Detection of Chromosomal Aneuploidy Using Exome Sequencing
Abstract
1. Introduction
2. Methods
2.1. Study Design and Sample Size
2.2. Exome Sequencing
2.2.1. Sample Collection and Processing
2.2.2. Bioinformatic Analysis
2.3. Methodology for DNA Sequencing in Prenatal Samples (Amniotic Fluid or CVS)
2.4. Preliminary Observations on Suspected Aneuploidy by Physician
2.5. Results Confirmation
2.6. Ethical Approval
3. Results
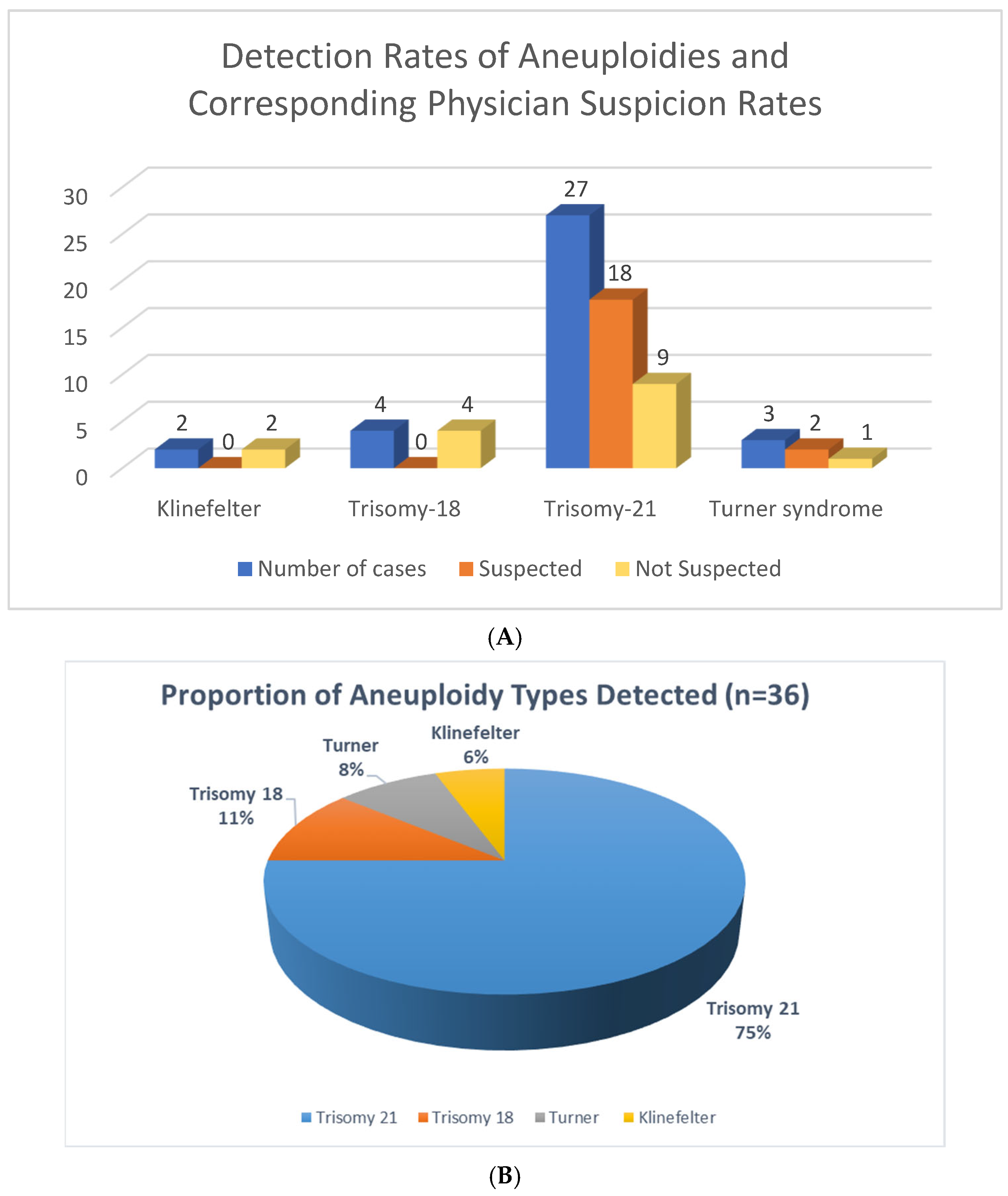
3.1. Overall Detection of Aneuploidies Using Exome Sequencing
3.2. Prenatal Exome Sequencing for Aneuploidies
3.3. Clinical Suspicion of Aneuploidy
3.4. Confirmation Status
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Wang, X.; Zhang, J.; Song, Z.; Wang, S.; Gao, Y.; Wang, J.; Luo, Y.; Niu, Z.; Yue, X.; et al. Detection of chromosomal aneuploidy in human preimplantation embryos by next-generation sequencing. Biol. Reprod. 2014, 90, 95. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.T.; Adam, M.P.; Aradhya, S.; Biesecker, L.G.; Brothman, A.R.; Carter, N.P.; Church, D.M.; Crolla, J.A.; Eichler, E.E.; Epstein, C.J.; et al. Consensus statement: Chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am. J. Hum. Genet. 2010, 86, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Speicher, M.R.; Gwyn Ballard, S.; Ward, D.C. Karyotyping human chromosomes by combinatorial multi-fluor FISH. Nat. Genet. 1996, 12, 368–375. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, M.; Zheng, D. Chromosomal mosaicism detected by karyotyping and chromosomal microarray analysis in prenatal diagnosis. J. Cell. Mol. Med. 2021, 25, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Meher, P.K.; Lundholm, L.; Wojcik, A. Fluorescence in situ hybridisation for interphase chromosomal aberration-based biological dosimetry. Radiat. Prot. Dosim. 2023, 199, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, L.G. American College of Medical Genetics Professional Practice and Guidelines Committee. American College of Medical Genetics guideline on the cytogenetic evaluation of the individual with developmental delay or mental retardation. Genet. Med. 2005, 7, 650–654. [Google Scholar] [CrossRef]
- Biesecker, L.G.; Green, R.C. Diagnostic clinical genome and exome sequencing. N. Engl. J. Med. 2014, 370, 2418–2425. [Google Scholar] [CrossRef]
- Taylor, A.M.; Shih, J.; Ha, G.; Gao, G.F.; Zhang, X.; Berger, A.C.; Schumacher, S.E.; Wang, C.; Hu, H.; Liu, J.; et al. Genomic and Functional Approaches to Understanding Cancer Aneuploidy. Cancer Cell 2018, 33, 676–689.e3. [Google Scholar] [CrossRef]
- Mackie, F.L.; Carss, K.J.; Hillman, S.C.; Hurles, M.E.; Kilby, M.D. Exome Sequencing in Fetuses with Structural Malformations. J. Clin. Med. 2014, 3, 747–762. [Google Scholar] [CrossRef]
- Borrell, A.; Ordoñez, E.; Pauta, M.; Otaño, J.; Paz-Y-Miño, F.; de Almeida, M.; León, M.; Cirigliano, V. Prenatal Exome Sequencing Analysis in Fetuses with Various Ultrasound Findings. J. Clin. Med. 2023, 13, 181. [Google Scholar] [CrossRef]
- Petrovski, S.; Aggarwal, V.; Giordano, J.L.; Stosic, M.; Wou, K.; Bier, L.; Spiegel, E.; Brennan, K.; Stong, N.; Jobanputra, V.; et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: A prospective cohort study. Lancet 2019, 393, 758–767. [Google Scholar] [CrossRef]
- Gilissen, C.; Hoischen, A.; Brunner, H.G.; Veltman, J.A. Unlocking Mendelian disease using exome sequencing. Genome Biol. 2011, 12, 228. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Zhang, Q.; Larson, D.E.; Shen, D.; McLellan, M.D.; Lin, L.; Miller, C.A.; Mardis, E.R.; Ding, L.; Wilson, R.K. VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012, 22, 568–576. [Google Scholar] [CrossRef]
- Malinowski, J.; Miller, D.T.; Demmer, L.; Gannon, J.; Pereira, E.M.; Schroeder, M.C.; Scheuner, M.T.; Tsai, A.C.H.; Hickey, S.E.; Shen, J. Systematic evidence-based review: Outcomes from exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability. Genet. Med. 2020, 22, 986–1004. [Google Scholar] [CrossRef]
- Kucharík, M.; Budiš, J.; Hýblová, M.; Minárik, G.; Szemes, T. Copy Number Variant Detection with Low-Coverage Whole-Genome Sequencing Represents a Viable Alternative to the Conventional Array-CGH. Diagnostics 2021, 11, 708. [Google Scholar] [CrossRef]
- Tommel, J.; Kenis, D.; Lambrechts, N.; Brohet, R.M.; Swysen, J.; Mollen, L.; Hoefmans, M.-J.F.; Pusparum, M.; Evers, A.W.M.; Ertaylan, G.; et al. Personal Genomes in Practice: Exploring Citizen and Healthcare Professionals’ Perspectives on Personalized Genomic Medicine and Personal Health Data Spaces Using a Mixed-Methods Design. Genes 2023, 14, 786. [Google Scholar] [CrossRef] [PubMed]
- Harisinghani, A.; Raffaele, G.; Zawatsky, C.B.; Santoro, S.L. Beyond chromosome analysis: Additional genetic testing practice in a Down syndrome clinic. Am. J. Med. Genet. C Semin. Med. Genet. 2023, 193, e32063. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.R.; Lee, K.; Pfau, R.B.; Reshmi, S.C.; Corsmeier, D.J.; Hashimoto, S.; Dave-Wala, A.; Jayaraman, V.; Koboldt, D.; Matthews, T.; et al. Disease-associated mosaic variation in clinical exome sequencing: A two-year pediatric tertiary care experience. Mol Case Stud. 2020, 6, a005231. [Google Scholar] [CrossRef]
- Lukaszuk, K.; Jakiel, G.; Kuczynski, W.; Pukszta, S.; Liss, J.; Plociennik, L.; Lukaszuk, A.; Pastuszek, E. Next generation sequencing for preimplantation genetic testing of blastocysts aneuploidies in women of different ages. Ann. Agric. Environ. Med. 2016, 23, 163–166. [Google Scholar] [CrossRef]
- Xiang, J.; Ding, Y.; Tang, H.; Zhang, W.; Mao, J.; He, Q.; Zhang, Q.; Wang, T. Genetic analysis of pregnancy loss and fetal structural anomalies by whole exome sequencing. Orphanet J. Rare Dis. 2024, 19, 330. [Google Scholar] [CrossRef]
- Zare, F.; Nabavi, S. Copy Number Variation Detection Using Total Variation. In Proceedings of the BCB ’19: 10th ACM International Conference on Bioinformatics, Computational Biology and Health Informatics, Niagara Falls, NY, USA, 7–10 September 2019; pp. 423–428. [Google Scholar]
- Magi, A.; Tattini, L.; Pippucci, T.; Torricelli, F.; Benelli, M. Read count approach for DNA copy number variants detection. Bioinformatics 2012, 28, 470–478. [Google Scholar] [CrossRef] [PubMed]
| No | Chromosomal Aneuploidy | Phenotype | Prenatal/Postnatal | Confirmed with Different Method | Suspected by Physician |
|---|---|---|---|---|---|
| 1 | Klinefelter | A three-year-old boy with high triglyceridemia | Postnatal | Yes | No |
| 2 | Klinefelter | Learning disability, hypospadias | Postnatal | Yes | No |
| 3 | Trisomy-18 | Abnormal fetus with strawberry-shaped head, polyhydramnios, cardiomegaly, aortic stenosis, and Trisomy 18 | Prenatal | Yes | No |
| 4 | Trisomy-18 | Abnormal fetus with cystic hygroma and abnormal lower limb extremities | Prenatal | No | No |
| 5 | Trisomy-18 | A fetus with multiple congenital anomalies | Prenatal | No | No |
| 6 | Trisomy-18 | Abnormal fetal development, including cystic hygroma and abnormal lower limb extremities | Prenatal | Yes | No |
| 7 | Trisomy-21 | 2-year-old boy known for cases of down syndrome with hypothyroidism, ASD and neutropenia, genetic workup for bone marrow transplant | Postnatal | Yes | Yes |
| 8 | Trisomy-21 | A 2-year-old girl with Down syndrome, pancytopenia, recurrent fever, thrombocytopenia, hypothyroidism, abnormal hemoglobin, leukopenia, and splenomegaly | Postnatal | Yes | Yes |
| 9 | Trisomy-21 | Known case of Down syndrome and unexplained recurrent chest infection | Postnatal | Yes | Yes |
| 10 | Trisomy-21 | Down syndrome and methemoglobinemia | Postnatal | Yes | Yes |
| 11 | Trisomy-21 | Anemia, neutropenia, pancytopenia, bone marrow failure and down syndrome | Postnatal | Yes | Yes |
| 12 | Trisomy-21 | Two-year-old female with Trisomy 21, consort heart disease, recurrent fungal infection, severe combined immunodeficiency | Postnatal | Yes | Yes |
| 13 | Trisomy-21 | A fetus at 17 weeks’ gestation with hydrops fetalis, abnormal heart, echogenic bowels | Prenatal | Yes | No |
| 14 | Trisomy-21 | Abnormal fetus with severe congenital diaphragmatic hernia and dilated loops of bowel | Prenatal | Yes | No |
| 15 | Trisomy-21 | A 9-year-old male diagnosed with Trisomy 21, autism spectrum disorder | Postnatal | Yes | Yes |
| 16 | Trisomy-21 | One year and 8 months female with atrial septal defect, ventricular septal defect, failure to thrive, microcephaly, IUGR, sepsis | Postnatal | No | NO |
| 17 | Trisomy-21 | Three years girl with hypotonia | Postnatal | Yes | No |
| 18 | Trisomy-21 | Five years boy with clinical features of Down syndrome | Postnatal | No | Yes |
| 19 | Trisomy-21 | A 12-year-old boy with Down syndrome, Trisomy 21, autistic spectrum disorder, congenital heart disease, and polycythemia | Postnatal | No | Yes |
| 20 | Trisomy-21 | A 4-year-old male diagnosed with Abnormal pigmentation, multiple café au lait spots, speech delay, and facial dysmorphism. A known case of Down syndrome with clinical features resembling neurofibromatosis | Postnatal | No | Yes |
| 21 | Trisomy-21 | Down syndrome, hypothyroidism, severe psoriasis, psoriatic arthritis, Mild macrocytosis | Postnatal | No | Yes |
| 22 | Trisomy-21 | An 8-year-old boy with a known case of Down Syndrome | Postnatal | No | Yes |
| 23 | Trisomy-21 | A 7-year-old girl with Down Syndrome. | Postnatal | No | Yes |
| 24 | Trisomy-21 | Abnormal fetus with dilated ventriculomegaly with partial agenesis of falx cerebri and mild hydronephrosis | Prenatal | Yes | No |
| 25 | Trisomy-21 | A 2-year-old male diagnosed with recurrent chest infections (pneumonia, bacteremia), developmental delay, brain atrophy, suspected Trisomy 21. | Postnatal | No | Yes |
| 26 | Trisomy-21 | Suspected Trisomy 21 based on non-invasive prenatal screening | Prenatal | Yes | Yes |
| 27 | Trisomy-21 | A 2-month-old female diagnosed with Atrial septal defect, hypothyroidism, hypertelorism, hypotonia, neonatal thrombocytopenia, and facial dysmorphism. Clinical features suggestive of Trisomy 21 | Postnatal | No | Yes |
| 28 | Trisomy-21 | A 2-year-old male diagnosed with Down syndrome, elevated liver enzymes, hepatomegaly, repeated infection (chest and chronic), chronic diarrhea, and recurrent vomiting | Postnatal | No | Yes |
| 29 | Trisomy-21 | 3-year-old boy with clinical features of Down syndrome | Postnatal | No | Yes |
| 30 | Trisomy-21 | Abnormal fetus with thick nuchal translucency and atrial septal defect (ASD) | Prenatal | Yes | No |
| 31 | Trisomy-21 | A 5-month-old male diagnosed with muscle weakness, facial dysmorphism, hypertelorism, cardiomyopathy, ventricular septal defect, failure to thrive, gross motor delay, hypotonia, aganglionic megacolon, possible Hirschsprung disease, and pneumonia | Postnatal | Yes | No |
| 32 | Trisomy-21 | Abnormal fetus with cystic hygroma | Prenatal | No | Yes |
| 33 | Trisomy-21 | A 7-month-old male diagnosed with Trisomy 21 | Postnatal | No | Yes |
| 34 | Turner syndrome | Fetus with cystic hygroma | Prenatal | Yes | No |
| 35 | Turner syndrome | Turner syndrome, hypogonadism | Postnatal | No | Yes |
| 36 | Turner syndrome | Short stature, aortic dilatation, a webbed neck, mental retardation, autism spectrum disorder, hepatomegaly, Turner syndrome, and a large hepatic adenoma | Postnatal | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hamed, M.H.; Maddirevula, S.; Moghrabi, N.; Aldahmesh, M.A.; Alfalah, A.H.; Khouj, E.; Altuwaijri, N.; Alhossiny, M.; Imtiaz, F.; Alfares, A. Detection of Chromosomal Aneuploidy Using Exome Sequencing. Genes 2025, 16, 992. https://doi.org/10.3390/genes16090992
Al-Hamed MH, Maddirevula S, Moghrabi N, Aldahmesh MA, Alfalah AH, Khouj E, Altuwaijri N, Alhossiny M, Imtiaz F, Alfares A. Detection of Chromosomal Aneuploidy Using Exome Sequencing. Genes. 2025; 16(9):992. https://doi.org/10.3390/genes16090992
Chicago/Turabian StyleAl-Hamed, Mohamed H., Sateesh Maddirevula, Nabil Moghrabi, Mohammed A. Aldahmesh, Abdullah H. Alfalah, Ebtissal Khouj, Norah Altuwaijri, Midrar Alhossiny, Faiqa Imtiaz, and Ahmed Alfares. 2025. "Detection of Chromosomal Aneuploidy Using Exome Sequencing" Genes 16, no. 9: 992. https://doi.org/10.3390/genes16090992
APA StyleAl-Hamed, M. H., Maddirevula, S., Moghrabi, N., Aldahmesh, M. A., Alfalah, A. H., Khouj, E., Altuwaijri, N., Alhossiny, M., Imtiaz, F., & Alfares, A. (2025). Detection of Chromosomal Aneuploidy Using Exome Sequencing. Genes, 16(9), 992. https://doi.org/10.3390/genes16090992







