Euchromatic Supernumerary Chromosomal Segments—Remnants of Ongoing Karyotype Restructuring in the Prospero autumnale Complex?
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Karyotyping and FISH (Fluorescence In Situ Hybridisation with 5S & 35S Ribosomal DNAs, Vertebrate-Type Telomeric Repeats, and Satellite DNA PaB6)
2.3. GISH (Genomic In Situ Hybridisation)
3. Results and Discussion
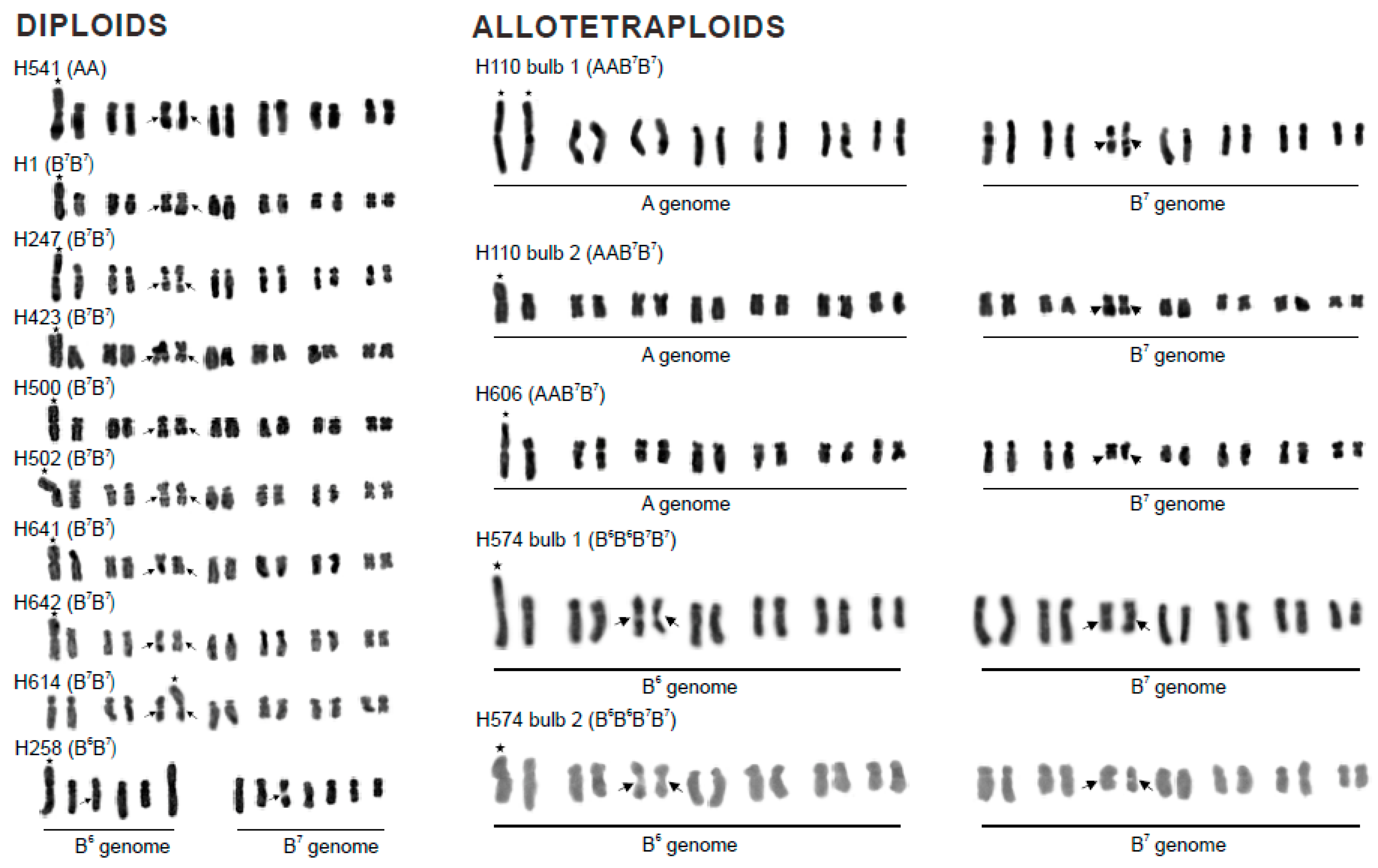
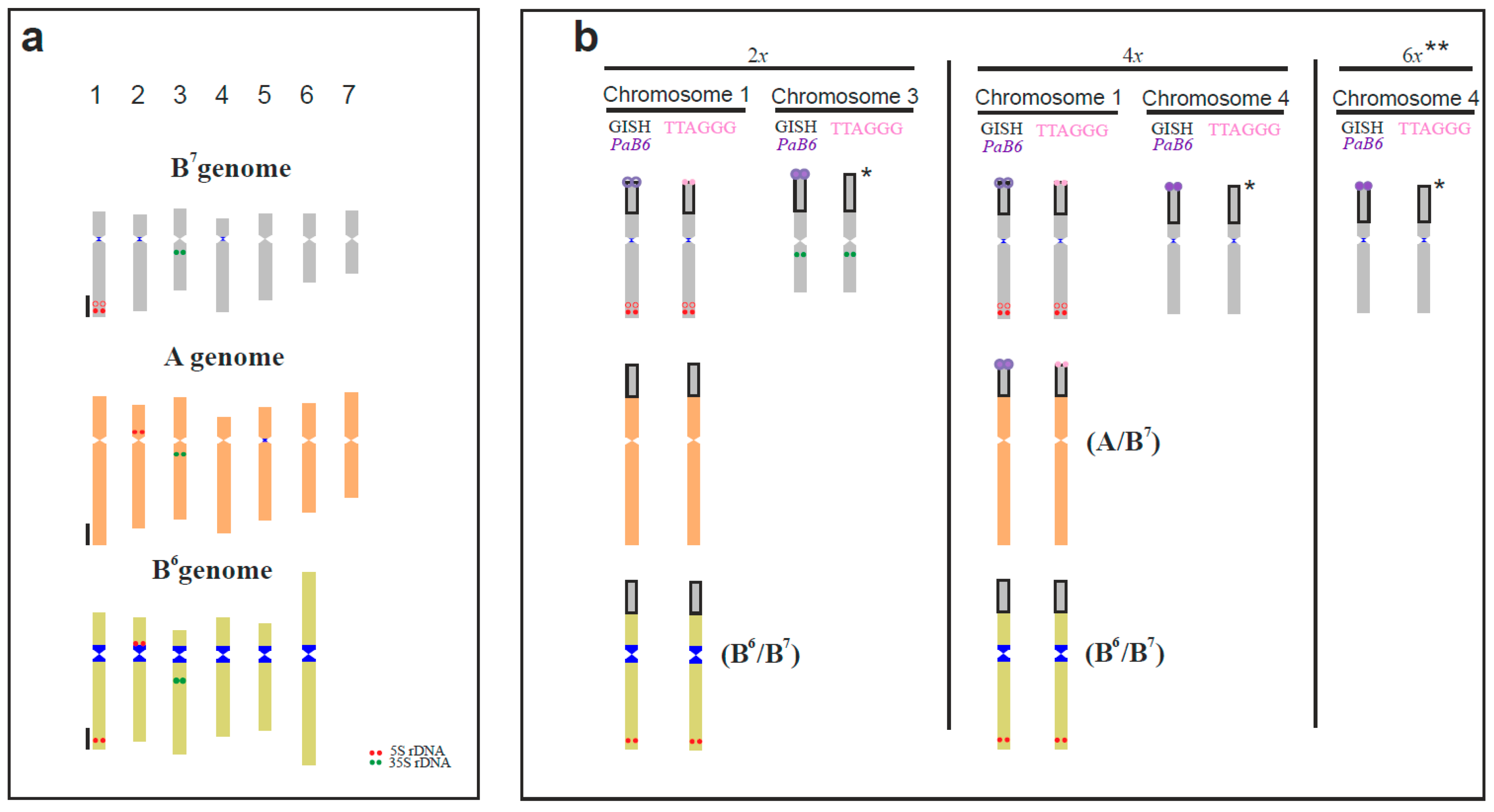
3.1. Karyotype Structure and Localisation of Supernumerary Chromosomal Segments
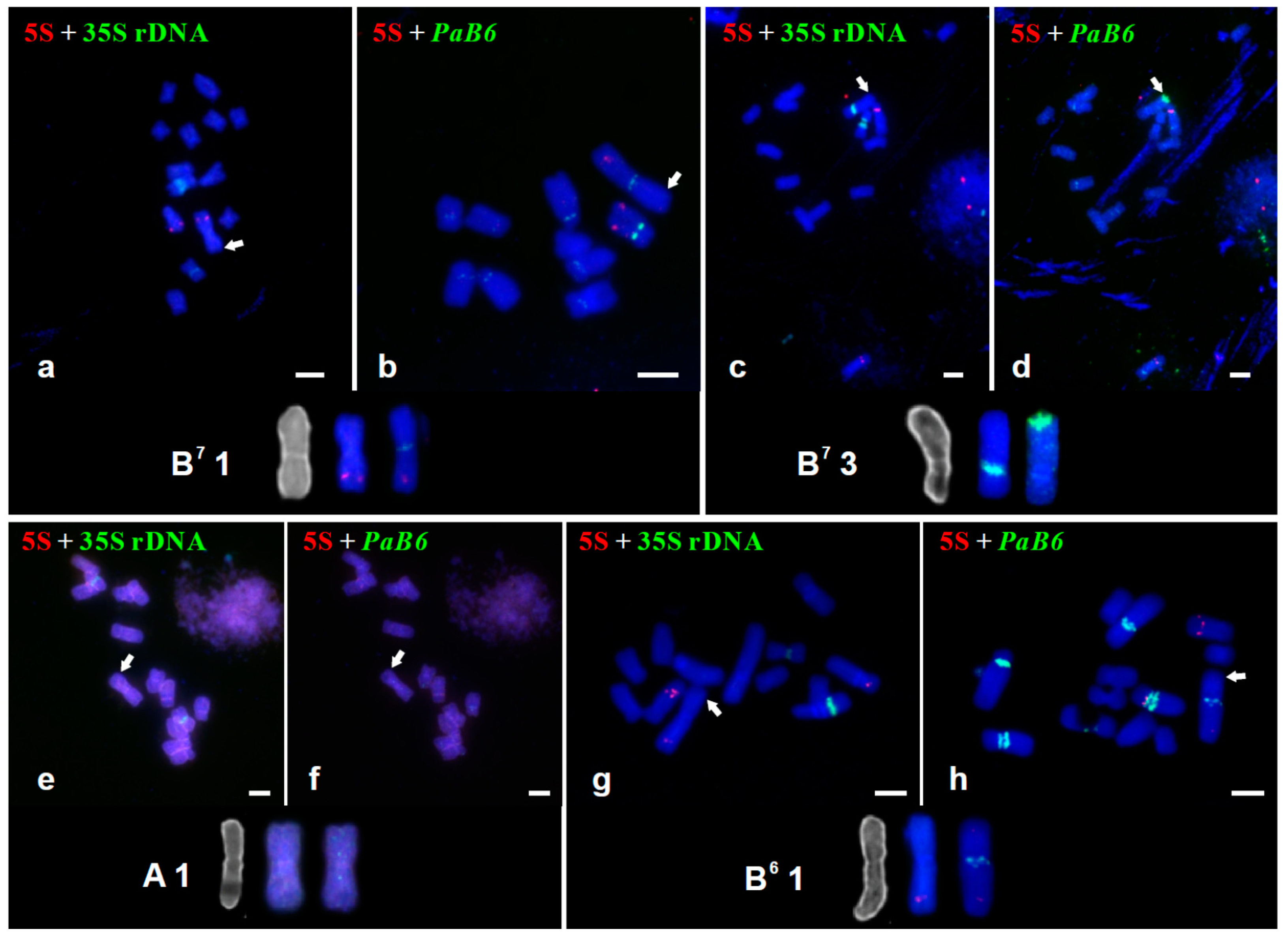
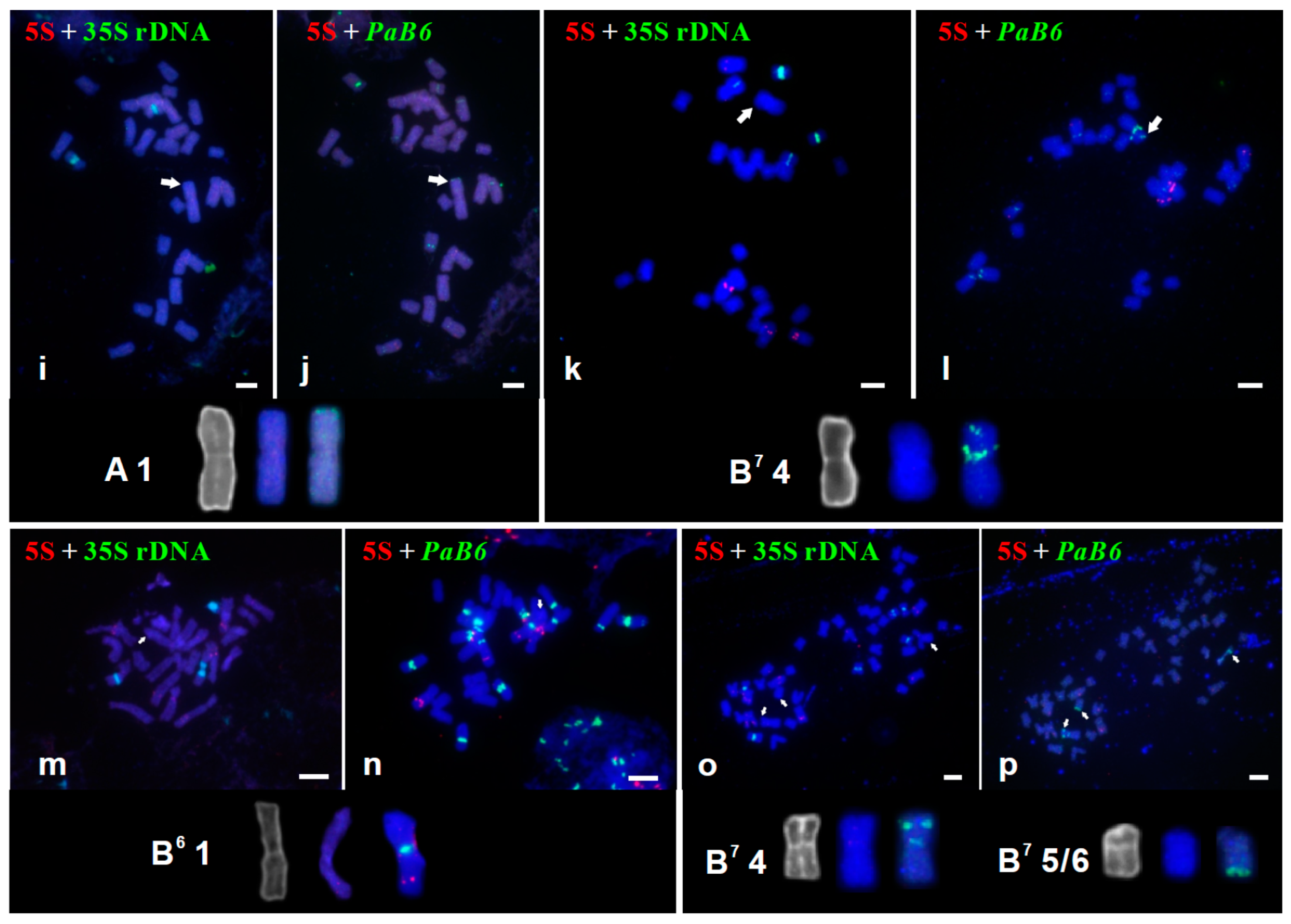
3.2. Tandem Repeats in Supernumerary Chromosomal Segments
3.3. Genomic DNA Affinities of the Supernumerary Chromosomal Segments (Genomic In Situ Hybridisation)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Houben, A.; Banaei-Moghaddam, A.M.; Klemme, S. Biology and evolution of B chromosomes. In Plant Genome Diversity, Vol 2, Physical Structure, Behavior and Evolution of Plant Genomes, 1st ed.; Leitch, I.J., Greilhuber, J., Dolezel, J., Wendel, J., Eds.; Springer Verlag: Vienna, Austria, 2013; pp. 149–165. [Google Scholar]
- Weiss-Schneeweiss, H.; Schneeweiss, G.M. Karyotype diversity and evolutionary trends in angiosperms. In Plant Genome Diversity, Vol 2, Physical Structure, Behavior and Evolution of Plant Genomes, 1st ed.; Leitch, I.J., Greilhuber, J., Dolezel, J., Wendel, J., Eds.; Springer Verlag: Vienna, Austria, 2013; pp. 209–230. [Google Scholar]
- Wilby, A.S.; Parker, J.S. The supernumerary segment systems of Rumex acetosa. Heredity 1988, 60, 109–117. [Google Scholar] [CrossRef]
- Greilhuber, J.; Speta, F. Quantitative analysis of the C-banded karyotypes and systematic in the cultivated species of the Scilla siberica group (Liliaceae). Plant Syst. Evol. 1978, 129, 63–109. [Google Scholar] [CrossRef]
- Ruiz Rejón, M.; Oliver, J.L. Genetic variability in Muscari comosum (Liliaceae). I. A comparative analysis of chromosome polymorphisms in Spanish and Aegean populations. Heredity 1981, 47, 403–407. [Google Scholar] [CrossRef]
- Parker, J.S.; Lozano, R.; Taylor, S.; Ruiz Rejon, M. Chromosomal structure of populations of Scilla autumnalis in the Iberian Peninsula. Heredity 1991, 67, 287–297. [Google Scholar] [CrossRef]
- Stevens, J.P.; Bougourd, S.M. The frequency and meiotic behaviour of structural chromosome variants in natural populations of Allium schoenoprasum L. (wild chives) in Europe. Heredity 1991, 66, 391–401. [Google Scholar] [CrossRef]
- Jamilena, M.; Martínez, F.; Garrido-Ramos, M.A.; Ruiz-Rejón, C.; Romero, A.T.; Camacho, J.P.M.; Parker, J.S.; Ruiz-Rejón, M. Inheritance and fitness effects analysis for a euchromatic supernumerary chromosome segment in Scilla autumnalis (Liliaceae). Bot. J. Linn. Soc. 1995, 118, 249–259. [Google Scholar] [CrossRef]
- Garrido-Ramos, M.A.; Jamilena, M.; de la Herrán, R.; Ruiz Rejón, C.; Camacho, J.P.M.; Ruiz-Rejón, M. Inheritance and fitness effects of a pericentric inversion and a supernumerary chromosome segment in Muscari comosum (Liliaceae). Heredity 1998, 80, 724–731. [Google Scholar] [CrossRef]
- Weiss-Schneeweiss, H.; Riha, K.; Jang, C.G.; Puizina, J.; Scherthan, H.; Schweizer, D. Chromosome termini of the monocot plant Othocallis siberica are maintained by telomerase, which specifically synthesizes vertebrate-type telomere sequences. Plant J. 2004, 37, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, C.C.; Parker, J.S.; Horton, D.M. Chromosome variation and evolution in Scilla autumnalis. In Kew Chromosome Conference II; Brandham, P.E., Bennett, M.D., Eds.; George Allen & Unwin: London, UK, 1983; pp. 261–268. [Google Scholar]
- Ebert, I.; Greilhuber, J.; Speta, F. Chromosome banding and genome size differentiation in Prospero (Hyacinthaceae): Diploids. Plant Syst. Evol. 1996, 203, 143–177. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Cabrero, J.; Camacho, J.P.M. Chiasma redistribution in bivalents carrying supernumerary chromosome segments in grasshoppers. Heredity 1985, 55, 245–248. [Google Scholar] [CrossRef]
- Ruiz Rejón, C.; Ruiz Rejón, M. Chromosomal polymorphism for a heterochromatic supernumerary segment in a natural population of Tulipa australis Link (Liliaceae). Can. J. Genet. Cytol. 1981, 27, 633–638. [Google Scholar] [CrossRef]
- Jang, T.-S.; Emadzade, K.; Parker, J.; Temsch, E.M.; Leitch, A.R.; Speta, F.; Weiss-Schneeweiss, H. Chromosomal diversification and karyotype evolution of diploids in the cytologically diverse genus Prospero (Hyacinthaceae). BMC Evol. Biol. 2013, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, C.C. The Population Cytology of Scilla autumnalis. Ph.D. Thesis, University of London, London, UK, 1980. [Google Scholar]
- Ebert, I. Systematische Karyologie und Embryologie von Prospero Salisb. und Barnardia Lindl. (Hyacinthaceae). Ph.D. Thesis, University of Vienna, Vienna, Austria, 1993. [Google Scholar]
- Speta, F. The autumn-flowering squills of the Mediterranean Region. In Proceedings of the 5th Optima Meeting, Istanbul, Turkey, 18–30 July 1993; University of Istanbul: Istanbul, Turkey, 1993; pp. 109–124. [Google Scholar]
- Speta, F. Beitrag zur Kenntnis der Gattung Prospero Salisb (Hyacinthaceae) auf der griechischen Insel Kreta. Linzer Biol. Beitr. 2000, 32, 1323–1326. [Google Scholar]
- Jang, T.-S.; Parker, J.; Emadzade, K.; Temsch, E.M.; Leitch, A.R.; Weiss-Schneeweiss, H. Multiple origins and nested cycles of hybridization result in high tetraploid diversity in the monocot Prospero. Front. Plant Sci. 2018, 9, e433. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S. Chromosomal Evolution of Scilla autumnalis. Ph.D. Thesis, University of London, London, UK, 1997. [Google Scholar]
- Vaughan, H.E.; Taylor, S.; Parker, J.S. The ten cytological races of the Scilla autumnalis species complex. Heredity 1997, 79, 371–379. [Google Scholar] [CrossRef]
- Jang, T.-S. Chromosomal Evolution in Prospero autumnale Complex. Ph.D. Thesis, University of Vienna, Vienna, Austria, 2013. [Google Scholar]
- Jang, T.-S.; Parker, J.; Weiss-Schneeweiss, H. Structural polymorphisms and distinct genomic composition suggest recurrent origin and ongoing evolution of B chromosomes in the Prospero autumnale complex (Hyacinthaceae). New Phytol. 2016, 210, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Shibata, F.; Hizume, M.; Kuroki, Y. Molecular cytogenetic analysis of supernumerary heterochromatic segments in Rumex acetosa. Genome 2000, 43, 391–397. [Google Scholar] [CrossRef] [PubMed]
- John, B. The cytogenetic systems of grasshoppers and locusts II. The origin and evolution of supernumerary segments. Chromosoma 1973, 44, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.P.M.; Cabrero, J. New hypotheses about the origin of supernumerary chromosome segments in grasshoppers. Heredity 1987, 58, 341–343. [Google Scholar] [CrossRef]
- Emadzade, K.; Jang, T.-S.; Macas, J.; Kovařík, A.; Novák, P.; Parker, J.; Weiss-Schneeweiss, H. Differential amplification of satellite PaB6 in chromosomally hypervariable Prospero autumnale complex (Hyacinthaceae). Ann. Bot. 2014, 114, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.-S.; Weiss-Schneeweiss, H. Formamide-free genomic in situ hybridization (ff-GISH) allows unambiguous discrimination of highly similar parental genomes in diploid hybrids and allopolyploids. Cytogenet. Genome Res. 2015, 146, 325–331. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Jenkins, G.; Parker, J.S. Elimination of multivalents during meiotic prophase in Scilla autumnalis. I. Diploid and triploid. Genome 1988, 30, 930–939. [Google Scholar] [CrossRef]
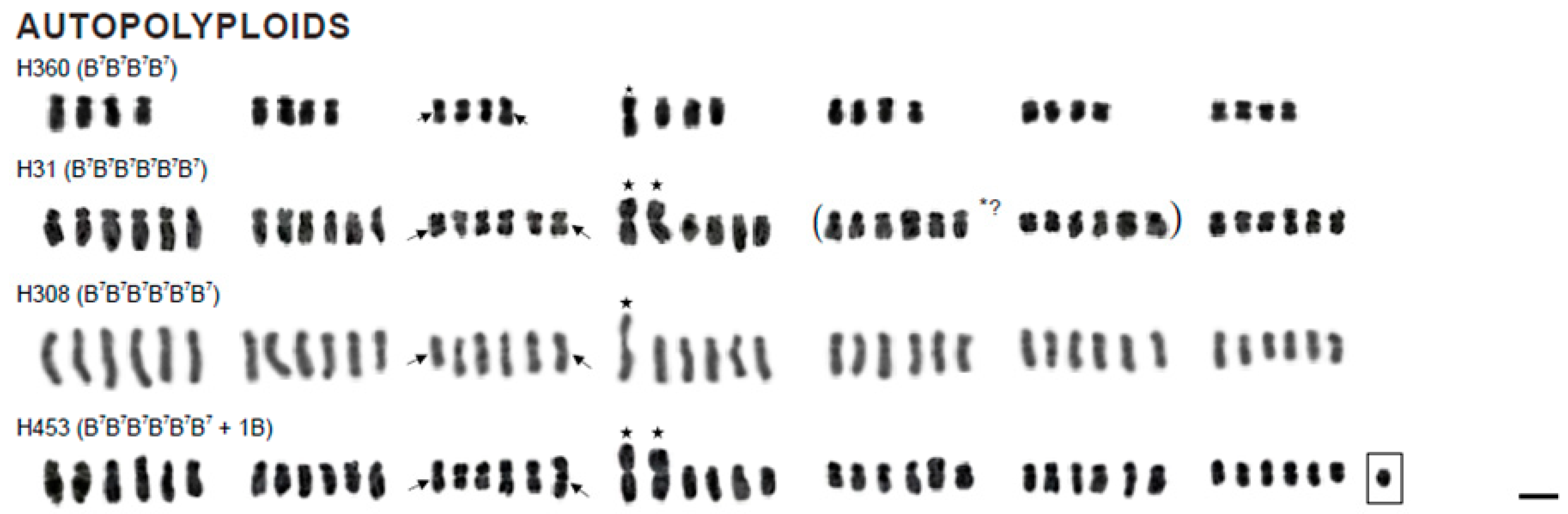
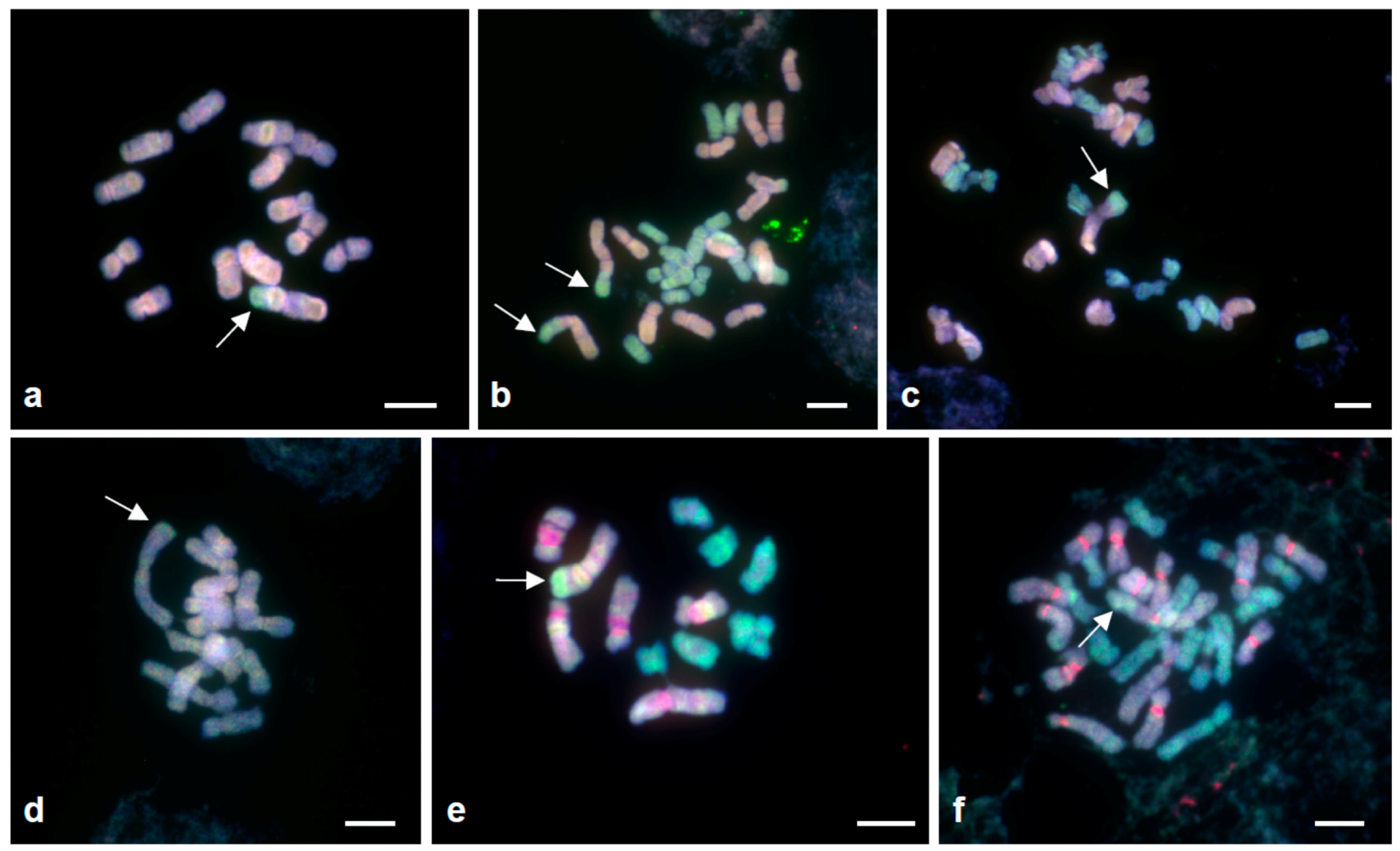
| Cytotype (Accession Number) | Locality; Collector | 2n | Genomic Location/Genomic Affinity of SCSs | SCS Length (µm) | Proportion of SCS (%) in the Chromosome † | Figure No. |
|---|---|---|---|---|---|---|
| Diploids with SCSs | ||||||
| AA (H541) | Spain, Huelva; J. Parker | 14 | A 1/genome B7 | 2.67 | 28.8 | Figure 1, Figure 2e,f, Figure 3a and Figure S1a |
| B7B7 (H1) | Greece, Rhodos; F. Speta | 14 | B7 1 | 2.02 | 28.8 | Figure 1 and Figure 2a,b |
| B7B7 (H247) | Greece, Crete; F. Speta | 14 | B7 1 | 2.02 | 24.5 | Figure 1 |
| B7B7 (H423) | Montenegro; F. Speta | 14 | B7 1 | 2.07 | 31.3 | Figure 1 |
| B7B7 (H500) | Greece, Crete; F. Speta | 14 | B7 1 | 2.02 | 30.6 | Figure 1 |
| B7B7 (H502) | Greece, Crete; F. Speta | 14 | B7 1 | 2.07 | 33.3 | Figure 1 |
| B7B7 (H614) | Israel, HaCarmel Park; J. Parker | 14 | B7 3 | 2.29 | 36.4 | Figure 1 and Figure 2c,d |
| B7B7 (H641) | Spain; J. Parker | 14 | B7 1 | 1.98 | 32.0 | Figure 1, Figure 3d and Figure S1d |
| B7B7 (H642) | Spain; J. Parker | 14 | B7 1 | 2.07 | 27.8 | Figure 1 |
| B6B7 (H258) | Greece, Crete; F. Speta | 13 | B6 1 | 2.11 | 23.4 | Figure 1, Figure 2g,h,e and Figure S1e |
| Polyploids with SCSs | ||||||
| AAB7B7 (H110–1) | Portugal, Cheleiros; F. Speta | 28 | A 1 * | 3.16 | 21.4 | Figure 1, Figure 3b and Figure S1b |
| AAB7B7 (H110–2) | Portugal, Cheleiros; F. Speta | 28 | A 1 | 2.63 | 33.3 | Figure 1, Figure 2i,j, Figure 3c and Figure S1c |
| AAB7B7 (H606) | Portugal, Castro Marin; J. Parker | 28 | A 1 | 2.89 | 26.2 | Figure 1 |
| B6B6B7B7 (H574–1) | Greece, Naxos; F. Speta | 28 | B6 1 | 3.85 | 27.3 | Figure 1, Figure 2m,n, Figure 3f and Figure S1f |
| B6B6B7B7 (H574–2) | Greece, Naxos; F. Speta | 28 | B6 1 | 3.21 | 31.3 | Figure 1 |
| B7B7B7B7 (H360) | Greece, Kos; F. Speta | 28 | B7 4 | 1.94 | 35.3 | Figure 1 and Figure 2k,l |
| B7B7B7B7B7B7 (H31) | No information; F. Speta | 42 | B7 4 * and (5 or 6) § | 1.92 § | 33.3 § | Figure 1 and Figure 2o,p |
| B7B7B7B7B7B7 (H308) | Croatia, Solta; F. Speta | 42 | B7 4 | 2.88 | 34.6 | Figure 1 |
| B7B7B7B7B7B7 (H453) | Croatia, Mosor; F. Speta | 42 + 1B | B7 4 * | 2.56 | 33.3 | Figure 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, T.-S.; Parker, J.S.; Weiss-Schneeweiss, H. Euchromatic Supernumerary Chromosomal Segments—Remnants of Ongoing Karyotype Restructuring in the Prospero autumnale Complex? Genes 2018, 9, 468. https://doi.org/10.3390/genes9100468
Jang T-S, Parker JS, Weiss-Schneeweiss H. Euchromatic Supernumerary Chromosomal Segments—Remnants of Ongoing Karyotype Restructuring in the Prospero autumnale Complex? Genes. 2018; 9(10):468. https://doi.org/10.3390/genes9100468
Chicago/Turabian StyleJang, Tae-Soo, John S. Parker, and Hanna Weiss-Schneeweiss. 2018. "Euchromatic Supernumerary Chromosomal Segments—Remnants of Ongoing Karyotype Restructuring in the Prospero autumnale Complex?" Genes 9, no. 10: 468. https://doi.org/10.3390/genes9100468
APA StyleJang, T.-S., Parker, J. S., & Weiss-Schneeweiss, H. (2018). Euchromatic Supernumerary Chromosomal Segments—Remnants of Ongoing Karyotype Restructuring in the Prospero autumnale Complex? Genes, 9(10), 468. https://doi.org/10.3390/genes9100468




