Phosphatidic Acid Reverses Obesity Induced by a High-Fat, High-Sugar Diet at the Transcriptional Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
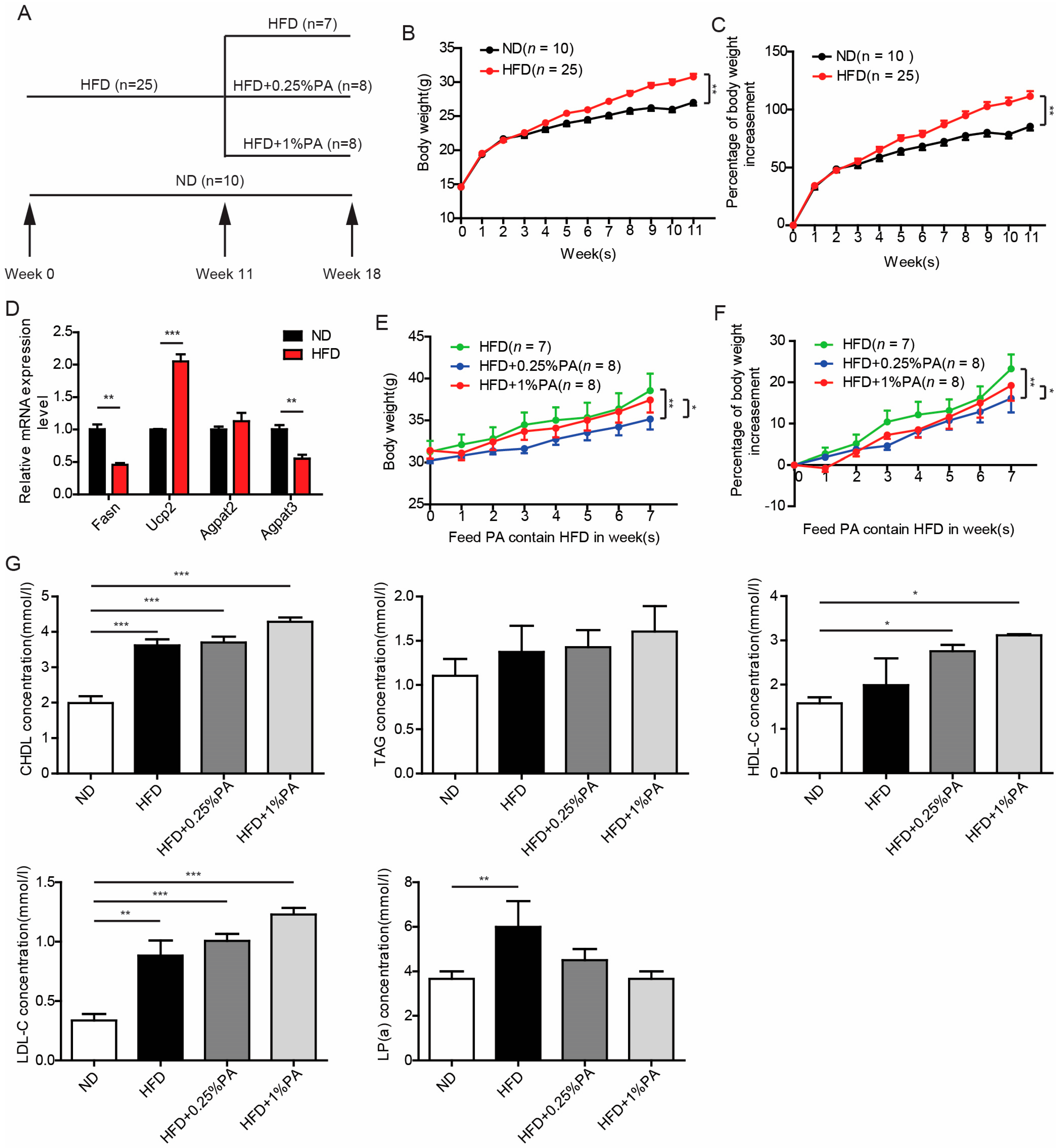
2.2. Mice and HFD-Induced Obesity Model
2.3. Detection of Biochemical Markers in Serum Samples
2.4. Immunohistochemistry of Tissue Samples
2.5. Oil Red Staining
2.6. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR
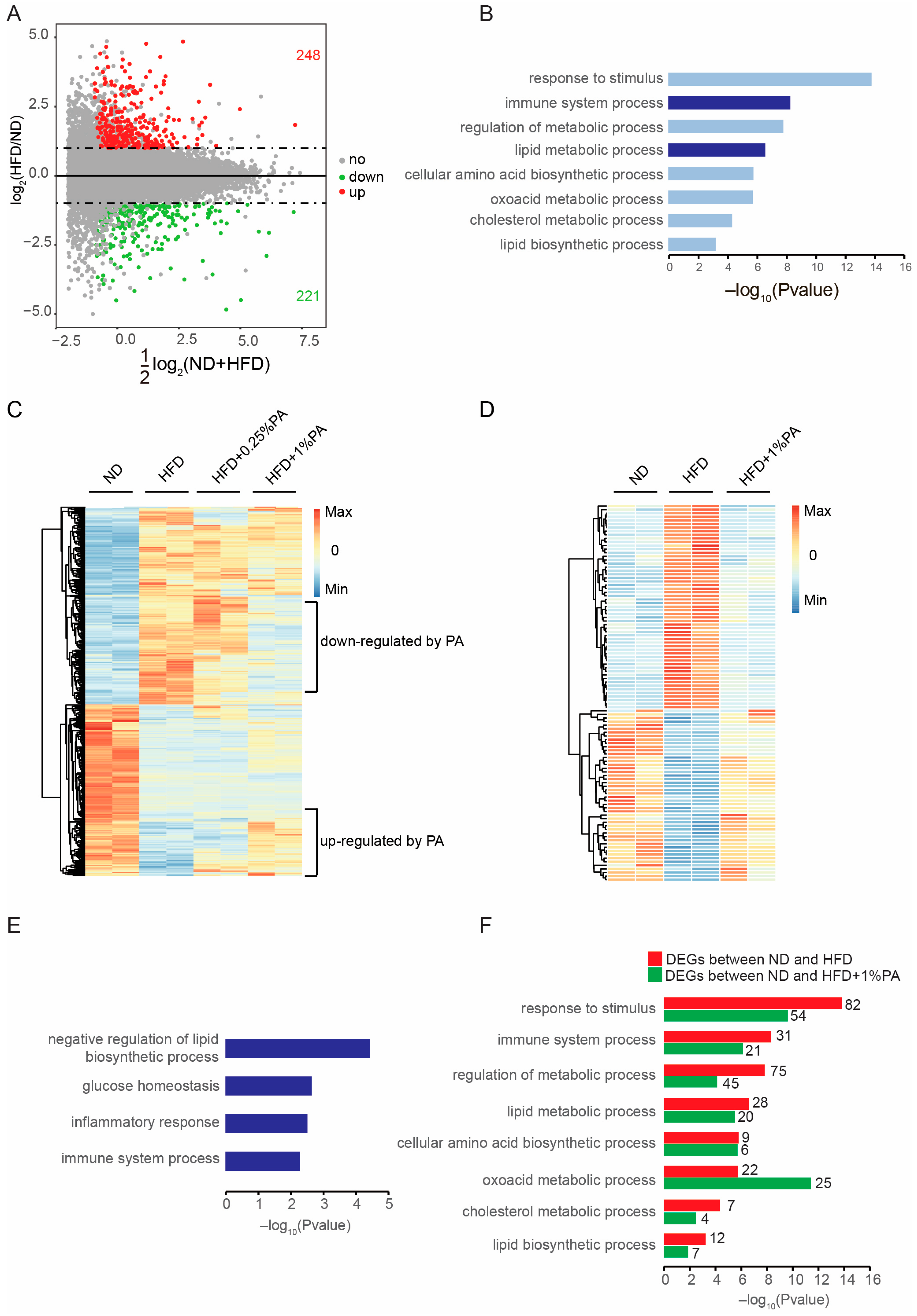
2.7. RNA-Seq Analysis
2.8. Statistical Analysis
3. Results
3.1. Effect of PA on Obesity Induced by High-Fat, High-Sugar Diet
3.2. PA Reversed HFD-Induced Obesity at the Transcriptomic Level
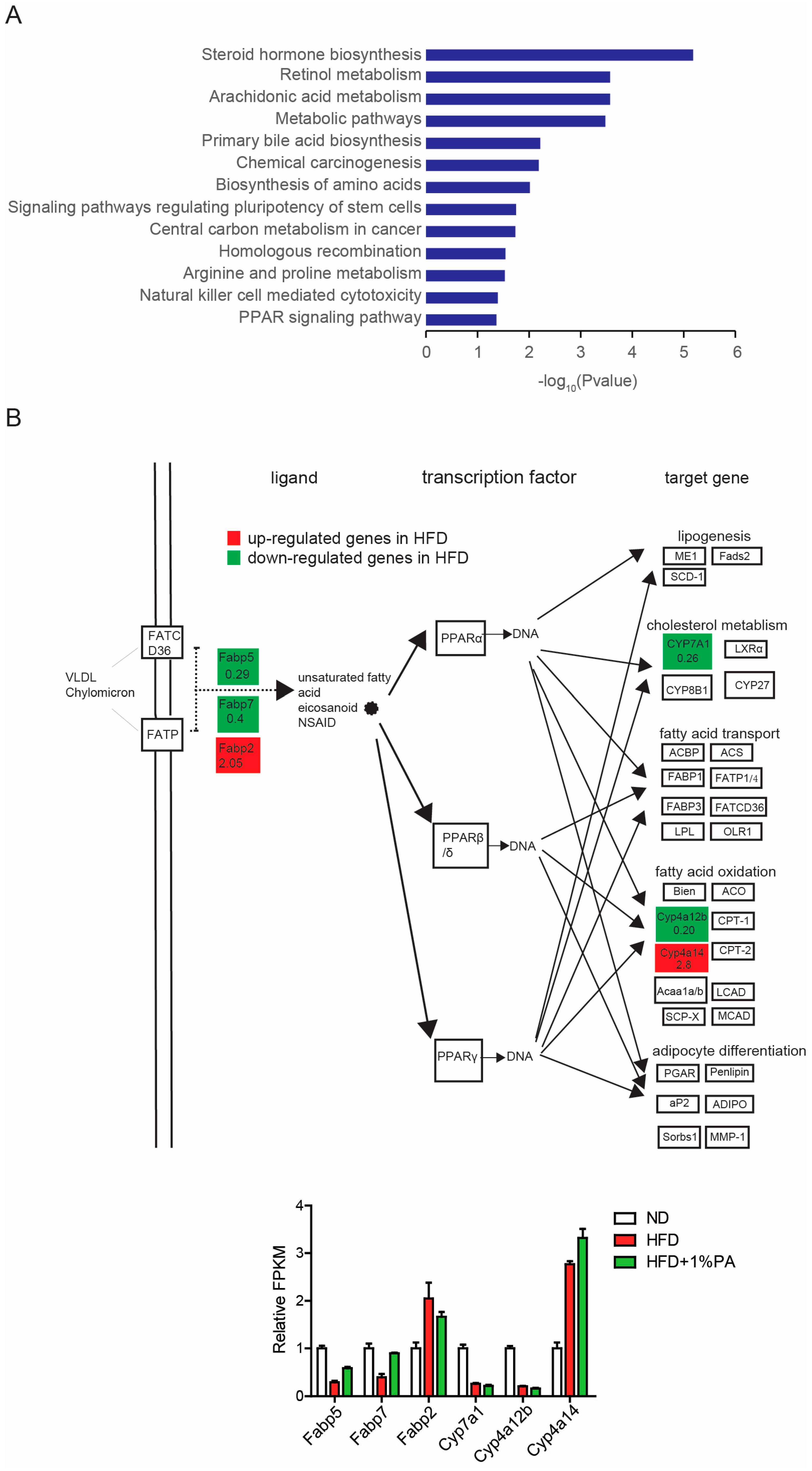
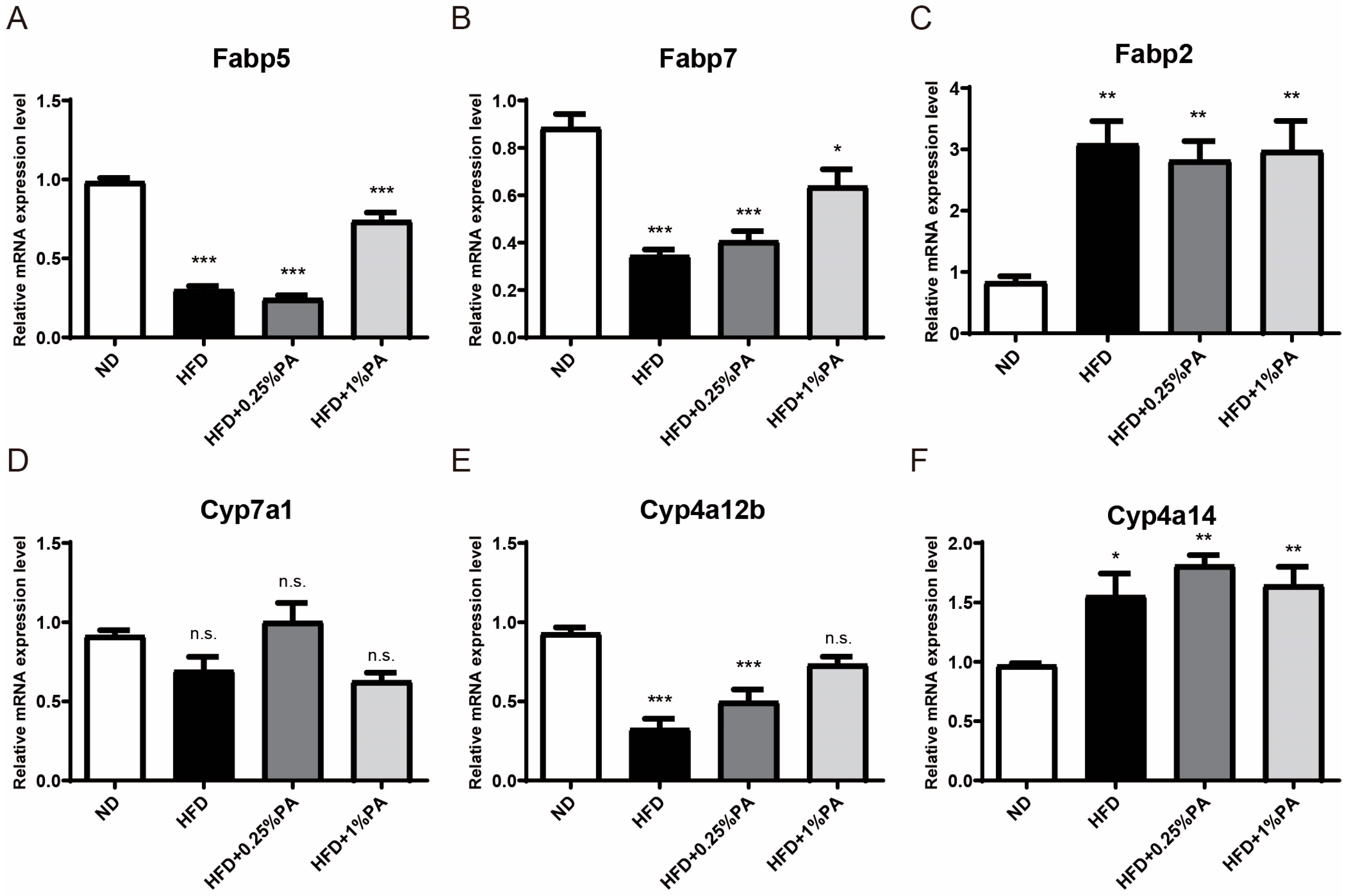
3.3. PA Reverses Aberrant Expression of Key Genes in the PPAR Signaling Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PA | phosphatidic acid |
| HFD | high-fat, high-sugar diet |
| ND | normal diet |
| TAG | triglycerides |
| CHDL | total cholesterol |
| HDL-C | high-density lipoprotein |
| LDL-C | low-density lipoprotein |
| APA | apolipoprotein A |
| APB | apolipoprotein B |
| LP(a) | lipoprotein a |
References
- Carr, D.B.; Utzschneider, K.M.; Hull, R.L.; Kodama, K.; Retzlaff, B.M.; Brunzell, J.D.; Shofer, J.B.; Fish, B.E.; Knopp, R.H.; Kahn, S.E. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes 2004, 53, 2087–2094. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Mohanty, B.P.; Mahanty, A.; Ganguly, S.; Mitra, T.; Karunakaran, D.; Anandan, R. Nutritional composition of food fishes and their importance in providing food and nutritional security. Food Chem. 2019, 293, 561–570. [Google Scholar] [CrossRef]
- Jarocki, M.; Turek, K.; Saczko, J.; Tarek, M.; Kulbacka, J. Lipids associated with autophagy: Mechanisms and therapeutic targets. Cell Death Discov. 2024, 10, 460. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling pathways in obesity: Mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- Richard, A.J.; White, U.; Elks, C.M.; Stephens, J.M. Adipose Tissue: Physiology to Metabolic Dysfunction. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Batacan, R.B., Jr.; Duncan, M.J.; Dalbo, V.J.; Tucker, P.S.; Fenning, A.S. Effects of high-intensity interval training on cardiometabolic health: A systematic review and meta-analysis of intervention studies. Br. J. Sports Med. 2017, 51, 494–503. [Google Scholar] [CrossRef]
- O’Neil, P.M.; Birkenfeld, A.L.; McGowan, B.; Mosenzon, O.; Pedersen, S.D.; Wharton, S.; Carson, C.G.; Jepsen, C.H.; Kabisch, M.; Wilding, J.P.H. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: A randomised. double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018, 392, 637–649. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; le Roux, C.W.; Ortiz, R.V.; Jensen, C.B.; et al. Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Hurren, K.M.; Berlie, H.D. Lorcaserin: An investigational serotonin 2C agonist for weight loss. Am. J. Health Syst. Pharm. 2011, 68, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Vest, A.R.; Heneghan, H.M.; Schauer, P.R.; Young, J.B. Surgical management of obesity and the relationship to cardiovascular disease. Circulation 2013, 127, 945–959. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Wu, Y.; Wang, L.; Ding, S.; Hou, B.; Zhao, H.; Liu, W.; Li, P. Lipid homeostasis in diabetic kidney disease. Int. J. Biol. Sci. 2024, 20, 3710–3724. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Y.; Zhao, X.; Wu, S.; Li, F.; Wang, Y.; Liu, B.; Zhang, Y.; Gao, X.; Wang, Y.; et al. Peroxisome proliferator-activated receptors: A key link between lipid metabolism and cancer progression. Clin. Nutr. 2024, 43, 332–345. [Google Scholar] [CrossRef]
- Fan, W.; Lam, S.M.; Xin, J.; Yang, X.; Liu, Z.; Liu, Y.; Wang, Y.; Shui, G.; Huang, X. Drosophila TRF2 and TAF9 regulate lipid droplet size and phospholipid fatty acid composition. PLoS Genet. 2017, 13, e1006664. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Garg, A. Congenital generalized lipodystrophy: Significance of triglyceride biosynthetic pathways. Trends Endocrinol. Metab. 2003, 14, 214–221. [Google Scholar] [CrossRef]
- Fernandez-Murray, J.P.; McMaster, C.R. Lipid synthesis and membrane contact sites: A crossroads for cellular physiology. J. Lipid Res. 2016, 57, 1789–1805. [Google Scholar] [CrossRef]
- Coleman, R.A.; Lee, D.P. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 2004, 43, 134–176. [Google Scholar] [CrossRef]
- Exton, J.H. Phospholipase D-structure, regulation and function. Rev. Physiol. Biochem. Pharmacol. 2002, 144, 1–94. [Google Scholar] [CrossRef]
- Carman, G.M.; Han, G.S. Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. J. Biol. Chem. 2009, 284, 2593–2597. [Google Scholar] [CrossRef]
- Athenstaedt, K.; Daum, G. Phosphatidic acid, a key intermediate in lipid metabolism. Eur. J. Biochem. 1999, 266, 1–16. [Google Scholar] [CrossRef]
- Buckland, A.G.; Wilton, D.C. Anionic phospholipids, interfacial binding and the regulation of cell functions. Biochim. Biophys. Acta 2000, 1483, 199–216. [Google Scholar] [CrossRef]
- Yang, C.Y.; Frohman, M.A. Mitochondria: Signaling with phosphatidic acid. Int. J. Biochem. Cell Biol. 2012, 44, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Carman, G.M.; Henry, S.A. Phosphatidic acid plays a central role in the transcriptional regulation of glycerophospholipid synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 2007, 282, 37293–37297. [Google Scholar] [CrossRef] [PubMed]
- Bond, P. Phosphatidic acid: Biosynthesis; pharmacokinetics, mechanisms of action and effect on strength and body composition in resistance-trained individuals. Nutr. Metab. 2017, 14, 12. [Google Scholar] [CrossRef]
- Yao, S.; Kim, S.C.; Li, J.; Tang, S.; Wang, X. Phosphatidic acid signaling and function in nuclei. Prog. Lipid Res. 2024, 93, 101267. [Google Scholar] [CrossRef]
- Joy, J.M.; Gundermann, D.M.; Lowery, R.P.; Jager, R.; McCleary, S.A.; Purpura, M.; Roberts, M.D.; Wilson, S.M.; Hornberger, T.A.; Wilson, J.M. Phosphatidic acid enhances mTOR signaling and resistance exercise induced hypertrophy. Nutr. Metab. 2014, 11, 29. [Google Scholar] [CrossRef]
- Pascual, F.; Carman, G.M. Phosphatidate phosphatase, a key regulator of lipid homeostasis. Biochim. Biophys. Acta 2013, 1831, 514–522. [Google Scholar] [CrossRef]
- Carman, G.M.; Han, G.S. Fat-regulating phosphatidic acid phosphatase: A review of its roles and regulation in lipid homeostasis. J. Lipid Res. 2019, 60, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Institute of Laboratory Animal Resources (U.S.). Committee on Care and Use of Laboratory Animals. In Guide for the Care and Use of Laboratory Animals; NIH Publication, U.S. Dept. of Health and Human Services, Public Health Service: Bethesda, MD, USA, 2011. [Google Scholar]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, Y.J.; Ryu, R.; Cho, S.J.; Kwon, E.Y.; Choi, M.S. Effect of Green Tea Extract on Systemic Metabolic Homeostasis in Diet-Induced Obese Mice Determined via RNA-Seq Transcriptome Profiles. Nutrients 2016, 8, 640. [Google Scholar] [CrossRef]
| Primer Names | Primer Sequences | Expected Amplicon Size | GENCODE Gene Names |
|---|---|---|---|
| Tubulin-F | AGCTTTGGCGGGGGAACTGG | 167 bp | Tuba1a |
| Tubulin-R | AGGGTGGTGTGGGTGGTGAGGAT | ||
| Fasn-F | CTGGCCCCGGAGTCGCTTGAGTAT | 101 bp | Fasn |
| Fasn-R | AAGGCGCACAGGGACCGAGTAATG | ||
| Ucp2-F | TCGCCTCCCCTGTTGATGTGGTC | 112 bp | Ucp2 |
| Ucp2-R | GCGCGGGGTCCCTCCTTCC | ||
| Agpat2-F | CATCAACCGCCAGCAAGCCAGAAC | 199 bp | Agpat2 |
| Agpat2-R | AGACGAGTACACCACGGGGATGAT | ||
| Agpat3-F | CCGCTTGGCCTACTCGCTCTG | 189 bp | Agpat3 |
| Agpat3-R | ACGCCAAACCGCTCGCACAT | ||
| Fabp5-F | GGAAGTGGCGCCTGATGGA | 142 bp | Fabp5 |
| Fabp5-R | GTTTTGACCGTGATGTTGTTGC | ||
| Fabp7-F | AAGTGGGATGGCAAAGAAACAAAT | 101 bp | Fabp7 |
| Fabp7-R | TCATAACAGCGAACAGCAACGATA | ||
| Fabp2-F | CGGCACGTGGAAAGTAGACC | 189 bp | Fabp2 |
| Fabp2-R | ACACCGAGCTCAAACACAACATCA | ||
| Cyp7a1-F | GCCTTCTGCTACCGAGTGATGTTT | 188 bp | Cyp7a1 |
| Cyp7a1-R | CGGGCTTTATGTGCGGTCTTG | ||
| Cyp4a12b-F | CCCCCTGTACCAAGTGTGAG | 183 bp | Cyp4a12b |
| Cyp4a12b-R | GCTGTGCCGGGAAGACC | ||
| Cyp4a14-F | TGCAGAAGGCCAGGAAGAAGAGA | 180 bp | Cyp4a14 |
| Cyp4a14-R | GGGTGGCCAGAGCATAGAAAATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, H.; Cheng, Q.; Tian, X.; Liao, Y. Phosphatidic Acid Reverses Obesity Induced by a High-Fat, High-Sugar Diet at the Transcriptional Level. Genes 2025, 16, 1112. https://doi.org/10.3390/genes16091112
Xie H, Cheng Q, Tian X, Liao Y. Phosphatidic Acid Reverses Obesity Induced by a High-Fat, High-Sugar Diet at the Transcriptional Level. Genes. 2025; 16(9):1112. https://doi.org/10.3390/genes16091112
Chicago/Turabian StyleXie, Hao, Qian Cheng, Xingyi Tian, and Yanlin Liao. 2025. "Phosphatidic Acid Reverses Obesity Induced by a High-Fat, High-Sugar Diet at the Transcriptional Level" Genes 16, no. 9: 1112. https://doi.org/10.3390/genes16091112
APA StyleXie, H., Cheng, Q., Tian, X., & Liao, Y. (2025). Phosphatidic Acid Reverses Obesity Induced by a High-Fat, High-Sugar Diet at the Transcriptional Level. Genes, 16(9), 1112. https://doi.org/10.3390/genes16091112






