamichi, R.; Iwasaki, Y.; Tanabe, A.; Fujiwara, A.; Yasuike, M.; Shiraishi, M.; Saitoh, K. A new resource of single nucleotide polymorphisms in the Japanese eel Anguilla japonica derived from restriction site-associated DNA. Ichthyol. Res. 2016, 63, 496. [Google Scholar] [CrossRef]
- Tseng, M.C.; Tzeng, W.N.; Lee, S.C. Population genetic structure of the Japanese eel Anguilla japonica in the North-west Pacific Ocean: Evidence of non-panmictic populations. Mar. Ecol. Prog. Ser. 2016, 308, 221–230. [Google Scholar] [CrossRef]
- Gagnaire, P.A.; Normandeau, E.; Côté, C.; Hansen, M.M.; Bernatchez, L. The genetic consequences of spatially varying selection in the panmictic American eel (Anguilla rostrata). Genetics 2012, 190, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Pavey, S.A.; Gaudin, J.; Normandeau, E.; Dionne, M.; Castonguay, M.; Audet, C.; Bernatchez, L. RAD Sequencing highlights polygenic discrimination of habitat ecotypes in the panmictic American eel. Curr. Biol. 2015, 25, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Pujolar, J.M.; Jacobsen, M.W.; Bekkevold, D.; Lobón-Cervià, J.; Jónsson, B.; Bernatchez, L.; Hansen, M.M. Signatures of natural selection between life cycle stages separated by metamorphosis in European eel. BMC Genom. 2015, 16, 600. [Google Scholar] [CrossRef] [PubMed]
- Chevreux, B.; Wetter, T.; Suhai, S. Genome sequence assembly using trace signals and additional sequence information. Comput. Sci. Biol. 1999, 99, 45–56. [Google Scholar]
- Inoue, J.G.; Miya, M.; Aoyama, J.; Ishikawa, S.; Tsukamoto, K.; Nishida, M. Complete mitochondrial DNA sequence of the Japanese eel Anguilla japonica. Fish Sci. 2001, 67, 118–125. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, Y.; Aoyama, J.; Inoue, J.G.; Miya, M.; Nishida, M.; Tsukamoto, K. Molecular phylogeny and evolution of the freshwater eels genus Anguilla based on the whole mitochondrial genome sequences. Mol. Phylogenet. Evol. 2005, 34, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Doi, H.; Yamanoue, Y.; Kinoshita, S.; Ishibashi, T.; Ushio, H.; Asakawa, S.; Nishida, M.; Watabe, S. Molecular phylogenetic relationship of Tetraodon pufferfish based on mitochondrial DNA analysis. Fish Sci. 2013, 79, 243–250. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Hannonlab. FASTX-Toolkit. Available online: http://hannonlab.cshl.edu/fastx_toolkit/ (accessed on 15 October 2015).
- Henkel, C.V.; Dirks, R.P.; de Wijze, D.L.; Minegishi, Y.; Aoyama, J.; Jansen, H.J.; Turner, B.; Knudsen, B.; Bundgaard, M.; Hvam, K.L.; et al. First draft genome sequence of the Japanese eel, Anguilla japonica. Gene 2012, 511, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Henkel, C.V.; Burgerhout, E.; de Wijze, D.L.; Dirks, R.P.; Minegishi, Y.; Jansen, H.J.; Spaink, H.P.; Dufour, S.; Weltzien, F.A.; Tsukamoto, K.; et al. Primitive duplicate hox clusters in the European eel’s genome. PLoS ONE 2012, 7, e32231. [Google Scholar] [CrossRef] [PubMed]
- Pavey, S.A.; Laporte, M.; Normandeau, E.; Gaudin, J.; Letourneau, L.; Boisvert, S.; Corbeil, J.; Audet, C.; Bernatchez, L. Draft genome of the American Eel (Anguilla rostrata). Mol. Ecol. Resour. 2017, 17, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Wysoker, A.; Tibbetts, K.; Homer, N.; Fennell, T.; van der Auwera, G.A. Picard. Available online: http://broadinstitute.github.io/picard/ (accessed on 18 October 2015).
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Suzuki, R.; Shimodaira, H. Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 2006, 22, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Kai, W.; Nomura, K.; Fujiwara, A.; Nakamura, Y.; Yasuike, M.; Ojima, N.; Masaoka, T.; Ozaki, A.; Kazeto, Y.; Gen, K.; et al. ddRAD-based genetic map and its integration with the genome assembly of Japanese eel (Anguilla japonica) provides insights into genome evolution after the teleost-specific genome duplication. BMC Genom. 2014, 15, 233. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yasuike, M.; Mekuchi, M.; Iwasaki, Y.; Ojima, N.; Fujiwara, A.; Chow, S.; Saitoh, K. Rhodopsin gene copies in Japanese eel originated at the teleost-specific genome duplication. Zool. Lett. 2017, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Foll, M.; Gaggiotti, O. A genome-scan method to identify selected loci appropriate for both dominant and codominant markers: A Bayesian perspective. Genetics 2008, 180, 977–993. [Google Scholar] [CrossRef] [PubMed]
- Antao, T.; Lopes, A.; Lopes, R.J.; Beja-Pereira, A.; Luikart, G. LOSITAN: A workbench to detect molecular adaptation based on a FST-outlier method. BMC Bioinform. 2008, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, M.W.; Smedegaard, L.; Sørensen, S.R.; Pujolar, J.M.; Munk, P.; Jónsson, B.; Magnussen, E.; Hansen, M.M. Assessing pre- and post-zygotic barriers between North Atlantic eels (Anguilla anguilla and A. rostrata). Heredity 2017, 118, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, K.A.; Gaines, S.D.; Caselle, J.E.; Warner, R.R. Current shifts and kin aggregation explain genetic patchiness in fish recruits. Ecology 2006, 87, 3082–3094. [Google Scholar] [CrossRef]
- Pujolar, J.M.; Bevacqua, D.; Andrello, M.; Capoccioni, F.; Ciccotti, E.; De Leo, G.A.; Zane, L. Genetic patchiness in European eel adults evidenced by molecular genetics and population dynamics modelling. Mol. Phylogenet. Evol. 2011, 58, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Hedgecock, D.; Pudovkin, A.I. Sweepstakes reproductive success in highly fecund marine fish and shellfish: A review and commentary. Bull. Mar. Sci. 2011, 87, 971–1002. [Google Scholar] [CrossRef]
- Kawecki, T.J.; Ebert, D. Conceptual issues in local adaptation. Ecol. Lett. 2004, 7, 1225–1241. [Google Scholar] [CrossRef]
- Fraser, D.J.; Weir, L.K.; Bernatchez, L.; Hansen, M.M.; Taylor, E.B. Extend and scale of local adaptation in salmonid fishes: Review and meta-analysis. Heredity 2011, 106, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.; Butkauskas, D. The formation of the population genetic structure of the European eel Anguilla anguilla (L.): A short review. Ekologija 2013, 59, 143–154. [Google Scholar] [CrossRef]
- Maes, G.E.; Volckaert, F.A.M. Clinal genetic variation and isolation by distance in the European eel Anguilla anguilla (L.). Biol. J. Linn. Soc. Lond. 2002, 77, 509–521. [Google Scholar] [CrossRef]
- Baltazar-Soares, M.; Biastoch, A.; Harrod, C.; Hanel, R.; Marohn, L.; Prigge, E.; Evans, D.; Bodles, K.; Behrens, E.; Böning, C.W.; et al. Recruitment collapse and population structure of the European eel shaped by local ocean current dynamics. Curr. Biol. 2014, 24, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Feder, J.L.; Nosil, P. The efficacy of divergence hitchhiking in generating genomic islands during ecological speciation. Evolution 2010, 64, 1729–1747. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, S.; Otto, S.P. Establishment and maintenance of adaptive genetic divergence under migration, selection and drift. Evolution 2010, 65, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, S.; Whitlock, M.C. The genetic architecture of adaptation under migration-selection balance. Evolution 2011, 65, 1897–1911. [Google Scholar] [CrossRef] [PubMed]
- Feder, J.L.; Egan, S.P.; Nosil, P. The genomics of speciation-with-gene-flow. Trends Genet. 2012, 28, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Edeline, E.; Dufour, S.; Elie, P. Role of glass eel salinity preference in the control of habitat selection and growth plasticity in Anguilla anguilla. Mar. Ecol. Prog. Ser. 2005, 304, 191–199. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igarashi, Y.; Zhang, H.; Tan, E.; Sekino, M.; Yoshitake, K.; Kinoshita, S.; Mitsuyama, S.; Yoshinaga, T.; Chow, S.; Kurogi, H.; et al. Whole-Genome Sequencing of 84 Japanese Eels Reveals Evidence against Panmixia and Support for Sympatric Speciation. Genes 2018, 9, 474. https://doi.org/10.3390/genes9100474
Igarashi Y, Zhang H, Tan E, Sekino M, Yoshitake K, Kinoshita S, Mitsuyama S, Yoshinaga T, Chow S, Kurogi H, et al. Whole-Genome Sequencing of 84 Japanese Eels Reveals Evidence against Panmixia and Support for Sympatric Speciation. Genes. 2018; 9(10):474. https://doi.org/10.3390/genes9100474
Chicago/Turabian StyleIgarashi, Yoji, Hong Zhang, Engkong Tan, Masashi Sekino, Kazutoshi Yoshitake, Shigeharu Kinoshita, Susumu Mitsuyama, Tatsuki Yoshinaga, Seinen Chow, Hiroaki Kurogi, and et al. 2018. "Whole-Genome Sequencing of 84 Japanese Eels Reveals Evidence against Panmixia and Support for Sympatric Speciation" Genes 9, no. 10: 474. https://doi.org/10.3390/genes9100474
APA StyleIgarashi, Y., Zhang, H., Tan, E., Sekino, M., Yoshitake, K., Kinoshita, S., Mitsuyama, S., Yoshinaga, T., Chow, S., Kurogi, H., Shinoda, A., Han, Y.-S., Wakiya, R., Mochioka, N., Yamamoto, T., Kuwada, H., Kaji, Y., Suzuki, Y., Gojobori, T., ... Asakawa, S. (2018). Whole-Genome Sequencing of 84 Japanese Eels Reveals Evidence against Panmixia and Support for Sympatric Speciation. Genes, 9(10), 474. https://doi.org/10.3390/genes9100474




