Chloroplast Genome Sequence of Clusterbean (Cyamopsis tetragonoloba L.): Genome Structure and Comparative Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Chloroplast DNA Isolation
2.2. Chloroplast Genome Sequencing, Assembly, and Annotation
2.3. Genome Analysis
2.4. Simple Sequence Repeats Analysis
3. Results and Discussion
3.1. Genome Features of Clusterbean Chloroplast Genome
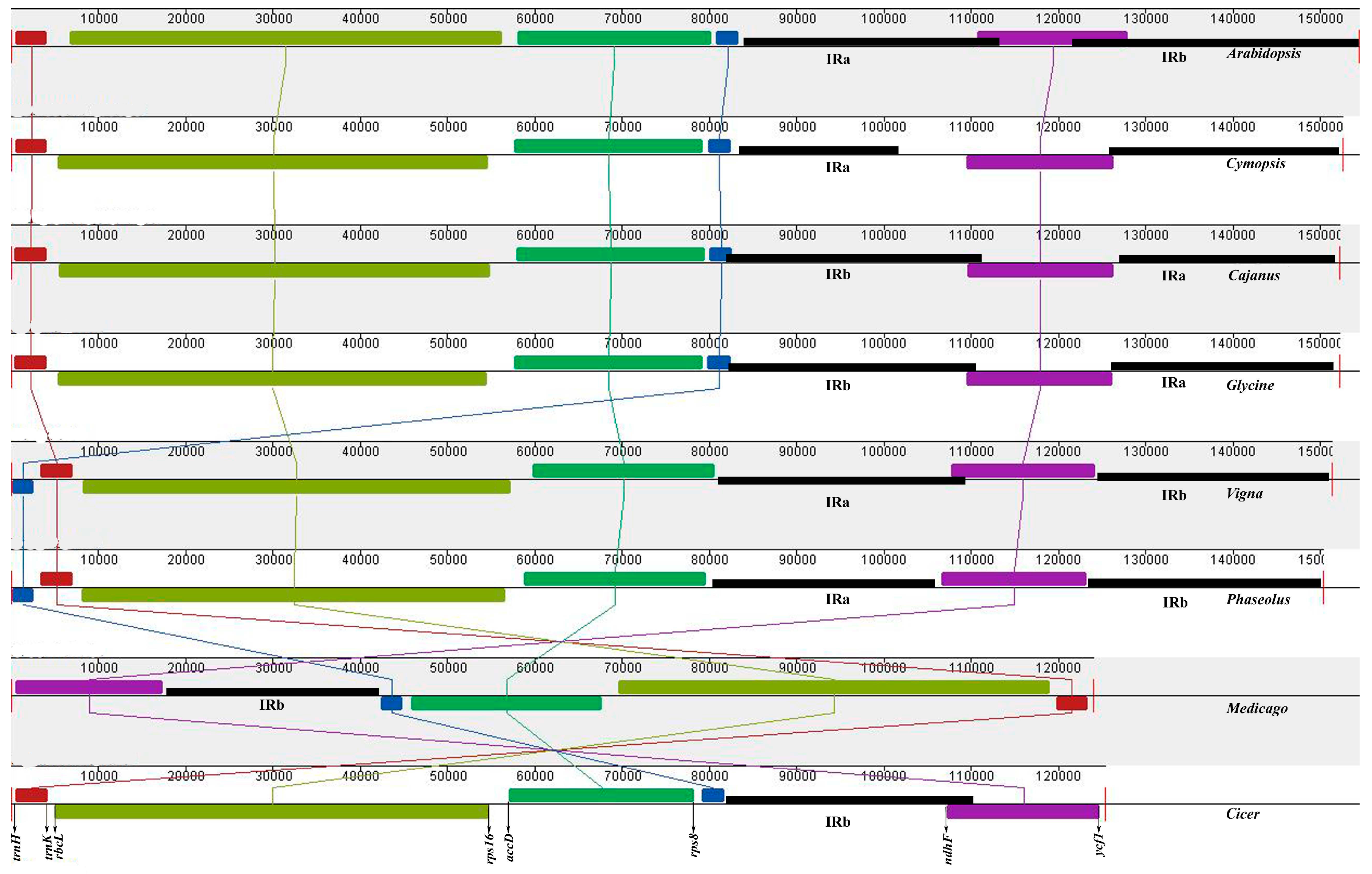
3.2. Gene Order
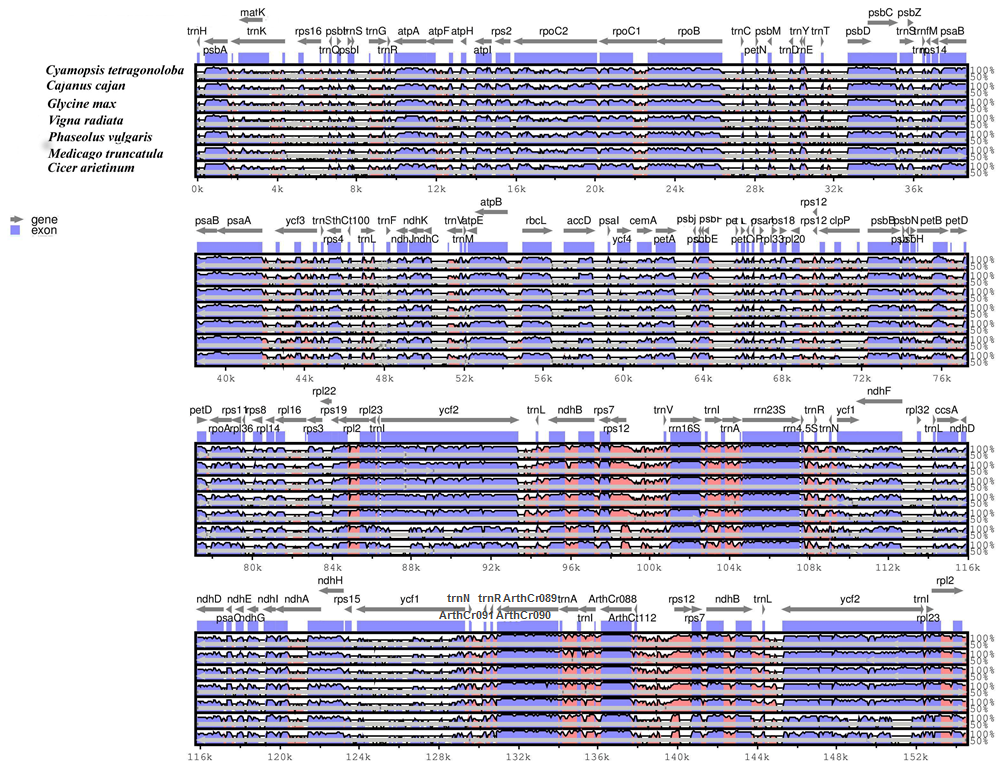
3.3. Plastid Genome Sequence Comparison
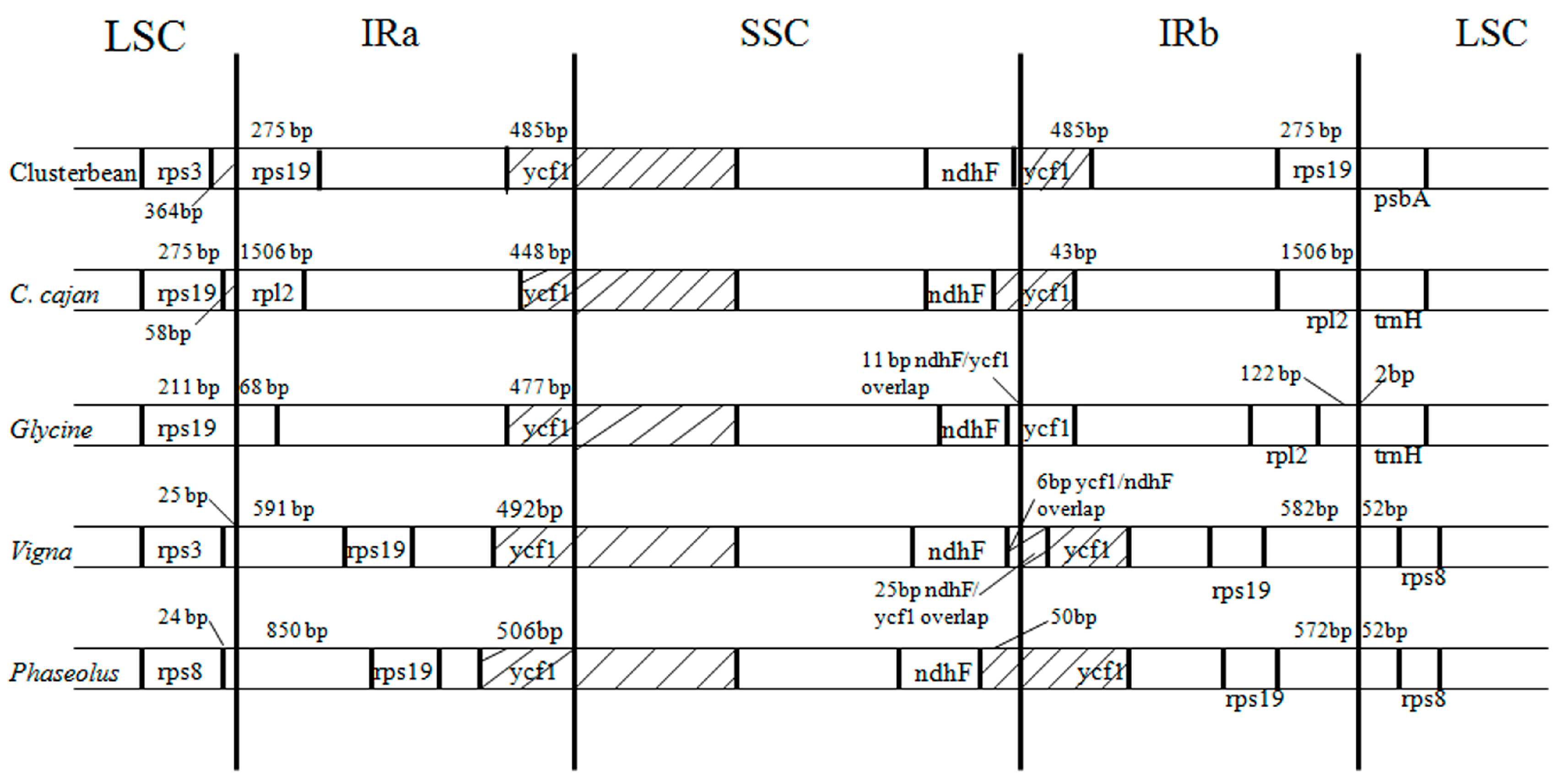
3.4. Comparison of Inverted Repeat Boundaries of Clusterbean with Other Closely Related Plastid Genomes
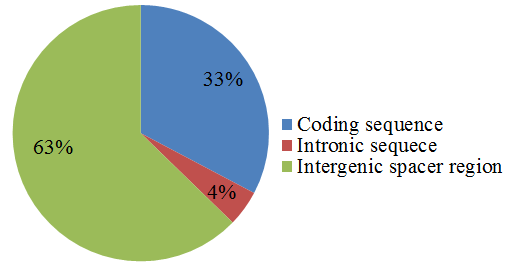
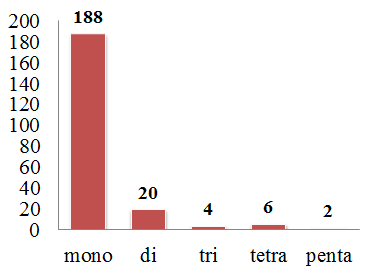
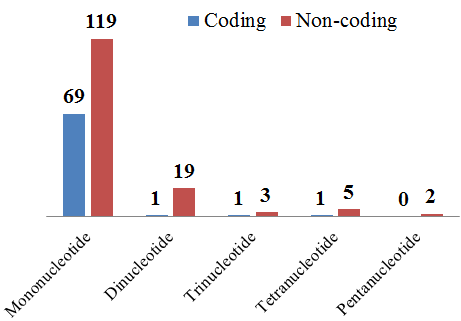
3.5. Simple Sequence Repeats Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Cp | chloroplast |
| LSC | large single-copy region |
| SSC | small single-copy region |
| IR | inverted repeat |
| DNA | deoxyribonucleic acid |
| bp | base pair(s) |
| GC | guanine–cytosine |
| AT | adenosine–thymine |
| kb | kilobase(s) |
| RNA | ribonucleic acid |
| rRNA | ribosomal RNA |
| SSR | simple sequence repeat(s) |
| tRNA | transfer RNA |
References
- Ashraf, M.Y.; Akhtar, K.; Sarwar, G.; Ashraf, M. Role of the rooting system in salt tolerance potential of different Guar accessions. Agron. Sustain. Dev. 2005, 25, 243–249. [Google Scholar] [CrossRef]
- Francois, L.E.; Donovan, T.J.; Maas, E.V. Salinity effects on emergence, vegetative growth and seed yield of Guar. Agron. J. 1990, 82, 587–592. [Google Scholar] [CrossRef]
- Gresta, F.; De Luca, A.I.; Strano, A.; Falcone, G.; Santonoceto, C.; Anastasi, U.; Gulisano, G. Economic and environmental sustainability analysis of guar (Cyamopsis tetragonoloba L.) farming process in a mediterranean area: Two case studies. Ital. J. Agron. 2014, 9, 20–24. [Google Scholar] [CrossRef]
- Undersander, D.J.; Putnam, D.H.; Kaminski, A.R.; Kelling, K.A.; Doll, J.D.; Oplinger, E.S.; Gunsolus, J.L. Guar. In Alternative Field Crops Manual; University of Wisconsin-Extension: Madison, WI, USA, 1991. [Google Scholar]
- Tucker, B.; Foraker, R. Cotton and grain sorghum yields following Guar and Cowpeas compared to continuous cropping. AGRIS 1975, 728, 24–27. [Google Scholar]
- Tripp, L.D.; Lovelace, D.A.; Boring, E.P., III. Keys to profitable Guar production; Texas Agricultural Experimental Station Bulletin: College Station, TX, USA, 2011; pp. 7–11. [Google Scholar]
- Elsheikh, E.A.E.; Ibrahim, K.A. The effect of Bradyrhizobium inoculation on yield and seed quality of guar (Cyamopsis tetragonoloba L.). Food Chem. 1999, 65, 183–187. [Google Scholar] [CrossRef]
- Kalyani, D.L. Performance of Cluster Bean Genotypes under Varied Time of Sowing. Legum. Res. Int. J. 2012, 35, 154–158. [Google Scholar]
- Aykroyd, U.R. Indian Council of Medical Research, Special Report; Vegetable, National Book Trust India: New Delhi, India, 1963; Volume 42, pp. 188–191. [Google Scholar]
- Jackson, K.J.; Doughton, J.A. Guar: A potential industrial crop for dry tropics of Australia. J. Aust. Inst. Agric. Sci. 1982, 42, 17–31. [Google Scholar]
- Miyazawa, T.; Funazukuri, T. Noncatalytic hydrolysis of guar gum under hydrothermal conditions. Carbohydr. Res. 2006, 341, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D. Locust bean gum: Processing, properties and food applications-A review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Sainy, M.I.; Paroda, R.S. Guar cultivation in Haryana, India. Department of Plant Breeding. Chaudhary Charan Singh Agric. J. 1984, 104, 199–203. [Google Scholar]
- Vaughna, S.F.; Berhowa, M.A.; Winkler-Mosera, J.; Lee, E. Formulation of a biodegradable, odor-reducing cat litter from solvent-extracted corn dried distillers grains. Ind. Crops Prod. 2011, 34, 999–1002. [Google Scholar] [CrossRef]
- Douglas, S.E. Plastid evolution: Origins, diversity, trends. Curr. Opin. Genet. Dev. 1990, 8, 655–661. [Google Scholar] [CrossRef]
- Raubeson, L.A.; Jansen, R.K. Chloroplast genomes of plants. In Plant Diversity and Evolution: Genotypic and Phenotypic Variation in Higher Plants; Henry, R.J., Ed.; CABI Press: Cambridge, MA, USA, 2005; pp. 45–68. [Google Scholar]
- Wicke, S.; Schneeweiss, G.M.; dePamphilis, C.W.; Müller, K.F.; Quandt, D. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol. 2011, 76, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M. The chloroplast genome. Plant Mol. Biol. 1992, 19, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Lin, H.C.; Lin, I.P.; Chow, T.Y.; Chen, H.H.; Chen, W.H.; Cheng, C.H.; Lin, C.Y.; Liu, S.M.; Chang, C.C.; et al. The chloroplast genome of Phalaenopsis aphrodite (Orchidaceae): Comparative analysis of evolutionary rate with that of grasses and its phylogenetic implications. Mol. Biol. Evol. 2006, 23, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.J.; Cheng, C.L.; Chang, C.C.; Wu, C.L.; Su, T.M.; Chaw, S.M. Dynamics and evolution of the inverted repeat-large single copy junctions in the chloroplast genomes of monocots. BMC Evol. Biol. 2008, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Bock, R. (Ed.) Structure, function, and inheritance of plastid genomes. In Cell and Molecular Biology of Plastids; Springer: Berlin/Heidelberg, Germany, 2007; pp. 29–63. [Google Scholar]
- Palmer, J.D.; Thompson, W.F. Rearrangements in the chloroplast genomes of mung bean and pea. Proc. Natl. Acad. Sci. USA 1981, 78, 5533–5537. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.K.; Ruhlman, T.A. Plastid genomes of seed plants. In Genomics of Chloroplasts and Mitochondria: Advances in Photosynthesis and Respiration; Bock, R., Knoop, V., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 35, pp. 103–126. [Google Scholar]
- Wojciechowski, M.F.; Sanderson, M.J.; Steele, K.P.; Liston, A. Molecular phylogeny of the “Temperate Herbaceous Tribes” of papilionoid legumes: A supertree approach. Adv. Legum. Syst. 2000, 9, 277–298. [Google Scholar]
- Strauss, S.H.; Palmer, J.D.; Howe, G.T.; Doerksen, A.H. Chloroplast genomes of two conifers lack a large inverted repeat and are extensively rearranged. Proc. Natl. Acad. Sci. USA 1988, 85, 3898–3902. [Google Scholar] [CrossRef] [PubMed]
- Tsudzuki, J.; Nakashima, K.; Tsudzuki, T.; Hiratsuka, J.; Shibata, M.; Wakasugi, T.; Sugiura, M. Chloroplast DNA of black pine retains a residual inverted repeat lacking rRNA genes: Nucleotide sequences of trnQ, trnK, psbA, trnI and trnH and the absence of rps16. Mol. Gen. Genet. 1992, 232, 206–214. [Google Scholar] [PubMed]
- Palmer, J.D.; Thompson, W.F. Chloroplast DNA rearrangements are more frequent when a large inverted repeat sequence is lost. Cell 1982, 29, 537–550. [Google Scholar] [CrossRef]
- Jansen, R.K.; Cai, Z.; Raubeson, L.A.; Daniell, H.; dePamphilis, C.W.; Leebens-Mack, J.; Muller, K.F.; Guisinger-Bellian, M.; Haberle, R.C.; Hansen, A.K.; et al. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proc. Natl. Acad. Sci. USA 2007, 104, 19369–19374. [Google Scholar] [CrossRef] [PubMed]
- Wicke, S.; Schneeweiss, G.M. Next-generation organellar genomics: Potentials and pitfalls of high-throughput technologies for molecular evolutionary studies and plant systematics. In Next-Generation Sequencing in Plant Systematics; Hörandl, E., Appelhans, M., Eds.; International Association for Plant Taxonomy (IAPT): Bratislava, Slovakia, 2015; pp. 1–42. ISBN 978-3-87-429492-8. [Google Scholar]
- Guisinger, M.M.; Kuehl, J.V.; Boore, J.L.; Jansen, R.K. Extreme reconfiguration of plastid genomes in the angiosperm family Geraniaceae: Rearrangements, repeats, and codon usage. Mol. Biol. Evol. 2011, 28, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Kaneko, T.; Sato, S.; Nakamura, Y.; Tabata, S. Complete structure of the chloroplast genome of a legume, Lotus japonicus. DNA Res. 2000, 7, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Castillo-Ramírez, S.; González, V.; Bustos, P.; Luís Fernández-Vázquez, J.; Santamaría, R.; Arellano, J.; Cevallos, M.A.; Dávila, G. Rapid evolutionary change of common bean (Phaseolus vulgaris L) plastome, and the genomic diversification of legume chloroplasts. BMC Genom. 2007, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Guisinger, M.; Kim, H.G.; Ruck, E.; Blazier, J.C.; McMurtry, V.; Kuehl, J.V.; Boore, J.; Jansen, R.K. Extensive reorganization of the plastid genome of Trifolium subterraneum (Fabaceae) is associated with numerous repeated sequences and novel DNA insertions. J. Mol. Evol. 2008, 67, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.K.; Wojciechowski, M.F.; Sanniyasi, E.; Lee, S.B.; Daniell, H. Complete plastid genome sequence of the chickpea (Cicer arietinum) and the phylogenetic distribution of rps12 and clpP intron losses among legumes (Leguminosae). Mol. Phylogenet. Evol. 2008, 48, 1204–1217. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Singh, G.P.; Pillay, D.T.N. Structure and sequence evolution of three chloroplast DNAs. Mol. Gen. Genet. 1983, 190, 13–19. [Google Scholar] [CrossRef]
- Spielmann, A.; Ortiz, W.; Stutz, E. The soybean chloroplast genome: Construction of a circular restriction site map and location of DNA regions encoding the genes for rRNAs, the large subunit of the ribulose-1,5-bisphosphate carboxylase and the 32 KD protein of the photosystem II reaction c. MGG Mol. Gen. Genet. 1983, 190, 5–12. [Google Scholar] [CrossRef]
- Kaila, T.; Chaduvla, P.K.; Saxena, S.; Bahadur, K.; Gahukar, S.J.; Chaudhury, A.; Sharma, T.R.; Singh, N.K.; Gaikwad, K. Chloroplast genome equence of Pigeonpea (Cajanus cajan (L.) Millspaugh) and Cajanus scarabaeoides (L.) Thouars: Genome organization and comparison with other legumes. Front. Plant Sci. 2016, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L.; Palmer, J.D. Multiple independent losses of two genes and one intron from legume chloroplast genomes. Syst. Bot. 1995, 20, 272–294. [Google Scholar] [CrossRef]
- Gantt, J.S.; Baldauf, S.L.; Calie, P.J.; Weeden, N.F.; Palmer, J.D. Transfer of rpl22 to the nucleus greatly preceded its loss from the chloroplast and involved the gain of an intron. EMBO J. 1991, 10, 3073–3078. [Google Scholar] [PubMed]
- Magee, A.M.; Aspinall, S.; Rice, D.W.; Cusack, B.P.; Semon, M.; Perry, A.S.; Stefanovic, S.; Milbourne, D.; Barth, S.; Palmer, J.D.; et al. Localized hypermutation and associated gene losses in legume chloroplast genomes. Genome Res. 2010, 20, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, J.; Shimada, H.; Whittier, R.; Ishibashi, T.; Sakamoto, M.; Mori, M.; Kondo, C.; Honji, Y.; Sun, C.R.; Meng, B.Y.; et al. The complete sequence of the rice (Oryza sativa) chloroplast genome: Intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. MGG Mol. Gen. Genet. 1989, 217, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.M.; Neckermann, K.; Igloi, G.L.; Kössel, H. Complete sequence of the maize chloroplast genome: Gene content, hotspots of divergence and fine tuning of genetic information by transcript editing. J. Mol. Biol. 1995, 251, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Massenet, O.; Dorne, A.M.; Briat, J.F.; Mache, R. Expression of the rpl23, rpl2, and rps19 genes in spinach chloroplasts. Nucleic Acids Res. 1988, 16, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Ohme, M.; Tanaka, M.; Wakasugi, T.; Hayashida, N.; Matsubayashi, T.; Zaita, N.; Chunwongse, J.; Obokata, J.; Yamaguchi-Shinozaki, K.; et al. The complete nucleotide sequence of the tobacco chloroplast genome: Its gene organization and expression. EMBO J. 1986, 5, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, K.; Fukuzawa, H.; Kohchi, T.; Shirai, H.; Sano, T.; Sano, S.; Umesono, K.; Shiki, Y.; Takeuchi, M.; Chang, Z.; et al. Chloroplast gene organization deduced from complete sequence of liverwort Marchantia polymorpha chloroplast DNA. Nature 1986, 322, 572–574. [Google Scholar] [CrossRef]
- Birky, C.W. The inheritance of genes in mitochondria and chloroplasts: Laws, Mechanisms, and Models. Annu. Rev. Genet. 2001, 35, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, J.L.; Coleman, A.W.; Corriveau, J.L.; Coleman, A.W. Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Am. J. Bot. 2009, 75, 1443–1458. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y. Sodmergen Examination of the cytoplasmic DNA in male reproductive cells to determine the potential for cytoplasmic inheritance in 295 angiosperm species. Plant Cell Physiol. 2003, 44, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Provan, J.; Powell, W.; Hollingsworth, P.M. Chloroplast microsatellites: New tools for studies in plant ecology and evolution. Trends Ecol. Evol. 2001, 16, 142–147. [Google Scholar] [CrossRef]
- Ravi, V.; Khurana, J.P.; Tyagi, A.K.; Khurana, P. An update on chloroplast genomes. Plant Syst. Evol. 2008, 271, 101–122. [Google Scholar] [CrossRef]
- Wolfe, K.H.; Li, W.H.; Sharp, P.M. Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc. Natl. Acad. Sci. USA 1987, 84, 9054–9058. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, A.; Folta, K.M. ASAP: Amplification, sequencing & annotation of plastomes. BMC Genom. 2005, 6, 176. [Google Scholar] [CrossRef]
- Cronn, R.; Liston, A.; Parks, M.; Gernandt, D.S.; Shen, R.; Mockler, T. Multiplex sequencing of plant chloroplast genomes using Solexa sequencing-by-synthesis technology. Nucleic Acids Res. 2008, 36. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Xu, C.; Cheng, T.; Lin, K.; Zhou, S. Sequencing angiosperm plastid genomes made easy: A complete set of universal primers and a case study on the phylogeny of saxifragales. Genome Biol. Evol. 2013, 5, 989–997. [Google Scholar] [CrossRef] [PubMed]
- McPherson, H.; van der Merwe, M.; Delaney, S.K.; Edwards, M.A.; Henry, R.J.; McIntosh, E.; Rymer, P.D.; Milner, M.L.; Siow, J.; Rossetto, M. Capturing chloroplast variation for molecular ecology studies: A simple next generation sequencing approach applied to a rainforest tree. BMC Ecol. 2013, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Velasco, R.; Zharkikh, A.; Troggio, M.; Cartwright, D.A.; Cestaro, A.; Pruss, D.; Pindo, M.; FitzGerald, L.M.; Vezzulli, S.; Reid, J.; et al. A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE 2007, 2, e1326. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Dhingra, A.; Soltis, P.S.; Shaw, R.; Farmerie, W.G.; Folta, K.M.; Soltis, D.E. Rapid and accurate pyrosequencing of angiosperm plastid genomes. BMC Plant Biol. 2006, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Jordan, W.C.; Courtney, M.W.; Neigel, J.E. Low Levels of Intraspecific Genetic Variation at a Rapidly Evolving Chloroplast DNA Locus in North American Duckweeds (Lemnaceae). Am. J. Bot. 1996, 83, 430–439. [Google Scholar] [CrossRef]
- Uthaipaisanwong, P.; Chanprasert, J.; Shearman, J.R.; Sangsrakru, D.; Yoocha, T.; Jomchai, N.; Jantasuriyarat, C.; Tragoonrung, S.; Tangphatsornruang, S. Characterization of the chloroplast genome sequence of oil palm (Elaeis guineensis Jacq). Gene 2012, 500, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wei, C.L.; Liu, H.W.; Wu, J.L.; Li, Z.G.; Zhang, L.; Jian, J.B.; Li, Y.Y.; Tai, Y.L.; Zhang, J.; et al. Genetic divergence between Camellia sinensis and its wild relatives revealed via genome-wide SNPs from RAD sequencing. PLoS ONE 2016, 11, e0151424. [Google Scholar] [CrossRef] [PubMed]
- Bakker, F.T.; Lei, D.; Yu, J.; Mohammadin, S.; Wei, Z.; van de Kerke, S.; Gravendeel, B.; Nieuwenhuis, M.; Staats, M.; Alquezar-Planas, D.E.; et al. Herbarium genomics: Plastome sequence assembly from a range of herbarium specimens using an iterative organelle genome assembly pipeline. Biol. J. Linn. Soc. 2016, 117, 33–43. [Google Scholar] [CrossRef]
- Bock, D.G.; Kane, N.C.; Ebert, D.P.; Rieseberg, L.H. Genome skimming reveals the origin of the Jerusalem Artichoke tuber crop species: Neither from Jerusalem nor an artichoke. New Phytol. 2014, 201, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, B.; Cheng, F.; Ramchiary, N.; Choi, S.R.; Lim, Y.P.; Wang, X.W. Sequencing of chloroplast genome using whole cellular DNA and Solexa sequencing technology. Front. Plant Sci. 2012, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Chen, J.J.W.; Huang, Y.; Chan, M.; Daniell, H.; Chang, W.; Hsu, C.; Liao, D.C.; Wu, F.; Lin, S.; et al. The location and translocation of ndh genes of chloroplast origin in the Orchidaceae family. Sci. Rep. 2015, 5, 9040. [Google Scholar] [CrossRef] [PubMed]
- Pan, I.C.; Liao, D.C.; Wu, F.H.; Daniell, H.; Singh, N.D.; Chang, C.; Shih, M.C.; Chan, M.T.; Lin, C.S. Complete chloroplast genome sequence of an orchid model plant candidate: Erycina pusilla apply in tropical oncidium breeding. PLoS ONE 2012, 7, e34738. [Google Scholar] [CrossRef] [PubMed]
- Kirti, P.B.; Narasimhulu, S.B.; Mohapatra, T.; Prakash, S.; Chopra, V.L. Correction of chlorophyll deficiency in alloplasmic male sterile Brassica juncea through recombination between chloroplast genomes. Gen. Res. 1993, 62, 11–14. [Google Scholar] [CrossRef]
- Wyman, S.K.; Jansen, R.K.; Boore, J.L. Automatic annotation of organellar genomes with DOGMA. Bioinformatics 2004, 20, 3252–3255. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Eddy, S.R. TRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1996, 25, 955–964. [Google Scholar] [CrossRef]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.C.E.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple alignment of conserved genomic sequence with rearrangements mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- DNA/RNA Base Composition Calculator. Available online: http://www.currentprotocols.com/WileyCDA/CurPro3Tool/toolId-7.html (accessed on 16 April 2017).
- MISA-MIcroSAtellite identification tool. Available online: http://pgrc.ipk-gatersleben.de/misa/ (accessed on 16 April 2017).
- Saski, C.; Lee, S.B.; Daniell, H.; Wood, T.C.; Tomkins, J.; Kim, H.G.; Jansen, R.K. Complete chloroplast genome sequence of Glycine max and comparative analyses with other legume genomes. Plant Mol. Biol. 2005, 59, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Tangphatsornruang, S.; Sangsrakru, D.; Chanprasert, J.; Uthaipaisanwong, P.; Yoocha, T.; Jomchai, N.; Tragoonrung, S. The chloroplast genome sequence of mungbean (Vigna radiata) determined by high-throughput pyrosequencing: Structural organization and phylogenetic relationships. DNA Res. 2010, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Tsudzuki, J.; Ito, S.; Tsudzuki, T.; Wakasugi, T.; Sugiura, M. A new gene encoding tRNAPro (GGG) is present in the chloroplast genome of black pine: A compilation of 32 tRNA genes from black pine chloroplasts. Curr. Genet. 1994, 26, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Tang, P.; Li, Z.; Li, D.; Liu, Y.; Huang, H. The first complete chloroplast genome sequences in Actinidiaceae: Genome structure and comparative analysis. PLoS ONE 2015, 10, e0129347. [Google Scholar] [CrossRef] [PubMed]
- Kazakoff, S.H.; Imelfort, M.; Edwards, D.; Koehorst, J.; Biswas, B.; Batley, J.; Scott, P.T.; Gresshoff, P.M. Capturing the Biofuel Wellhead and Powerhouse: The Chloroplast and Mitochondrial Genomes of the Leguminous Feedstock Tree Pongamia pinnata. PLoS ONE 2012, 7, e51687. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dang, Y.; Li, Q.; Lu, J.; Li, X.; Wang, Y. Complete chloroplast genome sequence of poisonous and medicinal plant Datura stramonium: Organizations and implications for genetic engineering. PLoS ONE 2014, 9, e110656. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Lai, X.; Li, X.; Wei, C.; Tan, X.; Zhang, Y. Analyses of the complete genome and gene expression of chloroplast of sweet potato [Ipomoea batata]. PLoS ONE 2015, 10, e0124083. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Matzke, A.J.M.; Matzke, M. Complete sequence and comparative analysis of the chloroplast genome of coconut palm (Cocos nucifera). PLoS ONE 2013, 8, e74736. [Google Scholar] [CrossRef] [PubMed]
- Salim, H.M.W.; Cavalcanti, A.R.O. Factors influencing codon usage bias in genomes. J. Braz. Chem. Soc. 2008, 19, 257–262. [Google Scholar] [CrossRef]
- Lee, J.; Kang, Y.; Shin, S.C.; Park, H.; Lee, H. Combined analysis of the chloroplast genome and transcriptome of the Antarctic vascular plant Deschampsia antarctica desv. PLoS ONE 2014, 9, e92501. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.H.; Gowri, G. Codon Usage in Higher Plants, Green Algae, and Cyanobacteria. Plant Physiol. 1990, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Wurdack, K.J.; Kanagaraj, A.; Lee, S.B.; Saski, C.; Jansen, R.K. The complete nucleotide sequence of the cassava (Manihot esculenta) chloroplast genome and the evolution of atpF in Malpighiales: RNA editing and multiple losses of a group II intron. Theor. Appl. Genet. 2008, 116, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Downie, S.R.; Olmstead, R.G.; Zurawski, G.; Soltis, D.E.; Soltis, P.S.; Watson, J.C.; Palmer, J.D. Six independent losses of the chloroplast DNA rpl2 intron in dicotyledons: Molecular and phylogenetic implications. Evolution 1991, 45, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.K.; Saski, C.; Lee, S.B.; Hansen, A.K.; Daniell, H. Complete plastid genome sequences of three rosids (Castanea, Prunus, Theobroma): Evidence for at least two independent transfers of rpl22 to the nucleus. Mol. Biol. Evol. 2011, 28, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Millen, R.S. Many parallel losses of infA from chloroplast DNA during angiosperm evolution with multiple independent transfers to the nucleus. Plant Cell 2001, 13, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Cusack, B.P.; Wolfe, K.H. When gene marriages don’t work out: Divorce by subfunctionalization. Trends Genet. 2007, 23, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Fujimoto, M.; Arimura, S.I.; Murata, J.; Tsutsumi, N.; Kadowaki, K.I. Loss of the rpl32 gene from the chloroplast genome and subsequent acquisition of a preexisting transit peptide within the nuclear gene in Populus. Gene 2007, 402, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Nishikawa, T.; Fujimoto, M.; Takanashi, H.; Arimura, S.I.; Tsutsumi, N.; Kadowaki, K.I. Substitution of the gene for chloroplast RPS16 was assisted by generation of a dual targeting signal. Mol. Biol. Evol. 2008, 25, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ueda, M.; Kadowaki, K.; Tsutsumi, N. Different status of the gene for ribosomal protein S16 in the chloroplast genome during evolution of the genus Arabidopsis and closely related species. Genes Genet. Syst. 2010, 85, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Osorio, B.; Thompson, W.F. Evolutionary significance of inversions in legume chloroplast DNAs. Curr. Genet. 1988, 14, 65–74. [Google Scholar] [CrossRef]
- Palmer, J.D. Chloroplast DNA exists in two orientations. Nature 1983, 301, 92–93. [Google Scholar] [CrossRef]
- Lavin, M.; Doyle, J.J.; Palmer, J.D. Evolutionary significance of the loss of the chloroplast-DNA inverted repeat in the leguminosae subfamily papilionoideae. Evolution 1990, 44, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Osorio, B.; Aldrich, J.; Thompson, W.F. Chloroplast DNA evolution among legumes: Loss of a large inverted repeat occurred prior to other sequence rearrangements. Curr. Genet. 1987, 11, 275–286. [Google Scholar] [CrossRef]
- Cronk, Q.; Ojeda, I.; Pennington, R.T. Legume comparative genomics: Progress in phylogenetics and phylogenomics. Curr. Opin. Plant Biol. 2006, 9, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, A.; Doyle, J.J.; Palmer, J.D. A Chloroplast DNA Inversion as a Subtribal Character in the Phaseoleae (Leguminosae). Syst. Bot. 1990, 15, 378–386. [Google Scholar] [CrossRef]
- Khakhlova, O.; Bock, R. Elimination of deleterious mutations in plastid genomes by gene conversion. Plant J. 2006, 46, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D. Plastid chromosomes: Structure and evolution. In Molecular Biology of Plastids; Bogorad, L., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 5–53. [Google Scholar]
- Chen, C.; Zhou, P.; Choi, Y.A.; Huang, S.; Gmitter, F.G. Mining and characterizing microsatellites from citrus ESTs. Theor. Appl. Genet. 2006, 112, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Ko, C.Y.; Kuo, C.I.; Liu, M.S.; Schafleitner, R.; Chen, L.F.O. Transcriptional slippage and RNA editing increase the diversity of transcripts in chloroplasts: Insight from deep sequencing of Vigna radiata genome and transcriptome. PLoS ONE 2015, 10, e0129396. [Google Scholar] [CrossRef] [PubMed]
- Curci, P.L.; Paola, D.D.; Danzi, D.; Vendramin, G.G.; Sonnante, G. Complete chloroplast genome of the multifunctional crop globe artichoke and comparison with other Asteraceae. PLoS ONE 2015, 10, e0120589. [Google Scholar] [CrossRef] [PubMed]
- Ozyigit, I.I.; Dogan, I.; Filiz, E. In silico analysis of simple sequence repeats (SSRs) in chloroplast genomes of Glycine species. Plant Omics J. 2015, 8, 24–29. [Google Scholar]
- Wheeler, G.L.; Dorman, H.E.; Buchanan, A.; Challagundla, L.; Wallace, L.E. A review of the prevalence, utility, and caveats of using chloroplast simple sequence repeats for studies of plant biology. Appl. Plant Sci. 2014, 2, 1400059. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Kim, K. Complete chloroplast genome sequences of important oilseed crop Sesamum indicum L. PLoS ONE 2012, 7, e35872. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Shi, C.; Liu, Y.; Mao, S.; Gao, L. Thirteen Camellia chloroplast genome sequences determined by high-throughput sequencin: Genome structure and phylogenetic relationships. BMC Evol. Boil. 2014, 14, 151. [Google Scholar] [CrossRef]
- Mariotti, R.; Cultrera, N.G.M.; Díez, C.M.; Baldoni, L.; Rubini, A. Identification of new polymorphic regions and differentiation of cultivated olives (Olea europaea L.) through plastome sequence comparison. BMC Plant Boil. 2010, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Liu, J.; Yu, J.; Wang, L.; Zhou, S. Highly variable chloroplast markers for evaluating plant phylogeny at low taxonomic levels and for DNA barcoding. PLoS ONE 2012, 7, e35071. [Google Scholar] [CrossRef] [PubMed]

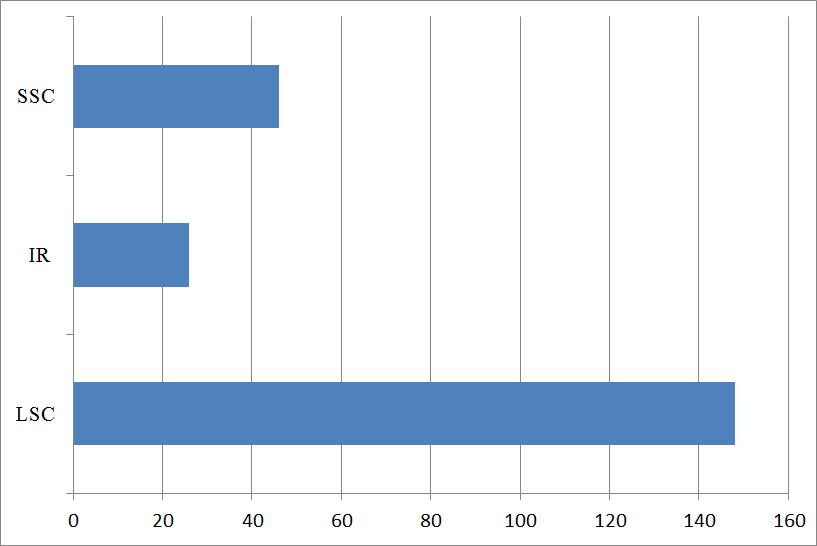
| Features | T/U% | C% | A% | G% | Length (bp) | AT% |
|---|---|---|---|---|---|---|
| Genome | 32 | 17 | 32 | 18 | 152,530 | 65 |
| LSC | 34 | 16 | 34 | 17 | 83,025 | 67 |
| SSC | 35 | 14 | 36 | 15 | 17,879 | 71 |
| IRa/IRb | 29 | 20 | 29 | 22 | 25,790 | 58 |
| Prt.Coding genes | 32 | 17 | 31 | 19 | 80,166 | 64 |
| tRNA | 26 | 23 | 22 | 29 | 3172 | 48 |
| rRNA | 19 | 23 | 26 | 31 | 9070 | 45 |
| First position | 24.1 | 18.4 | 31.5 | 25.8 | 26,722 | 55.6 |
| Second position | 33.2 | 19.9 | 29.6 | 17.1 | 26,722 | 62.8 |
| Third position | 39.1 | 12.9 | 30.0 | 14.7 | 26,722 | 69.1 |
| Category | Gene Name |
|---|---|
| Photosystem I | psaA,B,C,I,J,Ycf3 a |
| Photosystem II | psbA,B,C,D,E,F,H,I,J,K,L,M,N,T,Z/lhbA |
| Cytochrome b6/f | petA,B,D,G,L,N |
| ATP Synthase | atpA,B,E,F b,H,I |
| Rubisco | rbcL |
| NADH Oxidoreductase | ndhA,B b,c,C,D,E,F,G,H,I,J,K |
| Large subunit ribosomal proteins | rpl2 b,c,14,16,20,23 c,32,33,36 |
| Small subunit ribosomal proteins | rps2,3,4,7 c,8,11,12 c,d,14,15,16 e,18,19 |
| RNAP | rpoA, rpoB, C1 b, C2, |
| Other Proteins | accD, ccsA, matK, cemA, clpP a |
| Proteins of unknown Function | ycf1 c,, ycf2 b,c, ycf15 c,e, orf42 c, orf56 c, orf188 |
| Ribosomal RNAs | rrn23 c,16 c,5 c,4.5 c |
| Transfer RNAs | trnH(GUG), K(UUU) b, M(CAU), T(GGU), V(UAC) b, F(GAA), L(UAA) b, T(UGU), S(GGA), fM(CAU), G(UCC), S(UGA), E(UUC), Y(GUA), D(GUC), C(GCA), R(UCU), S(GCU), Q(UUG), W(CCA), P(UGG), P(GGG), I(CAU) c, L(CAA) c, V(GAC) c, I(GAU) b,c, A(UGC) b,c, R(ACG) c, N(GUU) c, L(UAG) |
| Amino Acid | Codon | Count | RSCU | tRNA |
|---|---|---|---|---|
| Ala | GCG | 127 | 0.09 | trnA-UGC |
| Ala | GCA | 396 | 0.29 | |
| Ala | GCT | 629 | 0.47 | |
| Ala | GCC | 193 | 0.14 | |
| Cys | TGT | 231 | 0.73 | trnC-GCA |
| Cys | TGC | 87 | 0.27 | |
| Asp | GAT | 836 | 0.80 | trnD-GUC |
| Asp | GAC | 211 | 0.20 | |
| Glu | GAG | 322 | 0.23 | trnE-UUC |
| Glu | GAA | 1051 | 0.77 | |
| Phe | TTT | 1106 | 0.68 | trnF-GAA |
| Phe | TTC | 509 | 0.32 | |
| Gly | GGG | 284 | 0.16 | trnG-UCC |
| Gly | GGA | 698 | 0.40 | |
| Gly | GGT | 588 | 0.34 | |
| Gly | GGC | 162 | 0.09 | |
| His | CAT | 511 | 0.79 | trnH-GUG |
| His | CAC | 136 | 0.21 | |
| Ile | ATA | 838 | 0.35 | trnI-GAU |
| Ile | ATT | 1188 | 0.49 | trnI-CAU |
| Ile | ATC | 401 | 0.17 | |
| Lys | AAG | 335 | 0.22 | trnK-UUU |
| Lys | AAA | 1185 | 0.78 | |
| Leu | TTG | 561 | 0.20 | trnL-UAA |
| Leu | TTA | 944 | 0.33 | trnL-CAA |
| Leu | CTG | 168 | 0.06 | trnL-UAG |
| Leu | CTA | 389 | 0.14 | |
| Leu | CTT | 590 | 0.21 | |
| Leu | CTC | 179 | 0.06 | |
| Met | ATG | 607 | 1.00 | trnM-CAU |
| Asn | AAT | 1051 | 0.78 | trnN-GUU |
| Asn | AAC | 291 | 0.22 | |
| Pro | CCG | 128 | 0.12 | trnP-GGG |
| Pro | CCA | 339 | 0.31 | trnP-UGG |
| Pro | CCT | 406 | 0.37 | |
| Pro | CCC | 211 | 0.19 | |
| Gln | CAG | 204 | 0.21 | trnQ-UUG |
| Gln | CAA | 764 | 0.79 | |
| Arg | AGG | 159 | 0.10 | trnR-UCU |
| Arg | AGA | 494 | 0.32 | trnR-ACG |
| Arg | CGG | 105 | 0.07 | |
| Arg | CGA | 364 | 0.23 | |
| Arg | CGT | 347 | 0.22 | |
| Arg | CGC | 91 | 0.06 | |
| Ser | AGT | 404 | 0.20 | trnS-UGA |
| Ser | AGC | 121 | 0.06 | trnS-GGA |
| Ser | TCG | 181 | 0.09 | trnS-GCU |
| Ser | TCA | 442 | 0.21 | |
| Ser | TCT | 604 | 0.29 | |
| Ser | TCC | 306 | 0.15 | |
| Thr | ACG | 136 | 0.10 | trnT-UGU |
| Thr | ACA | 424 | 0.31 | trnT-GGU |
| Thr | ACT | 577 | 0.43 | |
| Thr | ACC | 219 | 0.16 | |
| Val | GTG | 175 | 0.12 | trnV-UAC |
| Val | GTA | 540 | 0.38 | trnV-GAC |
| Val | GTT | 540 | 0.38 | |
| Val | GTC | 162 | 0.11 | |
| Trp | TGG | 448 | 1.00 | trnW-CCA |
| Tyr | TAT | 852 | 0.83 | trnY-GUA |
| Tyr | TAC | 170 | 0.17 | |
| Ter | TGA | 3 | 0.60 | |
| Ter | TAG | 2 | 0.40 | |
| Ter | TAA | 0 | 0.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaila, T.; Chaduvla, P.K.; Rawal, H.C.; Saxena, S.; Tyagi, A.; Mithra, S.V.A.; Solanke, A.U.; Kalia, P.; Sharma, T.R.; Singh, N.K.; et al. Chloroplast Genome Sequence of Clusterbean (Cyamopsis tetragonoloba L.): Genome Structure and Comparative Analysis. Genes 2017, 8, 212. https://doi.org/10.3390/genes8090212
Kaila T, Chaduvla PK, Rawal HC, Saxena S, Tyagi A, Mithra SVA, Solanke AU, Kalia P, Sharma TR, Singh NK, et al. Chloroplast Genome Sequence of Clusterbean (Cyamopsis tetragonoloba L.): Genome Structure and Comparative Analysis. Genes. 2017; 8(9):212. https://doi.org/10.3390/genes8090212
Chicago/Turabian StyleKaila, Tanvi, Pavan K. Chaduvla, Hukam C. Rawal, Swati Saxena, Anshika Tyagi, S. V. Amitha Mithra, Amolkumar U. Solanke, Pritam Kalia, T. R. Sharma, N. K. Singh, and et al. 2017. "Chloroplast Genome Sequence of Clusterbean (Cyamopsis tetragonoloba L.): Genome Structure and Comparative Analysis" Genes 8, no. 9: 212. https://doi.org/10.3390/genes8090212
APA StyleKaila, T., Chaduvla, P. K., Rawal, H. C., Saxena, S., Tyagi, A., Mithra, S. V. A., Solanke, A. U., Kalia, P., Sharma, T. R., Singh, N. K., & Gaikwad, K. (2017). Chloroplast Genome Sequence of Clusterbean (Cyamopsis tetragonoloba L.): Genome Structure and Comparative Analysis. Genes, 8(9), 212. https://doi.org/10.3390/genes8090212





