Exploring Folate Diversity in Wild and Primitive Potatoes for Modern Crop Improvement
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Potato Material
| Species: Spooner Classification [24] | Species: Old Classification | Number of Accessions Available from GRIN | Ploidy Level | Number of Accessions Tested | PI Number Tested | Origin |
|---|---|---|---|---|---|---|
| S. stipuloideum | S. circaeifolium | 14 | 2× | 3 | 498116 | Cochabamba, Bolivia |
| 498120 | Santa Cruz, Bolivia | |||||
| 545974 | La Paz, Bolivia | |||||
| S. chacoense | S. chacoense subsp. chacoense | 174 | 2× | 2 | 197760 | ? |
| 320293 | Salta, Argentina | |||||
| S. candolleanum | S. bukasovii | 176 | 2× | 3 | 265863 | Puno, Peru |
| 365321 | Huanuco, Peru | |||||
| 458379 | Apurimac, Peru | |||||
| S. acaule | S. acaule f. acaule | 424 | 4× | 3 | 175395, 472661 | Argentina |
| 473481 | Huancavelica, Peru | |||||
| S. demissum | S. demissum | 164 | 6× | 3 | 160208 | Mexico |
| 230589 | Huanuco, Peru | |||||
| 498232 | Apurimac, Peru | |||||
| S. microdontum | S. microdontum subsp. microdontum | 116 | 2× | 2 | 458355 | Jujuy, Argentina |
| 498123 | Chuquisaca, Bolivia | |||||
| S. okadae | S. venturii | 16 | 2× | 1 | 458368 | Salta, Argentina |
| S. okadae | 2× | 2 | 498130 | Cochabamba, Bolivia | ||
| 320327 | Salta, Argentina | |||||
| S. tuberosum subsp. andigenum | S. stenotomum subsp. stenotomum | 1006 | 2× | 2 | 195204 | Cuzco, Peru |
| 283141 | Colombia | |||||
| S. phureja subsp. phureja | 2× | 3 | 320355, 320377 | Narino, Colombia | ||
| 225710 | Cauca, Colombia | |||||
| S. tuberosum subsp. andigenum | 4× | 3 | 546023 | Potosi, Bolivia | ||
| 607886 | Cuzco, Peru | |||||
| 281034 | Mexico | |||||
| S. boliviense | S. megistacrolobum | 222 | 2× | 26 | 283082 | Bolivia |
| 283133 | Ecuador | |||||
| 275149, 435077, 500029, 500030 | Salta, Argentina | |||||
| 458347, 458348, 473110, 473112, 473113, 473124, 473129, 473130, 473138, 473141, 473144, 473149, 473160, 558094 | Jujuy, Argentina | |||||
| 545899, 568986 | Tarija, Bolivia | |||||
| 597689 | Oruro, Bolivia | |||||
| 597705, 597706, 597736 | Potosi, Bolivia | |||||
| S. vernei | S. vernei subsp. vernei | 36 | 2× | 18 | 320332 | Catamarca, Argentina |
| 230468, 458373, 473308 | Tucuman, Argentina | |||||
| 458374, 473306, 473310, 473311, 500045, 500062, 500063, 500065, 558147, 558148 | Salta, Argentina | |||||
| 500067, 500069, 558149, 558150 | Jujuy, Argentina | |||||
| S. vernei subsp. ballsii | 2× | 5 | 458369, 473303 | Jujuy, Argentina | ||
| 458370, 458371, 458372 | Salta, Argentina | |||||
| S. vernei | 2× | 1 | 500066 | Jujuy, Argentina |
2.3. Folate Analysis
2.4. Statistical Analysis
3. Results
| Plant Introduction Number | Species | Number of Individuals Tested | Individual Measurements | Mean ± SE | % DM |
|---|---|---|---|---|---|
| R. Burbank | S. tuberosum subsp. tuberosum | 3 | 1276-915-929 | 1040 ± 118 | 26 |
| 498116 | S. stipuloideum | 3 | 907-1115-1118 | 1046 ± 57 | 25 |
| 498120 | S. stipuloideum | 3 | 304-553-532 | 463 ± 65 | 25 |
| 545974 | S. stipuloideum | 4 | 415-824-1119-882 | 810 ± 127 | 23 |
| 197760 | S. chacoense | 3 | 591-653-653 | 632 ± 17 | 36 |
| 320293 | S. chacoense | 4 | 478-240-1198-408 | 581 ± 183 | 37 |
| 265863 | S. candolleanum | 3 | 1367-508-481 | 786 ± 206 | 25 |
| 365321 | S. candolleanum | 1 | 918 | 918 ± n.d. | 19 |
| 458379 | S. candolleanum | 1 | 1023 | 1023 ± n.d. | 19 |
| 175395 | S. acaule | 4 | 461-562-562-1017 | 651 ± 108 | 22 |
| 472661 | S. acaule | 4 | 480-490-517-1014 | 625 ± 113 | 23 |
| 473481 | S. acaule | 2 | 632-757 | 695 ± 44 | 25 |
| 160208 | S. demissum | 4 | 749-455-487-556 | 562 ± 57 | 23 |
| 230589 | S. demissum | 2 | 410-631 | 520 ± 78 | 22 |
| 498232 | S. demissum | 4 | 760-737-669-455 | 655 ± 60 | 30 |
| 458355 | S. microdontum | 3 | 703-694-650 | 682 ± 13 | 32 |
| 498123 | S. microdontum | 2 | 913-767 | 840 ± 51 | 36 |
| 320327 | S. okadae | 3 | 876-548-629 | 684 ± 80 | 35 |
| 458368 | S. okadae | 3 | 611-1317-991 | 973 ± 167 | 34 |
| 498130 | S. okadae | 3 | 723-660-806 | 730 ± 34 | 38 |
| 195204 | S. tuberosum subsp. andigenum | 4 | 410-836-499-794 | 635 ± 92 | 24 |
| 225710 | S. tuberosum subsp. andigenum | 1 | 2337 | 2337 ± n.d. | 22 |
| 281034 | S. tuberosum subsp. andigenum | 4 | 565-1030-1126-506 | 807 ± 137 | 18 |
| 283141 | S. tuberosum subsp. andigenum | 3 | 468-457-711 | 545 ± 68 | 18 |
| 320355 | S. tuberosum subsp. andigenum | 2 | 853-1400 | 1126 ± 193 | 28 |
| 320377 | S. tuberosum subsp. andigenum | 2 | 2198-1038 | 1618 ± 410 | 17 |
| 546023 | S. tuberosum subsp. andigenum | 4 | 985-700-333-626 | 661 ± 116 | 21 |
| 607886 | S. tuberosum subsp. andigenum | 4 | 404-553-622-361 | 485 ± 53 | 21 |
| 275149 | S. boliviense | 4 | 566-561-602-515 | 561 ± 15 | 24 |
| 283082 | S. boliviense | 1 | 934 | 934 ± n.d. | 23 |
| 283133 | S. boliviense | 4 | 891-1102-1097-351 | 860 ± 153 | 27 |
| 435077 | S. boliviense | 3 | 421-652-630 | 568 ± 60 | 26 |
| 458347 | S. boliviense | 3 | 779-1393-525 | 899 ± 210 | 21 |
| 458348 | S. boliviense | 4 | 585-759-666-679 | 672 ± 31 | 23 |
| 473110 | S. boliviense | 4 | 362-456-651-611 | 520 ± 58 | 18 |
| 473112 | S. boliviense | 4 | 517-897-450-688 | 638 ± 87 | 20 |
| 473113 | S. boliviense | 1 | 869 | 869 ± n.d. | 20 |
| 473124 | S. boliviense | 4 | 630-547-456-610 | 561 ± 34 | 20 |
| 473129 | S. boliviense | 4 | 816-997-722-584 | 780 ± 75 | 21 |
| 473130 | S. boliviense | 4 | 411-526-512-751 | 550 ± 62 | 24 |
| 473138 | S. boliviense | 4 | 1265-787-512-745 | 827 ± 137 | 21 |
| 473141 | S. boliviense | 4 | 460-524-630-385 | 500 ± 45 | 23 |
| 473144 | S. boliviense | 4 | 473-355-222-449 | 375 ± 49 | 22 |
| 473149 | S. boliviense | 4 | 628-523-826-780 | 689 ± 60 | 24 |
| 473160 | S. boliviense | 4 | 557-352-706-347 | 491 ± 75 | 22 |
| 500029 | S. boliviense | 4 | 684-461-610-622 | 594 ± 41 | 26 |
| 500030 | S. boliviense | 4 | 541-494-575-671 | 634 ± 32 | 24 |
| 545899 | S. boliviense | 4 | 647-426-1033-780 | 721 ± 110 | 22 |
| 558094 | S. boliviense | 3 | 350-473-542 | 455 ± 46 | 21 |
| 568986 | S. boliviense | 2 | 684-888 | 786 ± 72 | 19 |
| 597689 | S. boliviense | 4 | 570-1099-723-822 | 804 ± 96 | 22 |
| 597705 | S. boliviense | 4 | 332-749-366-707 | 539 ± 95 | 20 |
| 597706 | S. boliviense | 4 | 484-567-551-623 | 556 ± 25 | 23 |
| 597736 | S. boliviense | 4 | 713-1947-539-777 | 994 ± 279 | 33 |
| 230468 | S. vernei | 4 | 1377-1072-1416-1911 | 1444 ± 150 | 24 |
| 320332 | S. vernei | 4 | 1137-846-1985-1105 | 1268 ± 215 | 28 |
| 458369 | S. vernei | 2 | 1197-1002 | 1099 ± 69 | 22 |
| 458370 | S. vernei | 4 | 1207-1062-1073-817 | 1040 ± 70 | 20 |
| 458371 | S. vernei | 4 | 1940-786-881-1601 | 1302 ± 242 | 22 |
| 458372 | S. vernei | 4 | 1450-1801-1023-1110 | 1346 ± 154 | 23 |
| 458373 | S. vernei | 2 | 1316-1145 | 1230 ± 60 | 26 |
| 458374 | S. vernei | 4 | 891-838-851-908 | 872 ± 14 | 25 |
| 473303 | S. vernei | 3 | 1623-1099-774 | 1165 ± 202 | 21 |
| 473306 | S. vernei | 3 | 1968-1307-1703 | 1659 ± 157 | 18 |
| 473308 | S. vernei | 1 | 1117 | 1117 ± n.d. | 25 |
| 473310 | S. vernei | 3 | 649-973-1058 | 893 ± 102 | 21 |
| 473311 | S. vernei | 3 | 1589-1309-1122 | 1340 ± 111 | 23 |
| 500045 | S. vernei | 2 | 1361-826 | 1093 ± 189 | 26 |
| 500062 | S. vernei | 2 | 1287-818 | 1053 ± 166 | 25 |
| 500063 | S. vernei | 4 | 469-1282-776-725 | 813 ± 147 | 22 |
| 500065 | S. vernei | 3 | 829-1105-835 | 923 ± 74 | 25 |
| 500066 | S. vernei | 3 | 1178-1722-1372 | 1424 ± 112 | 20 |
| 500067 | S. vernei | 4 | 1219-1370-961-1035 | 1146 ± 280 | 22 |
| 500069 | S. vernei | 3 | 970-948-1294 | 1070 ± 91 | 24 |
| 558147 | S. vernei | 2 | 1117-1150 | 1133 ± 12 | 23 |
| 558148 | S. vernei | 4 | 853-974-1312-959 | 1024 ± 86 | 26 |
| 558149 | S. vernei | 4 | 1268-2211-1688-1355 | 1630 ± 185 | 24 |
| 558150 | S. vernei | 2 | 909-1620 | 1264 ± 252 | 22 |

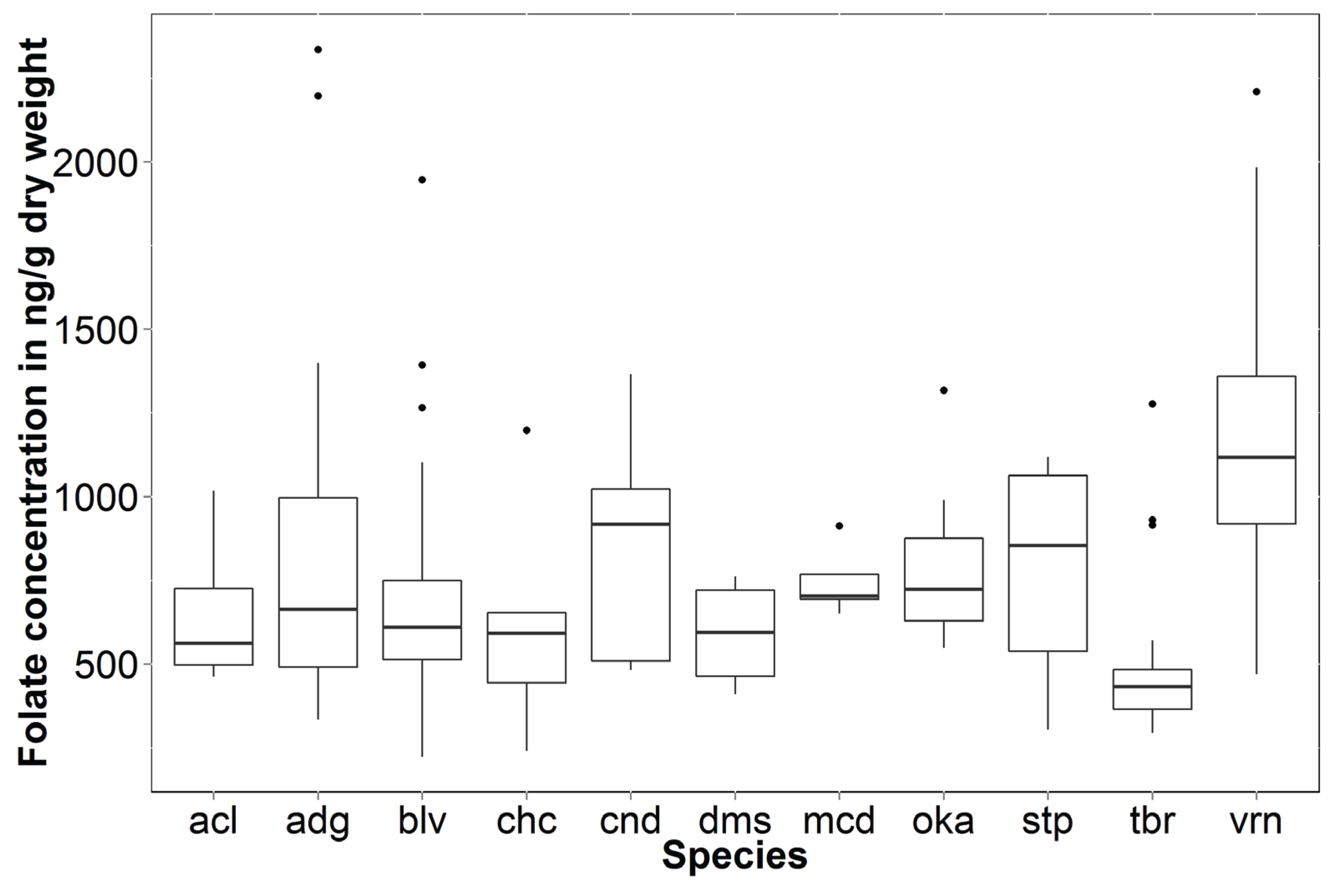
4. Discussion
5. Conclusions
Acknowledgments
Appendix
Author Contributions
Conflicts of Interest
References
- Bekaert, S.; Storozhenko, S.; Mehrshahi, P.; Bennett, M.J.; Lambert, W.; Gregory, J.F., 3rd; Schubert, K.; Hugenholtz, J.; van Der Straeten, D.; Hanson, A.D. Folate biofortification in food plants. Trends Plant Sci. 2008, 13, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Appling, D.R. Compartmentation of folate-mediated one-carbon metabolism in eukaryotes. FASEB J. 1991, 5, 2645–2651. [Google Scholar] [PubMed]
- Hyde, J.E. Exploring the folate pathway in plasmodium falciparum. Acta Trop. 2005, 94, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Shane, B.; Stokstad, E.L.R. Vitamin B12-folate interrelationships. Annu. Rev. Nutr. 1985, 5, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.B.; Rampersaud, G.C.; Kauwell, G.P.A. Folic acid supplements and fortification affect the risk for neural tube defects, vascular disease and cancer: Evolving science. J. Nutr. 2003, 133, 1961S–1968S. [Google Scholar] [PubMed]
- Beaudin, A.E.; Stover, P.J. Folate-mediated one-carbon metabolism and neural tube defects: Balancing genome synthesis and gene expression. Birth Defects Res. C Embryo Today 2007, 81, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.; Vupputuri, S.; Myers, L.; Whelton, P.K.; Kasner, S.E. Dietary intake of folate and risk of stroke in US men and women. Stroke 2002, 33, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.H.; Bedson, E.; Hughes, D.; Lloyd, K.; Moat, S.; Pirmohamed, M.; Slegg, G.; Tranter, R.; Whitaker, R.; Wilkinson, C.; et al. Folate augmentation of treatment—Evaluation for depression (folated): Protocol of a randomised controlled trial. BMC Psychiatry 2007. [Google Scholar] [CrossRef] [PubMed]
- Coppen, A.; Bolander-Gouaille, C. Treatment of depression: Time to consider folic acid and vitamin B12. J. PsychopharmacoL. 2005, 19, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Araújoa, J.R.; Martelb, F.; Borgesc, N.; Araújod, J.M.; Keating, E. Folates and aging: Role in mild cognitive impairment, dementia and depression. Ageing Res. Rev. 2015, 22, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Storozhenko, S.; Ravanel, S.; Zhang, G.-F.; Rébeillé, F.; Lambert, W.; van der Straeten, D. Folate enhancement in staple crops by metabolic engineering. Trends Food Sci. Technol. 2005, 16, 271–281. [Google Scholar] [CrossRef]
- Konings, E.J.M.; Roomans, H.H.S.; Dorant, E.; Goldbohm, R.A.; Saris, W.H.M.; van den Brandt, P.A. Folate intake of the dutch population according to newly established liquid chromatography data for foods. Am. J. Clin. Nutr. 2001, 765–776. [Google Scholar]
- Bentley, T.G.K.; Willett, W.C.; Weinstein, M.C.; Kuntz, K.M. Population-level changes in folate intake by age, gender, and race/ethnicity after folic acid fortification. Am. J. Public Health 2006, 96, 2040–2047. [Google Scholar] [CrossRef] [PubMed]
- Blancquaert, D.; de Steur, H.; Gellynck, X.; van der Straeten, D. Present and future of folate biofortification of crop plants. J. Exp. Bot. 2014, 65, 895–906. [Google Scholar] [CrossRef] [PubMed]
- CIP. Annual Report 2014. Cross-Cutting Efforts Optimize Food Security, Nutrition and Livelihood. International Potato Center: Lima, Peru, 2015; Volume 2015. [Google Scholar]
- Committee, P.C.G. Potato Vulnerability Statement 2014; USDA Agricultural Research Service Germplasm Resources Information Network. 2014. Available online: http://www.ars-grin.gov/ (accessed on 2 March 2015). [Google Scholar]
- Millam, S. Agrobacterium-mediated transformation of potato. In Transgenic Crops of the World: Essential Protocols; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 257–270. [Google Scholar]
- Alfthan, G.; Laurinen, M.S.; Valsta, L.M.; Pastinen, T.; Aro, A. Folate intake, plasma folate and homocysteine status in a random Finnish population. Eur. J. Clin. Nutr. 2003, 57, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Vahteristo, L.; Lehikoinen, K.; Ollilainen, V.; Varo, P. Application of an hplc assay for the determination of folate derivatives in some vegetables, fruits and berries consumed in finland. Food Chem. 1997, 59, 589–591. [Google Scholar] [CrossRef]
- Hatzis, C.M.; Bertsias, G.K.; Linardakis, M.; Scott, J.M.; Kafatos, A.G. Dietary and other lifestyle correlates of serum folate concentrations in a healthy adult population in Crete, Greece: A cross-sectional study. Nutr. J. 2006. [Google Scholar] [CrossRef] [PubMed]
- Goyer, A.; Navarre, D.A. Determination of folate concentrations in diverse potato germplasm using a trienzyme extraction and a microbiological assay. J. Agric. Food Chem. 2007, 55, 3523–3528. [Google Scholar] [CrossRef] [PubMed]
- Goyer, A.; Sweek, K. Genetic diversity of thiamin and folate in primitive cultivated and wild potato (solanum) species. J. Agric. Food Chem. 2011, 59, 13072–13080. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.M.; Rogers, L.M.; Gregory, J.F.I. Determination of folate in cereal-grain food products using trienzyme extraction and combined affinity and reversed-phase liquid chromatography. J. Agric. Food Chem. 1997, 45, 407–413. [Google Scholar] [CrossRef]
- Spooner, D.M.; Ghislain, M.; Simon, R.; Jansky, S.H.; Gavrilenko, T. Systematics, diversity, genetics, and evolution of wild and cultivated potatoes. Bot. Rev. 2014, 80, 283–383. [Google Scholar] [CrossRef]
- Horne, D.W.; Patterson, D. Lactobacillus casei microbiological assay of folic acid derivativesin 96-well microtiter plates. Clin. Chem. 1988, 34, 2357–2359. [Google Scholar] [PubMed]
- Van Daele, J.; Blancquaert, D.; Kiekens, F.; van Der Straeten, D.; Lambert, W.E.; Stove, C.P. Folate profiling in potato (Solanum tuberosum) tubers by ultrahigh-performance liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2014, 62, 3092–3100. [Google Scholar] [CrossRef] [PubMed]
- Goyer, A.; Navarre, D.A. Folate is higher in developmentally younger potato tubers. J. Sci. Food Agric. 2009, 89, 579–583. [Google Scholar] [CrossRef]
- Navarre, D.A.; Goyer, A.; Shakya, R. Nutritional value of potatoes: Vitamin, phytonutrient and mineral content. In Advances in Potato Chemistry and Technology; Singh, J., Lovedeep Kaur, L., Eds.; Elsevier Inc.: Philadelphia, United States, 2009; pp. 395–424. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, B.R.; Sathuvalli, V.; Bamberg, J.; Goyer, A. Exploring Folate Diversity in Wild and Primitive Potatoes for Modern Crop Improvement. Genes 2015, 6, 1300-1314. https://doi.org/10.3390/genes6041300
Robinson BR, Sathuvalli V, Bamberg J, Goyer A. Exploring Folate Diversity in Wild and Primitive Potatoes for Modern Crop Improvement. Genes. 2015; 6(4):1300-1314. https://doi.org/10.3390/genes6041300
Chicago/Turabian StyleRobinson, Bruce R., Vidyasagar Sathuvalli, John Bamberg, and Aymeric Goyer. 2015. "Exploring Folate Diversity in Wild and Primitive Potatoes for Modern Crop Improvement" Genes 6, no. 4: 1300-1314. https://doi.org/10.3390/genes6041300
APA StyleRobinson, B. R., Sathuvalli, V., Bamberg, J., & Goyer, A. (2015). Exploring Folate Diversity in Wild and Primitive Potatoes for Modern Crop Improvement. Genes, 6(4), 1300-1314. https://doi.org/10.3390/genes6041300







