Juvenile-Onset Diabetes and Congenital Cataract: “Double-Gene” Mutations Mimicking a Syndromic Diabetes Presentation
Abstract
1. Introduction
2. Patient, Family and Methods
2.1. Patient and Family
2.2. Whole-Exome Sequencing
2.3. Mutation Confirmation and Genotyping in the Whole Family
2.4. HLA Typing
2.5. Protein Structural Modeling
3. Results
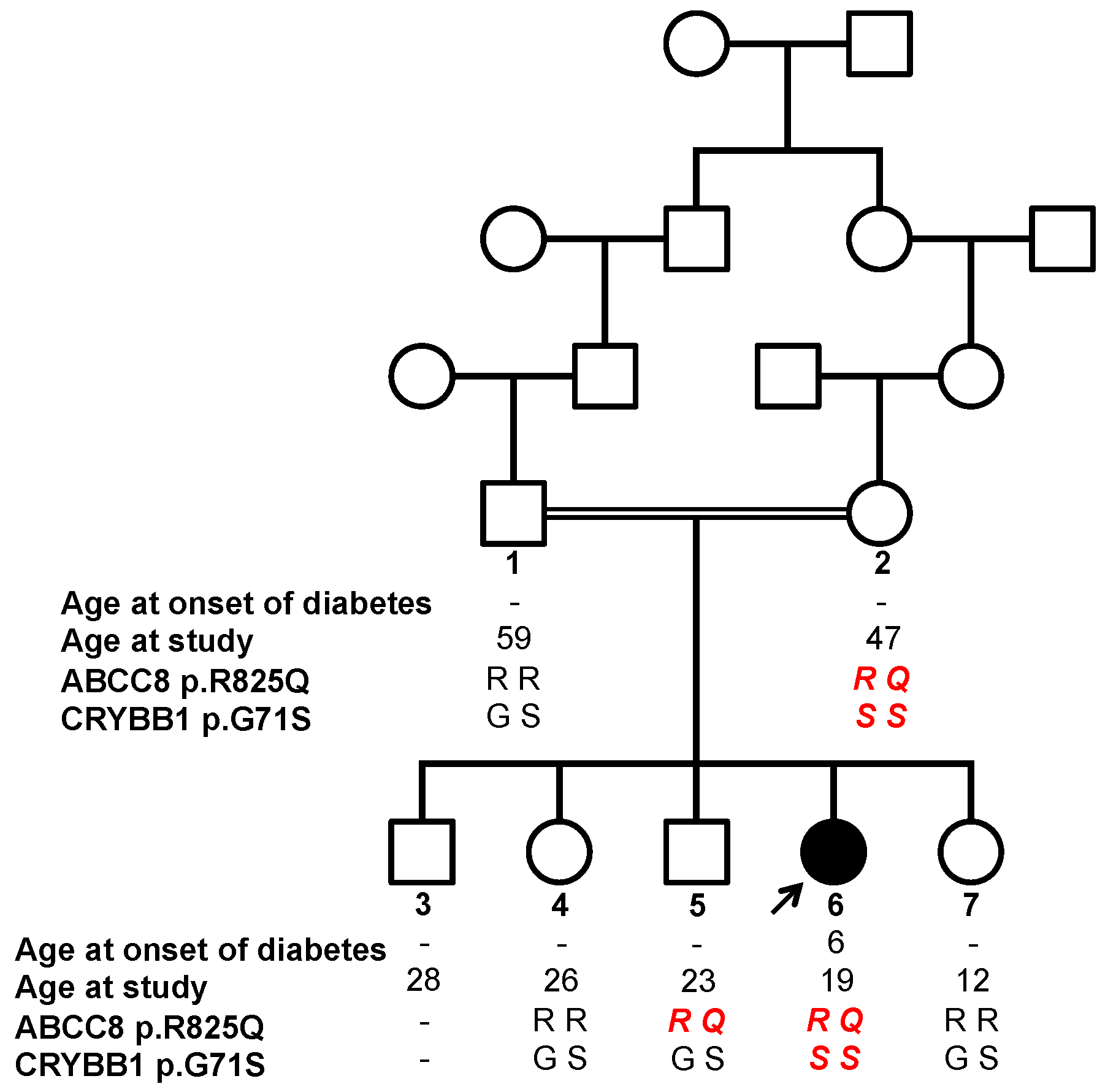
3.1. Description of an Apparently New Syndrome with Juvenile-Onset Diabetes and Congenital Cataract
3.2. Identification of a Heterozygous Mutation in the ABCC8 Gene Likely Responsible for Juvenile-Onset Diabetes
3.3. Identification of a Homozygous Mutation in the CRYBB1 Gene Responsible for Congenital Cataract
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murphy, R.; Ellard, S.; Hattersley, A.T. Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 200–213. [Google Scholar] [CrossRef]
- Schwitzgebel, V.M. Many faces of monogenic diabetes. J. Diabetes Investig. 2014, 5, 121–133. [Google Scholar] [CrossRef]
- Carroll, R.; Murphy, R. Monogenic diabetes: A diagnostic algorithm for clinicians. Genes 2013, 4, 522–535. [Google Scholar] [CrossRef]
- Inoue, H.; Tanizawa, Y.; Wasson, J.; Behn, P.; Kalidas, K.; Bernal-Mizrachi, E.; Mueckler, M.; Marshall, H.; Donis-Keller, H.; Crock, P.; et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (wolfram syndrome). Nat. Genet. 1998, 20, 143–148. [Google Scholar] [CrossRef]
- Delepine, M.; Nicolino, M.; Barrett, T.; Golamaully, M.; Lathrop, G.M.; Julier, C. Eif2ak3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with wolcott-rallison syndrome. Nat. Genet. 2000, 25, 406–409. [Google Scholar]
- Senee, V.; Chelala, C.; Duchatelet, S.; Feng, D.; Blanc, H.; Cossec, J.C.; Charon, C.; Nicolino, M.; Boileau, P.; Cavener, D.R.; et al. Mutations in glis3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat. Genet. 2006, 38, 682–687. [Google Scholar] [CrossRef]
- Zalloua, P.A.; Azar, S.T.; Delepine, M.; Makhoul, N.J.; Blanc, H.; Sanyoura, M.; Lavergne, A.; Stankov, K.; Lemainque, A.; Baz, P.; et al. Wfs1 mutations are frequent monogenic causes of juvenile-onset diabetes mellitus in lebanon. Hum. Mol. Genet. 2008, 17, 4012–4021. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and samtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Halees, A.; Itan, Y.; Spencer, E.G.; He, Y.; Azab, M.A.; Gabriel, S.B.; Belkadi, A.; Boisson, B.; Abel, L.; et al. Characterization of greater middle eastern genetic variation for enhanced disease gene discovery. Nat. Genet. 2016, 48, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Romanos, J.; Wijmenga, C. Comment on: Barker et al. (2008) two single nucleotide polymorphisms identify the highest-risk diabetes hla genotype: Diabetes 57:3152–3155, 2008. Diabetes 2008, 58, e1. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.M.; Triolo, T.M.; Aly, T.A.; Baschal, E.E.; Babu, S.R.; Kretowski, A.; Rewers, M.J.; Eisenbarth, G.S. Two single nucleotide polymorphisms identify the highest-risk diabetes hla genotype: Potential for rapid screening. Diabetes 2008, 57, 3152–3155. [Google Scholar] [CrossRef] [PubMed]
- Van Montfort, R.L.M.; Bateman, O.A.; Lubsen, N.H.; Slingsby, C. Crystal structure of truncated human βb1-crystallin. Protein Sci. 2009, 12, 2606–2612. [Google Scholar] [CrossRef] [PubMed]
- Feyfant, E.; Sali, A.; Fiser, A. Modeling mutations in protein structures. Protein Sci. 2007, 16, 2030–2041. [Google Scholar] [CrossRef] [PubMed]
- Tina, K.G.; Bhadra, R.; Srinivasan, N. Pic: Protein interactions calculator. Nucleic Acids Res. 2007, 35, W473–W476. [Google Scholar] [CrossRef] [PubMed]
- Sayle, R.A.; Milner-White, E.J. Rasmol: Biomolecular graphics for all. Trends Biochem. Sci. 1995, 20, 374–376. [Google Scholar] [CrossRef]
- Flanagan, S.E.; Clauin, S.; Bellanné-Chantelot, C.; De Lonlay, P.; Harries, L.W.; Gloyn, A.L.; Ellard, S. Update of mutations in the genes encoding the pancreatic beta-cell katpchannel subunits kir6.2 (kcnj11) and sulfonylurea receptor 1 (abcc8) in diabetes mellitus and hyperinsulinism. Hum. Mutat. 2009, 30, 170–180. [Google Scholar] [CrossRef] [PubMed]
- De Wet, H.; Proks, P.; Lafond, M.; Aittoniemi, J.; Sansom, M.S.P.; Flanagan, S.E.; Pearson, E.R.; Hattersley, A.T.; Ashcroft, F.M. A mutation (r826w) in nucleotide-binding domain 1 of abcc8 reduces atpase activity and causes transient neonatal diabetes. EMBO Rep. 2008, 9, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.E.; Patch, A.-M.; Mackay, D.J.G.; Edghill, E.L.; Gloyn, A.L.; Robinson, D.; Shield, J.P.H.; Temple, K.; Ellard, S.; Hattersley, A.T. Mutations in atp-sensitive k+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes 2007, 56, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Vaxillaire, M.; Dechaume, A.; Busiah, K.; Cavé, H.; Pereira, S.; Scharfmann, R.; De Nanclares, G.P.; Castano, L.; Froguel, P.; Polak, M.; et al. New abcc8 mutations in relapsing neonatal diabetes and clinical features. Diabetes 2007, 56, 1737–1741. [Google Scholar] [CrossRef] [PubMed]
- Klupa, T.; Kowalska, I.; Wyka, K.; Skupien, J.; Patch, A.-M.; Flanagan, S.E.; Noczynska, A.; Arciszewska, M.; Ellard, S.; Hattersley, A.T.; et al. Mutations in the abcc8 (sur1 subunit of the k atp channel) gene are associated with a variable clinical phenotype. Clin. Endocrinol. 2009, 71, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Riveline, J.P.; Rousseau, E.; Reznik, Y.; Fetita, S.; Philippe, J.; Dechaume, A.; Hartemann, A.; Polak, M.; Petit, C.; Charpentier, G.; et al. Clinical and metabolic features of adult-onset diabetes caused by abcc8 mutations. Diabetes Care 2012, 35, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Abujbara, M.A.; Liswi, M.I.; El-Khateeb, M.S.; Flanagan, S.E.; Ellard, S.; Ajlouni, K.M. Permanent neonatal diabetes mellitus in jordan. J. Pediatr. Endocrinol. Metab. 2014, 27, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Gong, C.; Wu, D.; Lu, C.; Liu, F.; Liu, X.; Zhang, Y.; Gu, Y.; Qi, Z.; Li, X.; et al. Genetic analysis and follow-up of 25 neonatal diabetes mellitus patients in china. J. Diabetes Res. 2016, 2016, 6314368. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.A.; Valdes, A.M. Genetics of the hla region in the prediction of type 1 diabetes. Curr. Diabetes Rep. 2011, 11, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Bloemendal, H.; de Jong, W.; Jaenicke, R.; Lubsen, N.H.; Slingsby, C.; Tardieu, A. Ageing and vision: Structure, stability and function of lens crystallins. Prog. Biophys. Mol. Biol. 2004, 86, 407–485. [Google Scholar] [CrossRef] [PubMed]
- Shiels, A.; Hejtmancik, J.F. Molecular Genetics of Cataract. Prog. Mol. Biol. Transl. Sci. 2015, 134, 203–218. [Google Scholar] [PubMed]
- Blundell, T.; Lindley, P.; Miller, L.; Moss, D.; Slingsby, C.; Tickle, I.; Turnell, B.; Wistow, G. The molecular structure and stability of the eye lens: X-ray analysis of gamma-crystallin ii. Nature 1981, 289, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. Gamma-s crystallin gene (crygs) mutation causes dominant progressive cortical cataract in humans. J. Med. Genet. 2005, 42, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Piszczek, G.; Wingfield, P.T.; Sergeev, Y.V.; Hejtmancik, J.F. The g18v crygs mutation associated with human cataracts increases? S-crystallin sensitivity to thermal and chemical stress. Biochemistry 2009, 48, 7334–7341. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, W.D.; Freites, J.A.; Golchert, K.J.; Shapiro, R.A.; Morikis, V.; Tobias, D.J.; Martin, R.W. Separating instability from aggregation propensity in gammas-crystallin variants. Biophys. J. 2011, 100, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Khago, D.; Wong, E.K.; Kingsley, C.N.; Freites, J.A.; Tobias, D.J.; Martin, R.W. Increased hydrophobic surface exposure in the cataract-related g18v variant of human γs-crystallin. BBA—Gen. Subj. 2016, 1860, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Klee, P.; Bellanne-Chantelot, C.; Depret, G.; Llano, J.P.; Paget, C.; Nicolino, M. A novel abcc8 mutation illustrates the variability of the diabetes phenotypes associated with a single mutation. Diabetes Metab. 2012, 38, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, M.; Shields, B.; Hammersley, S.; Hudson, M.; McDonald, T.J.; Colclough, K.; Oram, R.A.; Knight, B.; Hyde, C.; Cox, J.; et al. Systematic population screening, using biomarkers and genetic testing, identifies 2.5% of the u.K. Pediatric diabetes population with monogenic diabetes. Diabetes Care 2016, 39, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.B.; Irgens, H.U.; Molnes, J.; Sztromwasser, P.; Aukrust, I.; Juliusson, P.B.; Søvik, O.; Levy, S.; Skrivarhaug, T.; Joner, G.; et al. Targeted next-generation sequencing reveals mody in up to 6.5% of antibody-negative diabetes cases listed in the norwegian childhood diabetes registry. Diabetologia 2017, 60, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Sanyoura, M.; Woudstra, C.; Halaby, G.; Baz, P.; Senée, V.; Guillausseau, P.-J.; Zalloua, P.; Julier, C. A novel alms1 splice mutation in a non-obese juvenile-onset insulin-dependent syndromic diabetic patient. Eur. J. Hum. Genet. 2014, 22, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, C.-H.; Wang, E.; Yao, K.; Gong, X. Screening, genetics, risk factors, and treatment of neonatal cataracts. Birth Defects Res. 2017, 109, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Agarwal, T.; Kaur, P.; Kumar, M.; Khokhar, S.; Dada, R. Molecular and structural analysis of genetic variations in congenital cataract. Mol. Vis. 2013, 19, 2436–2450. [Google Scholar] [PubMed]
- De Franco, E.; Flanagan, S.E.; Yagi, T.; Abreu, D.; Mahadevan, J.; Johnson, M.B.; Jones, G.; Acosta, F.; Mulaudzi, M.; Lek, N.; et al. Dominant er stress-inducing wfs1 mutations underlie a genetic syndrome of neonatal/infancy-onset diabetes, congenital sensorineural deafness, and congenital cataracts. Diabetes 2017, 66, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Berry, V.; Gregory-Evans, C.; Emmett, W.; Waseem, N.; Raby, J.; Prescott, D.; Moore, A.T.; Bhattacharya, S.S. Wolfram gene (wfs1) mutation causes autosomal dominant congenital nuclear cataract in humans. Eur. J. Hum. Genet. 2013, 21, 1356–1360. [Google Scholar] [CrossRef] [PubMed]

| Chromosome Position (hg19) | Gene | dbSNP rsID | cDNA | Protein | Allele Counts WT/Mutated (MAF) in Public Databases | Estimated MAF * | In silico Pathogenicity Based on Annovar Prediction Programs | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Refseq | Nucleotide Change | Refseq | Amino Acid Change | gnomAD (N = 138,632) | EVS (N = 6503) | GME (N = 2497) | gnomAD, EVS, GME | ||||
| chr11: g.17434942G>A | ABCC8 | rs375172221 | NM_000352 | c.2474G>A | NP_000343 | p.Arg825Gln | 246,266/0 | 12,985/1 (0.000077) | 1986/0 | 3.4 × 10-6 | deleterious (9/11) |
| chr22: g.27008124G>A | CRYBB1 | NA | NM_001887 | c.211G>A | NP_001878 | p.Gly71Ser | Absent | Absent | Absent | Absent | deleterious (11/11) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenfant, C.; Baz, P.; Degavre, A.; Philippi, A.; Senée, V.; Vandiedonck, C.; Derbois, C.; Nicolino, M.; Zalloua, P.; Julier, C. Juvenile-Onset Diabetes and Congenital Cataract: “Double-Gene” Mutations Mimicking a Syndromic Diabetes Presentation. Genes 2017, 8, 309. https://doi.org/10.3390/genes8110309
Lenfant C, Baz P, Degavre A, Philippi A, Senée V, Vandiedonck C, Derbois C, Nicolino M, Zalloua P, Julier C. Juvenile-Onset Diabetes and Congenital Cataract: “Double-Gene” Mutations Mimicking a Syndromic Diabetes Presentation. Genes. 2017; 8(11):309. https://doi.org/10.3390/genes8110309
Chicago/Turabian StyleLenfant, Caroline, Patrick Baz, Anne Degavre, Anne Philippi, Valérie Senée, Claire Vandiedonck, Céline Derbois, Marc Nicolino, Pierre Zalloua, and Cécile Julier. 2017. "Juvenile-Onset Diabetes and Congenital Cataract: “Double-Gene” Mutations Mimicking a Syndromic Diabetes Presentation" Genes 8, no. 11: 309. https://doi.org/10.3390/genes8110309
APA StyleLenfant, C., Baz, P., Degavre, A., Philippi, A., Senée, V., Vandiedonck, C., Derbois, C., Nicolino, M., Zalloua, P., & Julier, C. (2017). Juvenile-Onset Diabetes and Congenital Cataract: “Double-Gene” Mutations Mimicking a Syndromic Diabetes Presentation. Genes, 8(11), 309. https://doi.org/10.3390/genes8110309




