Abstract
Background: Syndromic inherited retinal diseases (IRDs) are a clinically and genetically heterogeneous group of disorders, involving the retina and additional organs. Over 80 forms of syndromic IRD have been described. Methods: We aimed to phenotypically and genotypically characterize a cohort of 171 individuals from 140 Israeli families with syndromic IRD. Ophthalmic examination included best corrected visual acuity, fundus examination, visual field testing, retinal imaging and electrophysiological evaluation. Most participants were also evaluated by specialists in fields relevant to their extra-retinal symptoms. Genetic analyses included haplotype analysis, homozygosity mapping, Sanger sequencing and next-generation sequencing. Results: In total, 51% of the families in the cohort were consanguineous. The largest ethnic group was Muslim Arabs. The most common phenotype was Usher syndrome (USH). The most common causative gene was USH2A. In 29% of the families, genetic analysis led to a revised or modified clinical diagnosis. This included confirmation of an atypical USH diagnosis for individuals with late-onset retinitis pigmentosa (RP) and/or hearing loss (HL); diagnosis of Heimler syndrome in individuals with biallelic pathogenic variants in PEX6 and an original diagnosis of USH or nonsyndromic RP; and diagnosis of a mild form of Leber congenital amaurosis with early-onset deafness (LCAEOD) in an individual with a heterozygous pathogenic variant in TUBB4B and an original diagnosis of USH. Novel genotype–phenotype correlations included biallelic pathogenic variants in KATNIP, previously associated with Joubert syndrome (JBTS), in an individual who presented with kidney disease and IRD, but no other features of JBTS. Conclusions: Syndromic IRDs are a highly heterogeneous group of disorders. The rarity of some of these syndromes on one hand, and the co-occurrence of several syndromic and nonsyndromic conditions in some individuals, on the other hand, complicates the diagnostic process. Genetic analysis is the ultimate way to obtain an accurate clinical diagnosis in these individuals.
1. Introduction
Inherited retinal diseases (IRDs) are a clinically and genetically heterogeneous group of diseases, causing visual loss due to the abnormal development, dysfunction or degeneration of photoreceptors or the retinal pigment epithelium [1,2]. The most common form of IRD is retinitis pigmentosa (RP), also known as rod-cone dystrophy. Other forms include cone and cone–rod dystrophy (CD/CRD), inherited macular dystrophies and more. IRDs are one of the most genetically heterogeneous group of disorders in humans. They can be inherited as autosomal recessive (AR), autosomal dominant (AD) or X-linked. Mitochondrial and digenic patterns of inheritance have also been described. To date, over 320 genes have been implicated in IRD (RetNet at https://sph.uth.edu/Retnet/, accessed on 1 May 2025).
Most IRD cases are nonsyndromic (isolated; involving only ophthalmic manifestations); nevertheless, over 80 forms of syndromic IRD have been described [3]. These syndromes are associated with variants in approximately 200 genes. Many forms of syndromic IRD exhibit marked phenotypic variability, and some genes are involved in both syndromic and nonsyndromic IRD forms, depending on the nature and combination of the causative allele/s. The most common form of syndromic IRD is Usher syndrome (USH) (prevalence of 4 to 17 cases per 100,000 individuals), an AR disorder characterized by the combination of RP and sensorineural hearing loss (SNHL), with or without vestibular areflexia. USH is traditionally classified into three major subtypes (USH1-3), the most severe of which is USH1, characterized by congenital profound SNHL, precluding normal development of speech, vestibular areflexia and onset of RP at the first decade of life. The most common form of USH worldwide is USH2, characterized by early-onset, moderate-to-severe, stable SNHL, most pronounced at the higher frequencies, and postpubertal onset of RP, with normal vestibular function. USH3 includes a variable phenotype, characterized by post-lingual progressive SNHL (usually diagnosed in the first decade), varying levels of vestibular dysfunction and average onset of RP in the second decade of life, although later onset may occur [4]. Nevertheless, in recent years, several genes have been reported to be associated with a clinical subtype called “atypical USH” that does not meet the canonical criteria for the three recognized USH subtypes [4].
Like USH, many forms of syndromic IRD are defined as ciliopathies: a group of genetic diseases caused by mutations in genes associated with the structure and function of primary cilia [5]. In the retina, the primary cilium serves as the connecting bridge between the photoreceptor inner and outer segments; its dysfunction leads to impaired photoreceptor function and progressive degeneration. Additional organs which are commonly affected in ciliopathies, besides the retina, are the central nervous system, inner ear, skeleton, kidney and liver [3]. One of the major forms of syndromic IRD that belongs to the ciliopathy group is Bardet–Biedl syndrome (BBS) (estimated prevalence of 1 in 100,000 to 1 in 160,000 individuals), an AR multisystemic disease characterized by retinal dystrophy, postaxial polydactyly, renal disease, intellectual disability and truncal obesity [6,7]. Another ciliopathy is Joubert syndrome (JBTS) (estimated prevalence of 1 in 80,000 to 1 in 100,000 individuals), an AR severe disorder classically characterized by three primary findings: a distinctive cerebellar and brainstem malformation called the molar tooth sign, hypotonia and cognitive impairment. These may be accompanied by breathing abnormalities, ataxia, renal disease, genitourinary abnormalities, retinal dystrophy, ocular colobomas, occipital encephalocele, hepatic fibrosis, polydactyly and endocrine abnormalities [8,9]. Senior–Løken syndrome (estimated prevalence of 1 in 1,000,000 individuals) is an AR condition characterized by the combination of retinal dystrophy and nephronophthisis [10].
A rare AD ciliopathy is Leber congenital amaurosis with early-onset deafness (LCAEOD), caused by pathogenic variants in the TUBB4B gene [11]. TUBB4B encodes for beta-tubulin 4b. Microtubules are dynamic polymeric structures consisting of heterodimers of alpha-tubulins and beta-tubulins, such as TUBB4B, which function in mitosis, intracellular transport, neuron morphology, and ciliary and flagellar motility. Of note, some pathogenic variants of TUBB4B are associated with a distinct phenotype, primary ciliary dyskinesia (PCD), a disorder mainly affecting the respiratory system [12]. Some patients with heterozygous pathogenic variants of TUBB4B are affected by both conditions simultaneously [12].
Another major group of IRDs is inborn errors of metabolism (IEM). IEMs are genetic disorders leading to the failure of carbohydrate metabolism, protein metabolism, fatty acid oxidation or glycogen storage. Many IEMs present with neurologic symptoms [13]. The retina is considered an extension of the brain. Therefore, neurodegeneration resulting from IEMs often involves retinal degeneration as well. One form of syndromic IRD that belongs to the IEM group is peroxisomal biogenesis disorders (PBDs) (aggregated frequency of approximately 1 in 50,000 live births) [14]. Two disorders included in the PBD group are peroxisomal biogenesis disorder 4A (PBD4A, Zellweger syndrome), a multiple congenital anomaly syndrome, and Heimler syndrome 2 (HMLR2), a less severe phenotype involving IRD, SNHL, dental anomalies (amelogenesis imperfecta) and nail abnormalities. Both PBD4A and HMLR2 are caused by biallelic pathogenic variants in the PEX6 gene [15,16].
Genotypic and phenotypic analyses of individuals with syndromic IRD from various ethnic backgrounds have been previously reported as part of large cohorts of individuals with both syndromic and non-syndromic IRD [17,18,19,20,21,22]. Other studies focused on individuals with one specific form of syndromic IRD (such as JBTS or BBS) [23,24]. One study reported a cohort of 100 Spanish individuals with syndromic IRD only but excluded USH patients [25]. Here, we describe phenotypic and genotypic characterization of a cohort of 171 Israeli patients with various forms of syndromic IRD.
2. Materials and Methods
2.1. Subjects
A total of 1435 Israeli families were recruited between the years 2004 and 2025 to our ongoing study on the genetic basis for IRD, conducted at the Rappaport Faculty of Medicine in the Technion-Israel Institute of Technology (the term “family” refers to a nuclear family; thus, each proband and his first-degree relatives, if recruited, were defined as a family). In 891 of these families, the underlying genetic cause was identified. Of these genetically solved families, 140 (16%) segregate a syndromic IRD type. These families, including 171 affected individuals, were defined as the syndromic IRD cohort described in this study (Figure 1A and Supplementary Table S1). Of the 544 unsolved families, 49 were defined as syndromic or possibly syndromic. Thirty of these families were analyzed by whole exome sequencing (WES) or whole genome sequencing (WGS) (Supplementary Figure S1). In three of them, one heterozygous pathogenic allele was identified in a syndromic IRD-causative gene compatible with their phenotype and inherited in an AR mode. A second heterozygous pathogenic allele in the same gene was not identified, despite the fact that two of these families were analyzed by WGS. Some of the families and/or pathogenic variants included in this study were previously reported by us, but not in the context of a syndromic IRD cohort [26,27,28,29,30,31,32,33,34,35,36,37] (Supplementary Table S1).
The study was performed according to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Boards (IRBs) of all participating institutions (see details in the Institutional Review Board Statement). Informed consent was obtained from all participants or their parents, as well as from unaffected family members.
2.2. Clinical Evaluation
Ophthalmic diagnoses were made based on complete ophthalmic examination, including best corrected visual acuity (BCVA), fundus examination, visual field testing, retinal imaging by optical coherence tomography (OCT) and fundus autofluorescence (FAF) and electrophysiological evaluation by full-field electroretinography (ffERG) and/or multi-focal electroretinography (mfERG). Most participants were also evaluated by specialists in fields relevant to their symptoms, including neurology, otolaryngology, nephrology, hepatology and/or medical genetics.
2.3. Genetic Analyses
Testing strategies used in each family are listed in Supplementary Table S1. Index patients from 59 families were tested for founder mutations prevalent in their ethnic group or village, by PCR amplification with specifically designed primers, followed by direct sequencing. Index patients from 56 families were subjected to WES as previously described [38]. Index patients from two families were subjected to WGS as previously described [28]. Index patients from six families were tested by targeted next generation sequencing (TNGS) of 108 or 113 known IRD genes using the Molecular Inversion Probes technique [39,40]. Eight families were analyzed by haplotype analysis, followed by PCR-amplification and Sanger sequencing of relevant candidate genes, as previously described [41]. Affected individuals from six families were analyzed by homozygosity mapping, followed by PCR-amplification and Sanger sequencing of relevant candidate genes, as previously described [35]. In three index patients, specific candidate genes were targeted based on the phenotype, and all their coding exons were PCR-amplified with or without consecutive Sanger sequencing.
2.4. Bioinformatics
Public databases used to determine population allele frequencies included gnomAD [42], ESP6500 [43], TOPMed BRAVO [44] and GME Variome [45].
The pathogenicity of missense variants was evaluated based on the Franklin by Genoox (https://franklin.genoox.com/) aggregated prediction score, which is based on scores obtained by multiple prediction tools including REVEL [46], MutationAssessor/r3 [47], SIFT [48], Polyphen-2 [49], MutationTaster 2021 [50], FATHMM (v2.3) [51], DANN (v3.19) [52], MetaLR [53], PrimateAI (v1.0) [54] and BayesDel (v1) [55]. The putative effect of certain variants on splicing was evaluated based on the following prediction tools: SpliceAI (v1.3.1) [56], dbscSNV_AdaBoost[57] and dbscSNV_RandomForest [58] (v1.1). Analysis of the evolutionary conservation of KATNIP Ser1571 was performed with The ConSurf Server (https://consurf.tau.ac.il/consurf_index.php) [59].
3. Results
3.1. Characteristics of the Syndromic IRD Cohort
Seventy-two of the families in the syndromic IRD cohort (51%) are consanguineous. Muslim Arabs constitute the largest ethnic group in the cohort (36%), followed by Ashkenazi Jews (20%), North African Jews (11%), Oriental Jews (11%), Jews of mixed ancestry (7%), Yemenite Jews (6%), Druze (5%), Christian Arabs (2%) and others (Figure 1B).
Individuals in this cohort were affected by 24 distinct syndromic IRD phenotypes, caused by 96 pathogenic variants in 41 different genes. Of these variants, 81 have been previously reported, while 15 are novel. The most common phenotype in the cohort was USH (65% of families), followed by BBS (6%) and hypotrichosis with juvenile macular dystrophy (4%) [60] (Figure 1C). Among families with USH, the most common type was USH2 (44%). The most common causative gene in the entire cohort was USH2A (NM_206933.2) (31%), followed by MYO7A (NM_000260.3) (10%) and CLRN1 (NM_001195794.1) (6%) (Figure 1D). The most common causative variant in the entire cohort was USH2A: c.236_239dup (Figure 1E), present in 20 alleles of 12 probands.
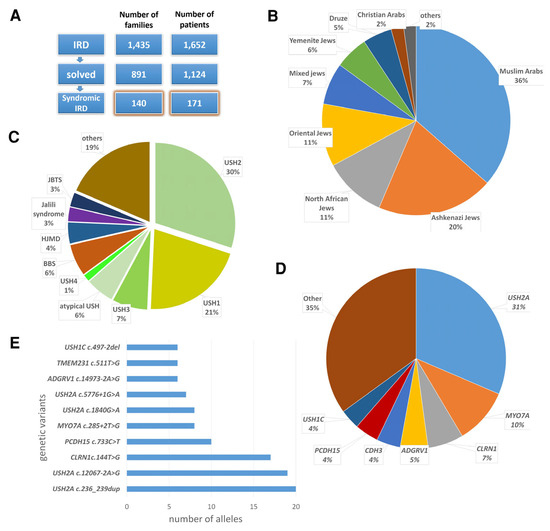
Figure 1.
Phenotypic and genotypic characteristics of the syndromic IRD patient cohort. (A) Assembly of the syndromic IRD cohort. (B) Ethnic distribution of the cohort. (C) Phenotypic distribution of the cohort. BBS, Bardet–Biedl syndrome; HJMD, hypotrichosis with juvenile macular dystrophy; JBTS, Joubert syndrome; USH, Usher syndrome. (D) Distribution of mutated genes in the cohort. (E) The most common causative variants identified in the cohort.
3.2. Revised Clinical Diagnosis Following Genetic Analysis
In 41 of the families (29%), genetic analysis led to a revised or modified clinical diagnosis (Supplementary Table S1). Most of these families (70%) were analyzed by WES. For example, in several individuals with a combination of RP and HL, late onset of one or both of these features led to uncertainty regarding a possible diagnosis of USH, since the phenotype did not fit any of the major USH types (USH1-3). Consequently, it was unclear if RP and HL in these cases are part of a syndrome or arose independently. Genetic analysis led to the identification of pathogenic variants in USH-related genes (mainly USH2A) in each of these cases, thus confirming a diagnosis of atypical USH (Supplementary Table S1 and Table 1).

Table 1.
Retinal, auditory and genetic characteristics of individuals in whom genetic analysis confirmed a diagnosis of atypical USH.
Interestingly, biallelic pathogenic variants in the USH2A gene were identified in two unrelated individuals from families TB120 and TB891, with an USH1 diagnosis due to congenital profound SNHL. One individual was homozygous for the common c.236_239dup allele, while the other was compound heterozygous for c.236_239dup and c.1840G>A; p.(Gly614Arg). Of note, the c.236_239dup allele was identified in homozygosity in seven additional individuals, six of them diagnosed with classic USH2 and one with atypical USH (Supplementary Table S1).
In individuals from two families, originally diagnosed with USH or with nonsyndromic RP, the identification of pathogenic variants in PEX6 (NM_000287) led to a revised clinical diagnosis. The participant from family TB725 was referred to clinical and genetic evaluation due to IRD with onset at the age of 30 y. WES revealed a likely homozygous variant in PEX6, c.1802G>A; p.(Arg601Gln). This variant is rare (gnomAD aggregated allele frequency (AF) of 0.3%) and predicted pathogenic (Franklin aggregated prediction score = 0.86). It has been previously reported in individuals with either HMLR2 or PBD4A [15,61,62]. The genetic finding led to focused clinical re-evaluation of this participant, which revealed late onset mild HL and a very mild dental abnormality. These clinical findings led to a revised clinical diagnosis of HMLR2. It should be noted that DNA from the parents of this individual was not available for segregation analysis, and that we did not perform a focused analysis to rule out the possibility that this individual is actually compound heterozygous for p.(Arg601Gln) and for a complete or partial deletion of the PEX6 gene. Such a deletion would be considered a null allele, and given the very mild phenotype of this patient, this option is less likely.
In the participant from family TB1003, the combination of congenital SNHL and late-onset RP led to a possible diagnosis of USH. WES revealed two heterozygous variants in PEX6, c.2094+2T>C and c.2534T>C;p.(Ile845Thr). Both variants are rare (c.2094+2T>C not present in public databases; c.2534T>C gnomAD aggregated AF of 0.003%), and they were both reported previously in compound heterozygosity in an Israeli individual with an USH-like phenotype [63]. A repeated clinical evaluation of our participant revealed that she had severe dental problems in childhood, probably amelogenesis imperfecta. Her clinical diagnosis was, thus, amended to HMLR2.
Diagnosis was also revised in an individual from family TB1134. This participant had SNHL since childhood and received a cochlear implant at the age of 36 y. She was diagnosed with RP at the age of 51 y. She also experienced multiple respiratory infections and had a family history of severe lung disease in her father. The combination of childhood-onset SNHL and RP led to a diagnosis of USH. WES revealed a heterozygous variant in TUBB4B (NM_006088.6), c.16C>T; p.(His6Tyr). This variant is rare (not present in public databases) and predicted pathogenic (Franklin aggregated prediction score = 0.7). To date, 33 individuals harboring heterozygous pathogenic variants of TUBB4B have been reported (including the current report). Of them, 30 (91%) had HL, 24 (73%) had IRD, 14 (42%) had PCD/recurrent respiratory infections, six (18%) had hydrocephalus, three had kidney disease, three had congenital heart disease and three had skeletal abnormalities (9% each) [11,12,64,65,66,67,68,69] (Figure 2).
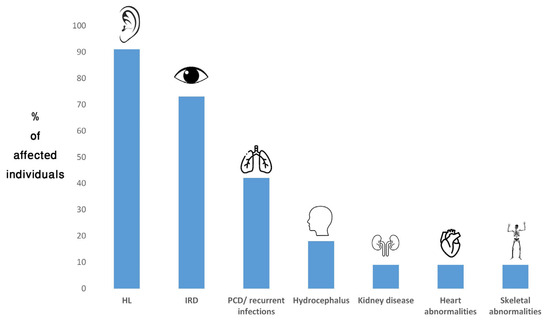
Figure 2.
Frequency of TUBB4B-associated phenotypes in affected individuals reported to date. HL, hearing loss; IRD, inherited retinal disease; PCD, primary ciliary dyskinesia.
3.3. Co-Occurrence of Syndromic IRD and Additional Phenotypes
The co-occurrence of non-syndromic IRD and additional non-syndromic phenotypes, leading to a false diagnosis of syndromic IRD, is not uncommon [70]. However, in some of the participants, we identified the co-occurrence of syndromic IRD with additional syndromic or non-syndromic conditions. For example, in family TB939, the proband had a combination of RP and HL (leading to an initial diagnosis of USH), as well as truncal obesity, hypoplastic kidney and diabetes mellitus. WES revealed homozygous pathogenic variants in two genes: BBS1 (NM_024649.4) c.437G>C; p.(Arg146Pro) (underlying BBS [71]) and CABP2 (NM_016366) c.419T>G; p.(Met140Arg) (underlying nonsyndromic HL [72]). The diagnosis was, thus, changed to BBS and HL. In family TB1199, the proband had multiple symptoms, including RP, HL, intellectual disability, short stature, truncal obesity and phalangeal abnormalities. WES revealed a homozygous pathogenic variant in ADGRV1 (NM_032119.3) (c.9335_9336inv; p.(Phe3112*)), accounting for RP and HL and establishing a diagnosis of USH2 [73]. In addition, the proband was heterozygous for a pathogenic variant of the SMARCA2 gene (NM_003070.5) (c.2657A>G; p.(Asn886Ser)). This variant is rare (not present in gnomAD) and predicted pathogenic (Franklin aggregated prediction score = 0.98). Heterozygous pathogenic variants in SMARCA2 are associated with Blepharophimosis-impaired intellectual development syndrome or with Nicolaides–Baraitser syndrome [74,75]. Both syndromes are inherited as AD, and they have partially overlapping phenotypes, which include intellectual disability, short stature and phalangeal abnormalities. This patient is, therefore, simultaneously affected by two distinct syndromes.
3.4. New Genotype–Phenotype Correlation: Biallelic Pathogenic Variants in KATNIP in an Individual with a JBTS26-Related Ciliopathy
Family TB398 is a non-consanguineous family of mixed Jewish origin. The proband is a female who was seen by us at the age of 24 y. She had normal growth and development, and normal cognitive function. At the age of 7 y, she was diagnosed with type 2 glomerulonephritis, and subsequently underwent two kidney transplantations, at the ages of 9 and 16 y. At the age of 15 y, she developed progressive SNHL, which was attributed to antibiotic toxicity. At the age of 22 y she was diagnosed with high intracranial pressure, which was treated pharmacologically (Diamox) and surgically (lumbar punctures). At the same age, she started experiencing visual disturbances with severe photophobia. BCVA was 6/7 in both eyes. Funduscopy revealed salt and pepper pigmentation of the retinal pigmented epithelium in the fovea and macula, surrounded by white dots and patches extending to the periphery. OCT revealed irregularity of the external band. ffERG indicated reduced responses under both photopic and scotopic conditions. mfERG demonstrated a reduced foveal peak with preserved peripheral responses. These findings led to a differential diagnosis of CRD versus macular dystrophy with peripheral involvement. Retinal involvement secondary to the kidney disease was also considered. WES revealed two rare heterozygous variants in the KATNIP (KIAA0556) gene (NM_015202.4): c.49C>T; p.(Arg17*) and c.4711A>G; p.(Ser1571Gly). c.49C>T is a nonsense variant, which is not present in gnomAD but was reported in ClinVar as pathogenic. c.4711A>G is a missense variant with a gnomAD aggregated AF of 0.008%. It affects a highly conserved amino acid (Supplementary Figure S2) and is predicted pathogenic (Franklin aggregated prediction score = 0.8), but it was reported in ClinVar as a VUS. Pathogenic variants in KATNIP have been associated with JBTS, a severe phenotype compatible with retinal degeneration and kidney disease, and usually involving developmental delay and intellectual disability [76]. HL has not been reported as part of the JBTS-associated phenotype. Of note, the proband was also found to be heterozygous for a likely-pathogenic variant in POLG (Supplementary Table S1). Pathogenic variants in POLG are mostly associated with a spectrum of AR mitochondrial conditions. However, AD inheritance has also been described, mainly associated with progressive external ophthalmoplegia, which may be accompanied by additional neurological features, including progressive SNHL [77]. The proband did not present with ophthalmoplegia, and whether her HL is caused by this POLG variant remains unclear.
4. Discussion
Over 80 forms of syndromic IRD have been described to date [3], over 20 of which were diagnosed in individuals from the cohort described here. The most common phenotype in the cohort was USH (and specifically USH2), and the most common causative gene was USH2A. These findings are similar to reported findings in other IRD cohorts worldwide [17,18,19,20,21,22]. Nevertheless, while this study confirms some previously reported findings, it also adds new insights, as detailed below.
Analysis of the USH cases in our cohort emphasizes the phenotypic variability associated with pathogenic variants in USH2A. While most individuals with biallelic pathogenic variants in USH2A are diagnosed with USH2 [78] or with nonsyndromic RP [79], some have atypical USH, with late onset of HL and/or RP (Table 1) (reviewed in [4]). In contrast, we also identified two individuals with biallelic pathogenic variants in USH2A who were phenotypically classified as USH1. Such cases have been reported previously but are quite rare [80]. Interestingly, one of these individuals was homozygous for a common allele in the Israeli population, c.236_239dup, which is usually associated with an USH2 phenotype. This finding suggests the involvement of additional genetic and/or environmental modifying factors.
Another interesting finding is the tendency to diagnose individuals with the combination of HL and RP with USH, while overlooking additional phenotypic features, which might point to the actual diagnosis. While USH is the most prevalent form of syndromic IRD and the most common cause for genetic deaf-blindness, at least 11 other syndromes involving RP and HL have been described [3]. One of them is Heimler syndrome. Our findings suggest that this syndrome is under-diagnosed, and some patients may obtain a diagnosis of USH or even nonsyndromic RP, if the dental anomalies are overlooked. Similarly, the participant with a heterozygous TUBB4B pathogenic variant was also diagnosed with USH, due to the combination of SNHL and RP. However, the very late onset of RP in this case, and the history of recurrent respiratory infections, suggested a different diagnosis, as, indeed, revealed by genetic testing.
To date, pathogenic variants in TUBB4B have been associated with two distinct AD phenotypes, LCAEOD and PCD, which may overlap in some patients [11,12]. The individual described here presented with early-onset SNHL, IRD and recurrent respiratory infections, which is in agreement with previous reports. However, our findings in this patient and the retrospective analysis of all patients reported to date demonstrate that pathogenic variants in TUBB4B are not necessarily associated with two distinct disorders, LCAEOD or PCD, but rather with a phenotypic spectrum, with different combinations of features found in both diseases. This conclusion was previously implied [12] but is further established in the current study. Moreover, we argue that the name LCAEOD is misleading. LCA is mostly characterized with severe visual impairment, which is either congenital or diagnosed within the first year of life [81]. Of the LCAEOD-individuals reported to date, only seven were reported to have LCA and/or diagnosed with visual impairment by the age of 1 y. Seven had early-onset IRD (ages 1–6 y), while five (including the individual described here) had adult-onset (ages 25–51 y) [11,12,64,65,66,67,68,69]. In addition, while most individuals presented with LCA or RP, at least two had CRD [64,69]. TUBB4B-associated disease is, therefore, a ciliopathy, which primarily affects the ear, the retina and the respiratory system, but it might affect additional systems as well; the associated retinal phenotype can appear from birth to late adulthood, and present as LCA, RP or CRD.
JBTS is an AR multisystemic ciliopathy, exhibiting both intra- and interfamilial clinical variation. At least 40 causative genes have been identified; one of them is KATNIP (underlying JBTS26) [76]. Biallelic pathogenic variants in KATNIP have been reported to date in only a few individuals worldwide, all diagnosed with JBTS [76,82,83,84,85,86]. Clinical data were provided for 10 of 11 reported individuals; nine of them were reported to have cognitive impairment, mostly severe. Ophthalmic symptoms in some of these individuals included oculomotor apraxia, nystagmus and ptosis; two siblings from one family had cone dystrophy/reduced cone function [86]. The individual reported here is heterozygous for two rare variants of KATNIP: a nulll variant (p.(Arg17*)) and a missense variant (p.(Ser1571Gly)), which is predicted pathogenic. She had normal cognitive function, with no hypotonia and no known brain abnormalities. Her main features were renal disease and RD. Renal disease was not previously reported in individuals with KATNIP pathogenic variants. Nevertheless, renal disease was found in individuals with JBTS due to pathogenic alleles in other genes [8,9]. Moreover, according to the Human Protein Atlas (www.proteinatlas.org), KATNIP is expressed in the kidney. The association of intracranial hypertension in a ciliopathy is a rare occurrence [87]. While cilia are involved in the central nervous system, and ciliary dysfunction may contribute to intracranial hypertension [88,89], the etiology of intracranial hypertension in this case may be also due to renal transplantation and/or post-renal transplant medications [90]. Nevertheless, this patient presents a milder JBTS26-associated phenotype, which extends the phenotypic spectrum associated with biallelic pathogenic variants of KATNIP.
Of the 140 families in the syndromic IRD cohort, 59 (42%) were genetically diagnosed by testing for common founder mutations and 56 (40%) by WES (Supplementary Figure S1). This indicates that in populations with strong founder effects, such as the Israeli population [91], the Dutch population [92], the Finnish population [93] and others, founder testing is a cost-effective approach for genetic diagnosis of inherited conditions, including syndromic IRD. WES is a highly effective approach for genetic analysis of syndromic IRD in all populations. While TNGS (gene panels) is commonly used in clinical settings, in Israel and in other Western countries, most commercially available IRD gene panels include mainly causative genes for nonsyndromic IRD (some of which, like USH2A, may also be associated with syndromic IRD). Given the high degree of genetic and clinical heterogeneity associated with syndromic IRD, WES is a broader approach with a higher chance to identify the causative variants, including in genes that are rare and hard to predict based on the phenotype alone. Patients who remain unsolved following WES should be subjected to more advanced genomic technologies, including WGS, long-read sequencing and optical genome mapping, which may identify variants in non-coding regions and complex structural aberrations [94,95,96].
In summary, the current study describes a relatively large cohort of 171 Israeli patients with syndromic IRD. While previous studies mainly reported findings on syndromic IRD as part of large IRD cohorts, or focused on specific types of syndromic IRD, our study included patients with various forms of syndromic IRD and focused on the unique challenges associated with clinical diagnosis of such patients. The availability of genetic data and comprehensive clinical data for these patients allowed us to perform a thorough genotype–phenotype correlation. This led to interesting outcomes, including a better definition of the TUBB4B-associated phenotype and broadening of the phenotypic spectrum associated with KATNIP pathogenic variants. Nevertheless, the application of WES and WGS on additional individuals with syndromic or possibly syndromic IRD from our general IRD cohort could increase the number of participants in the study. Also, it should be noted that most of the study participants were from Northern and Central Israel, while only a few were from Southern Israel and the Jerusalem area. Consequently, the relative frequencies of various phenotypes, genes and alleles in this cohort do not necessarily represent the entire Israeli population.
5. Conclusions
Syndromic IRD is a highly heterogeneous group of disorders, both clinically and genetically. The rarity of some of these syndromes, on one hand, and the co-occurrence of several syndromic and nonsyndromic conditions in some individuals, on the other hand, complicate the diagnostic process. This study further emphasizes these challenges and demonstrates how genetic analysis, combined with detailed phenotypic assessment, is the ultimate way to obtain an accurate clinical diagnosis in these individuals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes16070745/s1, Table S1: Demographic, phenotypic and genotypic characteristics of the syndromic IRD cohort; Figure S1: Composition of the entire IRD cohort and methods used for genetic analyses of syndromic IRD patients; Figure S2: Evolutionary conservation of KATNIP serine 1571.
Author Contributions
Conceptualization, T.B.-Y.; methodology, T.B.-Y., formal analysis, T.B.-Y., S.K., D.S., D.M.P. and S.R.; investigation, S.K. and D.M.P.; resources, R.L., H.N., M.E., N.G.-C., S.Z.-S., E.M., Y.R., I.D., D.Z. and E.C.; data curation, T.B.-Y. and S.K.; writing—original draft preparation, T.B.-Y.; writing—review and editing, S.K., R.L., H.N., M.E., N.G.-C., S.Z.-S., E.M., Y.R., I.D., DMP, D.Z., E.C., S.A.S., F.P.M.C., D.S. and S.R.; supervision, T.B.-Y., S.A.S., F.P.M.C. and S.R.; funding acquisition, T.B.-Y., F.P.M.C. and S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Israel Science Foundation, grant number 331/24, and the Dresner Research Fund in Life Sciences, to TBY; Foundation Fighting Blindness Career Development Award CDGE-0621-0809-RAD, the Algemene Nederlandse Vereniging ter voorkoming van Blindheid, Oogfonds, Landelijke Stichting voor Blinden en Slechtzienden, Rotterdamse Stichting Blindenbelangen, Stichting Blindenhulp, Stichting tot Verbetering van het Lot der Blinden, and Stichting Blinden-Penning, to SR.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the IRBs, as follows: Department of Ophthalmology, Rambam Health Care Campus (IRB approval #3019; approved on 8 November 2016); Division of Ophthalmology, Tel Aviv Sourasky Medical Center (IRB approval #0145-14-TLV, approved on 2 July 2018); the inherited retinal degeneration clinic at Schneider Children’s Medical Center of Israel and Department of Ophthalmology, Rabin Medical Center (IRB approval #0396-13-RMC, approved on 9 May 2023); Department of Ophthalmology, Bnai Zion Medical Center (IRB approval #0121-16-BN, approved on 17 March 2025); Goldschleger Eye Institute, Sheba Medical Center (IRB approval #0309-13-SMC, approved on 10 June 2024); Genetics Institute, Emek Medical Center (IRB approval #4610206, approved on 19 September 2012) (note that participants of this study were recruited over a period of 20 years, and IRB approval dates are relevant to the timing of patient recruitment from each participating institute).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study or their parents.
Data Availability Statement
All results obtained are presented in the text, the Figures or the Tables. Raw data can be obtained upon request. The identified pathogenic variants have been submitted to the Leiden Open Variation Database (LOVD) (http://www.lovd.nl, accessed on 4 April 2025) and/or to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/, accessed on 1 May 2025).
Acknowledgments
We are grateful to the patients for their participation in this study. We thank Leah Rizel for technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Autosomal dominant |
| AF | Allele frequency |
| AR | Autosomal recessive |
| BBS | Bardet–Biedl syndrome |
| BCVA | Best-corrected visual acuity |
| CRD | Cone–rod dystrophy |
| DD | Developmental delay |
| FAF | Fundus autofluorescence |
| ffERG | Full-field electroretinogram |
| HL | Hearing loss |
| HMLR2 | Heimler syndrome 2 |
| ID | Intellectual disability |
| IEM | Inborn errors of metabolism |
| IRB | Institutional review board |
| IRD | Inherited retinal disease |
| JBTS | Joubert syndrome |
| LCAEOD | Leber congenital amaurosis with early-onset deafness |
| mfERG | Multi-focal electroretinogram |
| OCT | Optical coherence tomography |
| PBD4A | Peroxisomal biogenesis disorder 4A |
| PCD | Primary ciliary dyskinesia |
| RP | Retinitis pigmentosa |
| SNHL | Sensorineural hearing loss |
| USH | Usher syndrome |
| WES | Whole exome sequencing |
| WGS | Whole genome sequencing |
| y | Years |
References
- Ben-Yosef, T. Inherited Retinal Diseases. Int. J. Mol. Sci. 2022, 23, 13467. [Google Scholar] [CrossRef] [PubMed]
- Schneider, N.; Sundaresan, Y.; Gopalakrishnan, P.; Beryozkin, A.; Hanany, M.; Levanon, E.Y.; Banin, E.; Ben-Aroya, S.; Sharon, D. Inherited retinal diseases: Linking genes, disease-causing variants, and relevant therapeutic modalities. Prog. Retin. Eye Res. 2021, 89, 101029. [Google Scholar] [CrossRef] [PubMed]
- Tatour, Y.; Ben-Yosef, T. Syndromic Inherited Retinal Diseases: Genetic, Clinical and Diagnostic Aspects. Diagnostics 2020, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Delmaghani, S.; El-Amraoui, A. The genetic and phenotypic landscapes of Usher syndrome: From disease mechanisms to a new classification. Hum. Genet. 2022, 141, 709–735. [Google Scholar] [CrossRef]
- Sreekumar, V.; Norris, D.P. Cilia and development. Curr. Opin. Genet. Dev. 2019, 56, 15–21. [Google Scholar] [CrossRef]
- Forsyth, R.; Gunay-Aygun, M. Bardet-Biedl Syndrome Overview. In GeneReviews((R)); Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2003. [Google Scholar]
- Forsythe, E.; Beales, P.L. Bardet-Biedl syndrome. Eur. J. Hum. Genet. 2013, 21, 8–13. [Google Scholar] [CrossRef]
- Glass, I.A.; Dempsey, J.C.; Parisi, M.; Doherty, D. Joubert Syndrome. In GeneReviews((R)); Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Parisi, M.A.; Doherty, D.; Chance, P.F.; Glass, I.A. Joubert syndrome (and related disorders) (OMIM 213300). Eur. J. Hum. Genet. 2007, 15, 511–521. [Google Scholar] [CrossRef]
- Tsang, S.H.; Aycinena, A.R.P.; Sharma, T. Ciliopathy: Senior-Loken Syndrome. Adv. Exp. Med. Biol. 2018, 1085, 175–178. [Google Scholar] [CrossRef]
- Luscan, R.; Mechaussier, S.; Paul, A.; Tian, G.; Gerard, X.; Defoort-Dellhemmes, S.; Loundon, N.; Audo, I.; Bonnin, S.; LeGargasson, J.F.; et al. Mutations in TUBB4B Cause a Distinctive Sensorineural Disease. Am. J. Hum. Genet. 2017, 101, 1006–1012. [Google Scholar] [CrossRef]
- Dodd, D.O.; Mechaussier, S.; Yeyati, P.L.; McPhie, F.; Anderson, J.R.; Khoo, C.J.; Shoemark, A.; Gupta, D.K.; Attard, T.; Zariwala, M.A.; et al. Ciliopathy patient variants reveal organelle-specific functions for TUBB4B in axonemal microtubules. Science 2024, 384, eadf5489. [Google Scholar] [CrossRef]
- Ferreira, C.R.; van Karnebeek, C.D.M. Inborn errors of metabolism. Handb. Clin. Neurol. 2019, 162, 449–481. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.J.; Dodt, G.; Raymond, G.V.; Braverman, N.E.; Moser, A.B.; Moser, H.W. Peroxisome biogenesis disorders. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2006, 1763, 1733–1748. [Google Scholar] [CrossRef] [PubMed]
- Ratbi, I.; Falkenberg, K.D.; Sommen, M.; Al-Sheqaih, N.; Guaoua, S.; Vandeweyer, G.; Urquhart, J.E.; Chandler, K.E.; Williams, S.G.; Roberts, N.A.; et al. Heimler Syndrome Is Caused by Hypomorphic Mutations in the Peroxisome-Biogenesis Genes PEX1 and PEX6. Am. J. Hum. Genet. 2015, 97, 535–545. [Google Scholar] [CrossRef]
- Yahraus, T.; Braverman, N.; Dodt, G.; Kalish, J.E.; Morrell, J.C.; Moser, H.W.; Valle, D.; Gould, S.J. The peroxisome biogenesis disorder group 4 gene, PXAAA1, encodes a cytoplasmic ATPase required for stability of the PTS1 receptor. EMBO J. 1996, 15, 2914–2923. [Google Scholar] [CrossRef]
- Carss, K.J.; Arno, G.; Erwood, M.; Stephens, J.; Sanchis-Juan, A.; Hull, S.; Megy, K.; Grozeva, D.; Dewhurst, E.; Malka, S.; et al. Comprehensive Rare Variant Analysis via Whole-Genome Sequencing to Determine the Molecular Pathology of Inherited Retinal Disease. Am. J. Hum. Genet. 2017, 100, 75–90. [Google Scholar] [CrossRef]
- Dockery, A.; Stephenson, K.; Keegan, D.; Wynne, N.; Silvestri, G.; Humphries, P.; Kenna, P.F.; Carrigan, M.; Farrar, G.J. Target 5000: Target Capture Sequencing for Inherited Retinal Degenerations. Genes 2017, 8, 304. [Google Scholar] [CrossRef]
- Karali, M.; Testa, F.; Di Iorio, V.; Torella, A.; Zeuli, R.; Scarpato, M.; Romano, F.; Onore, M.E.; Pizzo, M.; Melillo, P.; et al. Genetic epidemiology of inherited retinal diseases in a large patient cohort followed at a single center in Italy. Sci. Rep. 2022, 12, 20815. [Google Scholar] [CrossRef]
- Marta, A.; Marques-Couto, P.; Vaz-Pereira, S.; Costa, J.; Cabral, D.; Estrela-Silva, S.; Franca, M.; Marques, J.H.; Meneres, M.J.; Lemos, C.; et al. Clinical and genetic landscape of IRD in Portugal: Pooled data from the nationwide IRD-PT registry. NPJ Genom. Med. 2025, 10, 11. [Google Scholar] [CrossRef]
- Perea-Romero, I.; Gordo, G.; Iancu, I.F.; Del Pozo-Valero, M.; Almoguera, B.; Blanco-Kelly, F.; Carreno, E.; Jimenez-Rolando, B.; Lopez-Rodriguez, R.; Lorda-Sanchez, I.; et al. Genetic landscape of 6089 inherited retinal dystrophies affected cases in Spain and their therapeutic and extended epidemiological implications. Sci. Rep. 2021, 11, 1526. [Google Scholar] [CrossRef]
- Stone, E.M.; Andorf, J.L.; Whitmore, S.S.; DeLuca, A.P.; Giacalone, J.C.; Streb, L.M.; Braun, T.A.; Mullins, R.F.; Scheetz, T.E.; Sheffield, V.C.; et al. Clinically Focused Molecular Investigation of 1000 Consecutive Families with Inherited Retinal Disease. Ophthalmology 2017, 124, 1314–1331. [Google Scholar] [CrossRef]
- Gnanasekaran, H.; Chandrasekhar, S.P.; Kandeeban, S.; Periyasamy, P.; Bhende, M.; Khetan, V.; Gupta, N.; Kabra, M.; Namboothri, S.; Sen, P.; et al. Mutation profile of Bardet-Biedl syndrome patients from India: Implicative role of multiallelic rare variants and oligogenic inheritance pattern. Clin. Genet. 2023, 104, 443–460. [Google Scholar] [CrossRef] [PubMed]
- Kiraz, A.; Erdogan, M.; Balta, B.; Gumus, H.; Mutlu, M.B.; Mammadova, N.; Ozcelik, F.; Sahin, I.O.; Guven, A.S.; Kumandas, S.; et al. A case series of joubert syndrome evaluated with whole exome sequencing and the utility of optical genome mapping in the diagnosis. Neurogenetics 2025, 26, 47. [Google Scholar] [CrossRef] [PubMed]
- Perea-Romero, I.; Blanco-Kelly, F.; Sanchez-Navarro, I.; Lorda-Sanchez, I.; Tahsin-Swafiri, S.; Avila-Fernandez, A.; Martin-Merida, I.; Trujillo-Tiebas, M.J.; Lopez-Rodriguez, R.; Rodriguez de Alba, M.; et al. NGS and phenotypic ontology-based approaches increase the diagnostic yield in syndromic retinal diseases. Hum. Genet. 2021, 140, 1665–1678. [Google Scholar] [CrossRef]
- Auslender, N.; Bandah, D.; Rizel, L.; Behar, D.M.; Shohat, M.; Banin, E.; Allon-Shalev, S.; Sharony, R.; Sharon, D.; Ben-Yosef, T. Four USH2A founder mutations underlie the majority of Usher syndrome type 2 cases among non-Ashkenazi Jews. Genet. Test. 2008, 12, 289–294. [Google Scholar] [CrossRef]
- Ben-Yosef, T.; Asia Batsir, N.; Ali Nasser, T.; Ehrenberg, M. Retinal dystrophy as part of TTC21B-associated ciliopathy. Ophthalmic Genet. 2021, 42, 329–333. [Google Scholar] [CrossRef]
- Fadaie, Z.; Whelan, L.; Ben-Yosef, T.; Dockery, A.; Corradi, Z.; Gilissen, C.; Haer-Wigman, L.; Corominas, J.; Astuti, G.D.N.; de Rooij, L.; et al. Whole genome sequencing and in vitro splice assays reveal genetic causes for inherited retinal diseases. NPJ Genom. Med. 2021, 6, 97. [Google Scholar] [CrossRef]
- Goldenberg-Cohen, N.; Banin, E.; Zalzstein, Y.; Cohen, B.; Rotenstreich, Y.; Rizel, L.; Basel-Vanagaite, L.; Ben-Yosef, T. Genetic heterogeneity and consanguinity lead to a “double hit”: Homozygous mutations of MYO7A and PDE6B in a patient with retinitis pigmentosa. Mol. Vis. 2013, 19, 1565–1571. [Google Scholar]
- Khalaileh, A.; Abu-Diab, A.; Ben-Yosef, T.; Raas-Rothschild, A.; Lerer, I.; Alswaiti, Y.; Chowers, I.; Banin, E.; Sharon, D.; Khateb, S. The Genetics of Usher Syndrome in the Israeli and Palestinian Populations. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1095–1104. [Google Scholar] [CrossRef]
- Khateb, S.; Kowalewski, B.; Bedoni, N.; Damme, M.; Pollack, N.; Saada, A.; Obolensky, A.; Ben-Yosef, T.; Gross, M.; Dierks, T.; et al. A homozygous founder missense variant in arylsulfatase G abolishes its enzymatic activity causing atypical Usher syndrome in humans. Genet. Med. 2018, 20, 1004–1012. [Google Scholar] [CrossRef]
- Namburi, P.; Ratnapriya, R.; Khateb, S.; Lazar, C.H.; Kinarty, Y.; Obolensky, A.; Erdinest, I.; Marks-Ohana, D.; Pras, E.; Ben-Yosef, T.; et al. Bi-allelic Truncating Mutations in CEP78, Encoding Centrosomal Protein 78, Cause Cone-Rod Degeneration with Sensorineural Hearing Loss. Am. J. Hum. Genet. 2016, 99, 1222–1223. [Google Scholar] [CrossRef]
- Rizel, L.; Safieh, C.; Shalev, S.A.; Mezer, E.; Jabaly-Habib, H.; Ben-Neriah, Z.; Chervinsky, E.; Briscoe, D.; Ben-Yosef, T. Novel mutations of MYO7A and USH1G in Israeli Arab families with Usher syndrome type 1. Mol. Vis. 2011, 17, 3548–3555. [Google Scholar] [PubMed]
- Shalata, A.; Bar-Shai, M.; Hadid, Y.; Mahroum, M.; Mintz, H.; Shalata, Z.E.; Radzishevsky, E.; Genizi, J.; Lorber, A.; Ben-Yosef, T.; et al. Danon Disease: Entire LAMP2 Gene Deletion with Unusual Clinical Presentation-Case Report and Review of the Literature. Genes 2023, 14, 1539. [Google Scholar] [CrossRef] [PubMed]
- Sharon, D.; Ben-Yosef, T.; Goldenberg-Cohen, N.; Pras, E.; Gradstein, L.; Soudry, S.; Mezer, E.; Zur, D.; Abbasi, A.H.; Zeitz, C.; et al. A nationwide genetic analysis of inherited retinal diseases in Israel as assessed by the Israeli inherited retinal disease consortium (IIRDC). Hum. Mutat. 2020, 41, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.; Poulter, J.A.; Levin, A.V.; Capasso, J.E.; Price, S.; Ben-Yosef, T.; Sharony, R.; Newman, W.G.; Shore, R.C.; Brookes, S.J.; et al. Spectrum of PEX1 and PEX6 variants in Heimler syndrome. Eur. J. Hum. Genet. 2016, 24, 1565–1571. [Google Scholar] [CrossRef]
- Tatour, Y.; Sanchez-Navarro, I.; Chervinsky, E.; Hakonarson, H.; Gawi, H.; Tahsin-Swafiri, S.; Leibu, R.; Lopez-Molina, M.I.; Fernandez-Sanz, G.; Ayuso, C.; et al. Mutations in SCAPER cause autosomal recessive retinitis pigmentosa with intellectual disability. J. Med. Genet. 2017, 54, 698–704. [Google Scholar] [CrossRef]
- Ben Yosef, T.; Banin, E.; Chervinsky, E.; Shalev, S.A.; Leibu, R.; Mezer, E.; Rotenstreich, Y.; Goldenberg-Cohen, N.; Weiss, S.; Khan, M.I.; et al. Genetic causes of inherited retinal diseases among Israeli Jews of Ethiopian ancestry. Mol. Vis. 2023, 29, 1–12. [Google Scholar]
- Panneman, D.M.; Hitti-Malin, R.J.; Holtes, L.K.; de Bruijn, S.E.; Reurink, J.; Boonen, E.G.M.; Khan, M.I.; Ali, M.; Andreasson, S.; De Baere, E.; et al. Cost-effective sequence analysis of 113 genes in 1,192 probands with retinitis pigmentosa and Leber congenital amaurosis. Front. Cell Dev. Biol. 2023, 11, 1112270. [Google Scholar] [CrossRef]
- Weisschuh, N.; Feldhaus, B.; Khan, M.I.; Cremers, F.P.M.; Kohl, S.; Wissinger, B.; Zobor, D. Molecular and clinical analysis of 27 German patients with Leber congenital amaurosis. PLoS ONE 2018, 13, e0205380. [Google Scholar] [CrossRef]
- Auslender, N.; Sharon, D.; Abbasi, A.H.; Garzozi, H.J.; Banin, E.; Ben-Yosef, T. A common founder mutation of CERKL underlies autosomal recessive retinal degeneration with early macular involvement among Yemenite Jews. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5431–5438. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Fu, W.; O’Connor, T.D.; Jun, G.; Kang, H.M.; Abecasis, G.; Leal, S.M.; Gabriel, S.; Rieder, M.J.; Altshuler, D.; Shendure, J.; et al. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature 2013, 493, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.C.; Gogarten, S.M.; Fullerton, S.M.; Isasi, C.R.; Mitchell, B.D.; North, K.E.; Rich, S.S.; Taylor, M.R.G.; Zollner, S.; Sofer, T. Social and scientific motivations to move beyond groups in allele frequencies: The TOPMed experience. Am. J. Hum. Genet. 2022, 109, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Halees, A.; Itan, Y.; Spencer, E.G.; He, Y.; Azab, M.A.; Gabriel, S.B.; Belkadi, A.; Boisson, B.; Abel, L.; et al. Characterization of Greater Middle Eastern genetic variation for enhanced disease gene discovery. Nat. Genet. 2016, 48, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, N.M.; Rothstein, J.H.; Pejaver, V.; Middha, S.; McDonnell, S.K.; Baheti, S.; Musolf, A.; Li, Q.; Holzinger, E.; Karyadi, D.; et al. REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am. J. Hum. Genet. 2016, 99, 877–885. [Google Scholar] [CrossRef]
- Reva, B.; Antipin, Y.; Sander, C. Predicting the functional impact of protein mutations: Application to cancer genomics. Nucleic Acids Res. 2011, 39, e118. [Google Scholar] [CrossRef]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009, 4, 1073–1081. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods 2014, 11, 361–362. [Google Scholar] [CrossRef]
- Shihab, H.A.; Gough, J.; Cooper, D.N.; Stenson, P.D.; Barker, G.L.; Edwards, K.J.; Day, I.N.; Gaunt, T.R. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum. Mutat. 2013, 34, 57–65. [Google Scholar] [CrossRef]
- Quang, D.; Chen, Y.; Xie, X. DANN: A deep learning approach for annotating the pathogenicity of genetic variants. Bioinformatics 2015, 31, 761–763. [Google Scholar] [CrossRef]
- Dong, C.; Wei, P.; Jian, X.; Gibbs, R.; Boerwinkle, E.; Wang, K.; Liu, X. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum. Mol. Genet. 2015, 24, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, L.; Gao, H.; Padigepati, S.R.; McRae, J.F.; Li, Y.; Kosmicki, J.A.; Fritzilas, N.; Hakenberg, J.; Dutta, A.; Shon, J.; et al. Predicting the clinical impact of human mutation with deep neural networks. Nat. Genet. 2018, 50, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.J. PERCH: A Unified Framework for Disease Gene Prioritization. Hum. Mutat. 2017, 38, 243–251. [Google Scholar] [CrossRef]
- Jaganathan, K.; Kyriazopoulou Panagiotopoulou, S.; McRae, J.F.; Darbandi, S.F.; Knowles, D.; Li, Y.I.; Kosmicki, J.A.; Arbelaez, J.; Cui, W.; Schwartz, G.B.; et al. Predicting Splicing from Primary Sequence with Deep Learning. Cell 2019, 176, 535–548.e24. [Google Scholar] [CrossRef]
- Moles-Fernandez, A.; Duran-Lozano, L.; Montalban, G.; Bonache, S.; Lopez-Perolio, I.; Menendez, M.; Santamarina, M.; Behar, R.; Blanco, A.; Carrasco, E.; et al. Computational Tools for Splicing Defect Prediction in Breast/Ovarian Cancer Genes: How Efficient Are They at Predicting RNA Alterations? Front. Genet. 2018, 9, 366. [Google Scholar] [CrossRef]
- Liu, X.; Wu, C.; Li, C.; Boerwinkle, E. dbNSFP v3.0: A One-Stop Database of Functional Predictions and Annotations for Human Nonsynonymous and Splice-Site SNVs. Hum. Mutat. 2016, 37, 235–241. [Google Scholar] [CrossRef]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf 2016: An improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef]
- Sprecher, E.; Bergman, R.; Richard, G.; Lurie, R.; Shalev, S.; Petronius, D.; Shalata, A.; Anbinder, Y.; Leibu, R.; Perlman, I.; et al. Hypotrichosis with juvenile macular dystrophy is caused by a mutation in CDH3, encoding P-cadherin. Nat. Genet. 2001, 29, 134–136. [Google Scholar] [CrossRef]
- Trujillano, D.; Bertoli-Avella, A.M.; Kumar Kandaswamy, K.; Weiss, M.E.; Koster, J.; Marais, A.; Paknia, O.; Schroder, R.; Garcia-Aznar, J.M.; Werber, M.; et al. Clinical exome sequencing: Results from 2819 samples reflecting 1000 families. Eur. J. Hum. Genet. 2017, 25, 176–182. [Google Scholar] [CrossRef]
- Yik, W.Y.; Steinberg, S.J.; Moser, A.B.; Moser, H.W.; Hacia, J.G. Identification of novel mutations and sequence variation in the Zellweger syndrome spectrum of peroxisome biogenesis disorders. Hum. Mutat. 2009, 30, E467–E480. [Google Scholar] [CrossRef]
- Raas-Rothschild, A.; Wanders, R.J.; Mooijer, P.A.; Gootjes, J.; Waterham, H.R.; Gutman, A.; Suzuki, Y.; Shimozawa, N.; Kondo, N.; Eshel, G.; et al. A PEX6-defective peroxisomal biogenesis disorder with severe phenotype in an infant, versus mild phenotype resembling Usher syndrome in the affected parents. Am. J. Hum. Genet. 2002, 70, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Bodenbender, J.P.; Marino, V.; Philipp, J.; Tropitzsch, A.; Kernstock, C.; Stingl, K.; Kempf, M.; Haack, T.B.; Zuleger, T.; Mazzola, P.; et al. Comprehensive analysis of two hotspot codons in the TUBB4B gene and associated phenotypes. Sci. Rep. 2024, 14, 10551. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.L.; Liu, X.; Wang, G.; Liu, B.; Meng, X.H.; Liu, Y. The first Chinese case with LCAEOD syndrome caused by mutation of TUBB4B gene. Int. J. Ophthalmol. 2025, 18, 753–756. [Google Scholar] [CrossRef]
- Maasz, A.; Hadzsiev, K.; Ripszam, R.; Zsigmond, A.; Maka, E.; Knezy, K.; Lesch, B.; Nemeth, A.; Bene, J.; Galik, B.; et al. TUBB4B gene mutation in Leber phenotype of congenital amaurosis syndrome associated with early-onset deafness. Eur. J. Med. Genet. 2022, 65, 104471. [Google Scholar] [CrossRef]
- McFadden, J.R.; Tolete, C.D.P.; Huang, Y.; Macnamara, E.; Sept, D.; Nesterova, G.; Gahl, W.A.; Sackett, D.L.; Malicdan, M.C.V. Clinical, genetic, and structural characterization of a novel TUBB4B tubulinopathy. Mol. Genet. Metab. Rep. 2023, 36, 100990. [Google Scholar] [CrossRef]
- Medina, G.; Perry, J.; Oza, A.; Kenna, M. Hiding in plain sight: Genetic deaf-blindness is not always Usher syndrome. Cold Spring Harb. Mol. Case Stud. 2021, 7, a006088. [Google Scholar] [CrossRef]
- Scarpato, M.; Testa, F.; Nesti, A.; Zeuli, R.; Boccia, R.; Auletta, G.; Banfi, S.; Simonelli, F.; Karali, M. A Novel Variant in TUBB4B Causes Progressive Cone-Rod Dystrophy and Early Onset Sensorineural Hearing Loss. Mol. Genet. Genom. Med. 2025, 13, e70068. [Google Scholar] [CrossRef]
- Ehrenberg, M.; Weiss, S.; Orenstein, N.; Goldenberg-Cohen, N.; Ben-Yosef, T. The co-occurrence of rare non-ocular phenotypes in patients with inherited retinal degenerations. Mol. Vis. 2019, 25, 691–702. [Google Scholar]
- Mykytyn, K.; Nishimura, D.Y.; Searby, C.C.; Shastri, M.; Yen, H.J.; Beck, J.S.; Braun, T.; Streb, L.M.; Cornier, A.S.; Cox, G.F.; et al. Identification of the gene (BBS1) most commonly involved in Bardet-Biedl syndrome, a complex human obesity syndrome. Nat. Genet. 2002, 31, 435–438. [Google Scholar] [CrossRef]
- Schrauwen, I.; Helfmann, S.; Inagaki, A.; Predoehl, F.; Tabatabaiefar, M.A.; Picher, M.M.; Sommen, M.; Zazo Seco, C.; Oostrik, J.; Kremer, H.; et al. A mutation in CABP2, expressed in cochlear hair cells, causes autosomal-recessive hearing impairment. Am. J. Hum. Genet. 2012, 91, 636–645. [Google Scholar] [CrossRef]
- Weston, M.D.; Luijendijk, M.W.; Humphrey, K.D.; Moller, C.; Kimberling, W.J. Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am. J. Hum. Genet. 2004, 74, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, G.; Sayou, C.; Tanno, P.L.; Tisserant, E.; Bruel, A.L.; Kennani, S.E.; Sa, J.; Low, K.J.; Dias, C.; Havlovicova, M.; et al. De novo SMARCA2 variants clustered outside the helicase domain cause a new recognizable syndrome with intellectual disability and blepharophimosis distinct from Nicolaides-Baraitser syndrome. Genet. Med. 2020, 22, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, J.K.; Nowakowska, B.A.; Sousa, S.B.; van Schaik, B.D.; Seuntjens, E.; Avonce, N.; Sifrim, A.; Abdul-Rahman, O.A.; van den Boogaard, M.J.; Bottani, A.; et al. Heterozygous missense mutations in SMARCA2 cause Nicolaides-Baraitser syndrome. Nat. Genet. 2012, 44, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.A.; de Vrieze, E.; Alazami, A.M.; Alzahrani, F.; Malarkey, E.B.; Sorusch, N.; Tebbe, L.; Kuhns, S.; van Dam, T.J.; Alhashem, A.; et al. KIAA0556 is a novel ciliary basal body component mutated in Joubert syndrome. Genome Biol. 2015, 16, 293. [Google Scholar] [CrossRef]
- Cohen, B.H.; Chinnery, P.F.; Copeland, W.C. POLG-Related Disorders. In GeneReviews((R)); Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2010. [Google Scholar]
- van Wijk, E.; Pennings, R.J.; te Brinke, H.; Claassen, A.; Yntema, H.G.; Hoefsloot, L.H.; Cremers, F.P.; Cremers, C.W.; Kremer, H. Identification of 51 novel exons of the Usher syndrome type 2A (USH2A) gene that encode multiple conserved functional domains and that are mutated in patients with Usher syndrome type II. Am. J. Hum. Genet. 2004, 74, 738–744. [Google Scholar] [CrossRef]
- Rivolta, C.; Sweklo, E.A.; Berson, E.L.; Dryja, T.P. Missense mutation in the USH2A gene: Association with recessive retinitis pigmentosa without hearing loss. Am. J. Hum. Genet. 2000, 66, 1975–1978. [Google Scholar] [CrossRef]
- Fuster-Garcia, C.; Garcia-Garcia, G.; Jaijo, T.; Fornes, N.; Ayuso, C.; Fernandez-Burriel, M.; Sanchez-De la Morena, A.; Aller, E.; Millan, J.M. High-throughput sequencing for the molecular diagnosis of Usher syndrome reveals 42 novel mutations and consolidates CEP250 as Usher-like disease causative. Sci. Rep. 2018, 8, 17113. [Google Scholar] [CrossRef]
- Kumaran, N.; Moore, A.T.; Weleber, R.G.; Michaelides, M. Leber congenital amaurosis/early-onset severe retinal dystrophy: Clinical features, molecular genetics and therapeutic interventions. Br. J. Ophthalmol. 2017, 101, 1147–1154. [Google Scholar] [CrossRef]
- Aksu Uzunhan, T.; Erturk, B.; Aydin, K.; Ayaz, A.; Altunoglu, U.; Yarar, M.H.; Gezdirici, A.; Icagasioglu, D.F.; Gokpinar Ili, E.; Uyanik, B.; et al. Clinical and genetic spectrum from a prototype of ciliopathy: Joubert syndrome. Clin. Neurol. Neurosurg. 2023, 224, 107560. [Google Scholar] [CrossRef]
- Cauley, E.S.; Hamed, A.; Mohamed, I.N.; Elseed, M.; Martinez, S.; Yahia, A.; Abozar, F.; Abubakr, R.; Koko, M.; Elsayed, L.; et al. Overlap of polymicrogyria, hydrocephalus, and Joubert syndrome in a family with novel truncating mutations in ADGRG1/GPR56 and KIAA0556. Neurogenetics 2019, 20, 91–98. [Google Scholar] [CrossRef]
- Fujita, A.; Higashijima, T.; Shirozu, H.; Masuda, H.; Sonoda, M.; Tohyama, J.; Kato, M.; Nakashima, M.; Tsurusaki, Y.; Mitsuhashi, S.; et al. Pathogenic variants of DYNC2H1, KIAA0556, and PTPN11 associated with hypothalamic hamartoma. Neurology 2019, 93, e237–e251. [Google Scholar] [CrossRef] [PubMed]
- Niceta, M.; Dentici, M.L.; Ciolfi, A.; Marini, R.; Barresi, S.; Lepri, F.R.; Novelli, A.; Bertini, E.; Cappa, M.; Digilio, M.C.; et al. Co-occurrence of mutations in KIF7 and KIAA0556 in Joubert syndrome with ocular coloboma, pituitary malformation and growth hormone deficiency: A case report and literature review. BMC Pediatr. 2020, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Roosing, S.; Rosti, R.O.; Rosti, B.; de Vrieze, E.; Silhavy, J.L.; van Wijk, E.; Wakeling, E.; Gleeson, J.G. Identification of a homozygous nonsense mutation in KIAA0556 in a consanguineous family displaying Joubert syndrome. Hum. Genet. 2016, 135, 919–921. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.A.; Vincent, A.L. Senior-Loken syndrome and intracranial hypertension. Ophthalmic Genet. 2020, 41, 354–357. [Google Scholar] [CrossRef]
- Banizs, B.; Pike, M.M.; Millican, C.L.; Ferguson, W.B.; Komlosi, P.; Sheetz, J.; Bell, P.D.; Schwiebert, E.M.; Yoder, B.K. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 2005, 132, 5329–5339. [Google Scholar] [CrossRef]
- Louvi, A.; Grove, E.A. Cilia in the CNS: The quiet organelle claims center stage. Neuron 2011, 69, 1046–1060. [Google Scholar] [CrossRef]
- Francis, P.J.; Haywood, S.; Rigden, S.; Calver, D.M.; Clark, G. Benign intracranial hypertension in children following renal transplantation. Pediatr. Nephrol. 2003, 18, 1265–1269. [Google Scholar] [CrossRef]
- Zlotogora, J.; Chemke, J. Medical genetics in Israel. Eur. J. Hum. Genet. 1995, 3, 147–154. [Google Scholar] [CrossRef]
- Zeegers, M.P.; van Poppel, F.; Vlietinck, R.; Spruijt, L.; Ostrer, H. Founder mutations among the Dutch. Eur. J. Hum. Genet. 2004, 12, 591–600. [Google Scholar] [CrossRef]
- Uusimaa, J.; Kettunen, J.; Varilo, T.; Jarvela, I.; Kallijarvi, J.; Kaariainen, H.; Laine, M.; Lapatto, R.; Myllynen, P.; Niinikoski, H.; et al. The Finnish genetic heritage in 2022—From diagnosis to translational research. Dis. Models Mech. 2022, 15, dmm049490. [Google Scholar] [CrossRef]
- de Bruijn, S.E.; Rodenburg, K.; Corominas, J.; Ben-Yosef, T.; Reurink, J.; Kremer, H.; Whelan, L.; Plomp, A.S.; Berger, W.; Farrar, G.J.; et al. Optical genome mapping and revisiting short-read genome sequencing data reveal previously overlooked structural variants disrupting retinal disease-associated genes. Genet. Med. 2023, 25, 100345. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, K.; Van Gelder, R.N.; Chao, J.R.; Mustafi, D. Targeted adaptive long-read sequencing for discovery of complex phased variants in inherited retinal disease patients. Sci. Rep. 2023, 13, 8535. [Google Scholar] [CrossRef] [PubMed]
- Zeuli, R.; Karali, M.; de Bruijn, S.E.; Rodenburg, K.; Scarpato, M.; Capasso, D.; Astuti, G.D.N.; Gilissen, C.; Rodriguez-Hidalgo, M.; Ruiz-Ederra, J.; et al. Whole genome sequencing identifies elusive variants in genetically unsolved Italian inherited retinal disease patients. Hum. Genet. Genom. Adv. 2024, 5, 100314. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).