Phylo-Epigenetic Conservation and CpG Erosion in OCT4, SOX2, and hTERT Intragenic CpG Islands: A Waddingtonian Perspective on Mammalian Developmental Evolution
Abstract
1. Introduction
2. Material and Methods
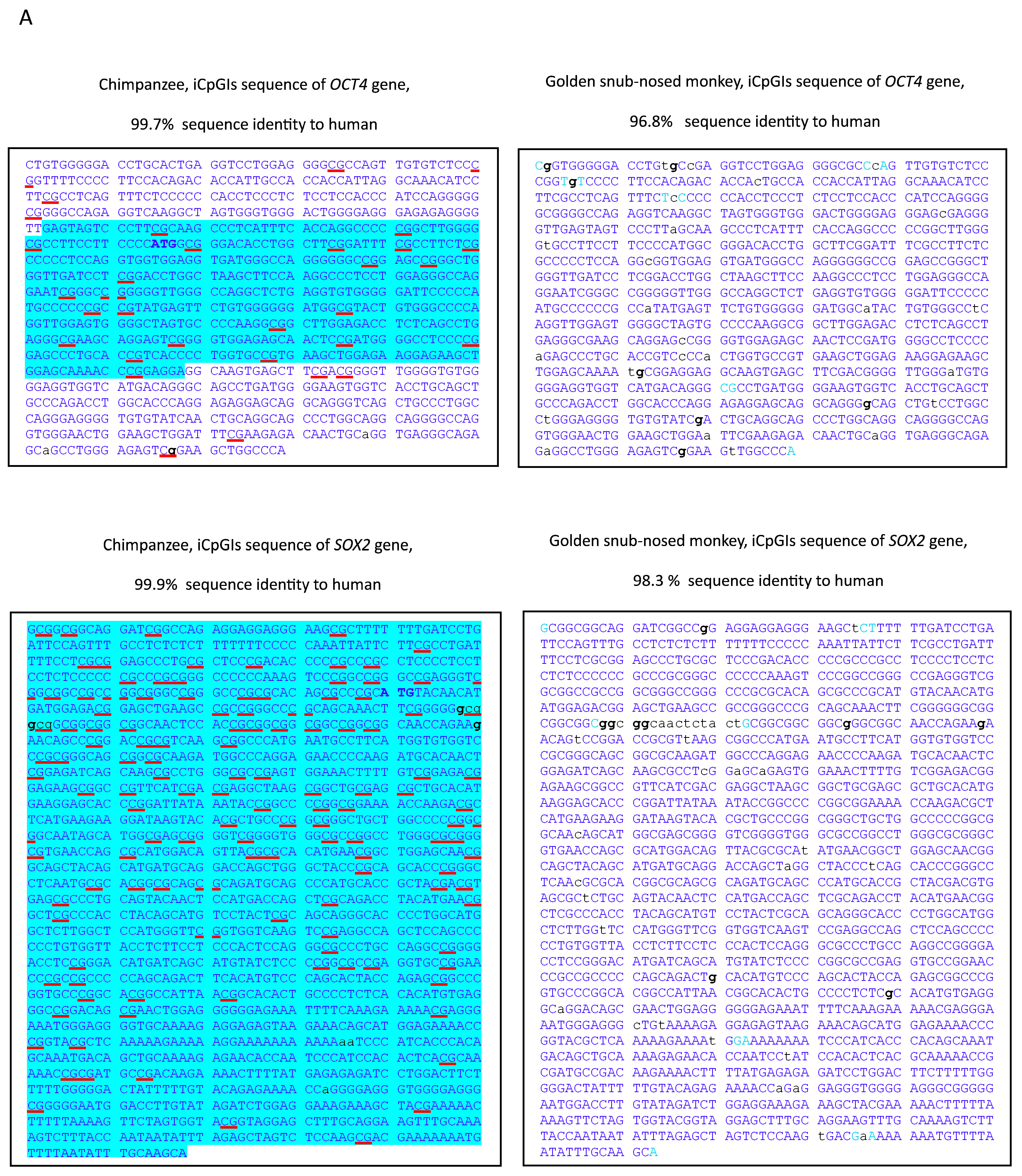
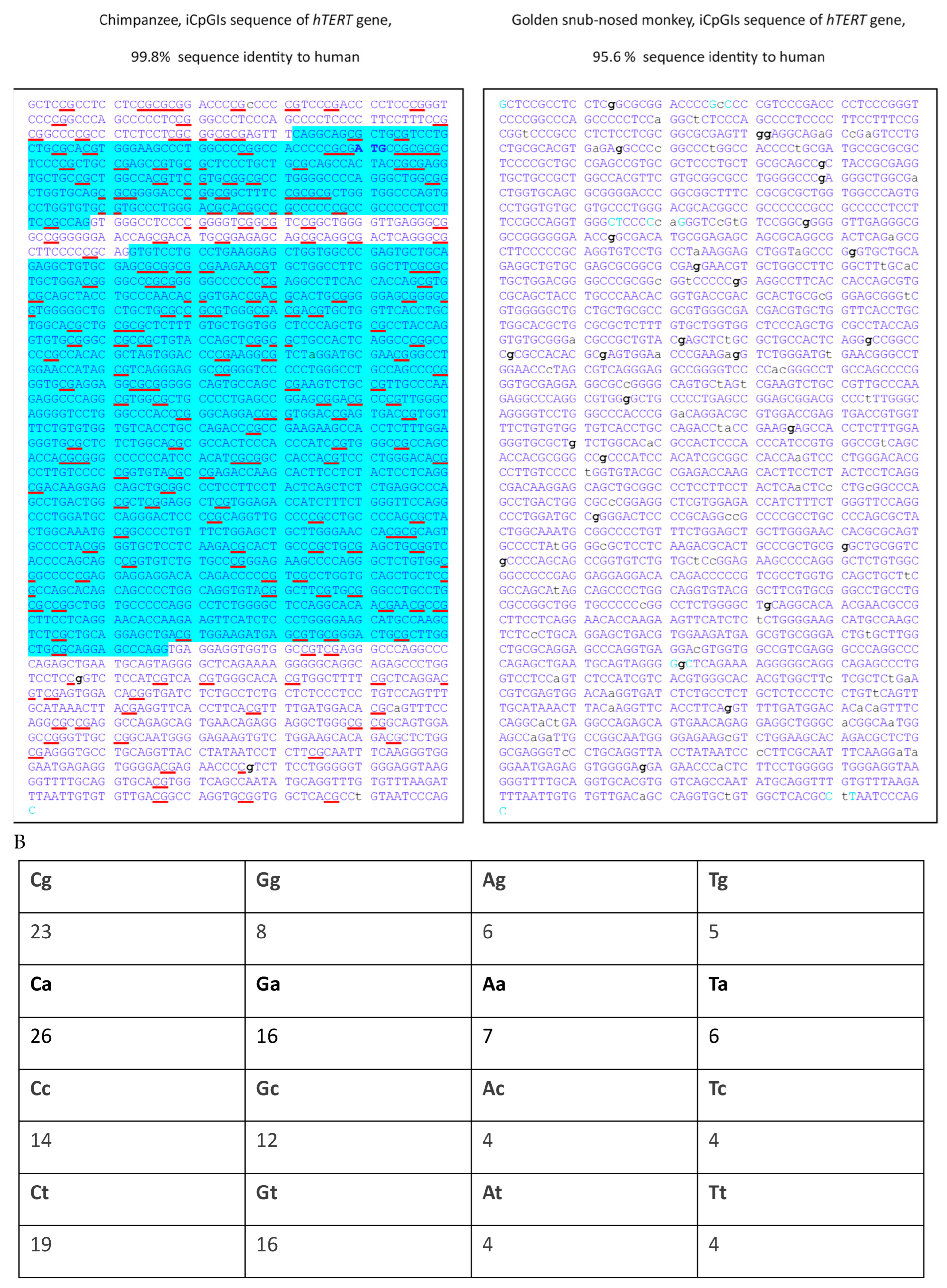
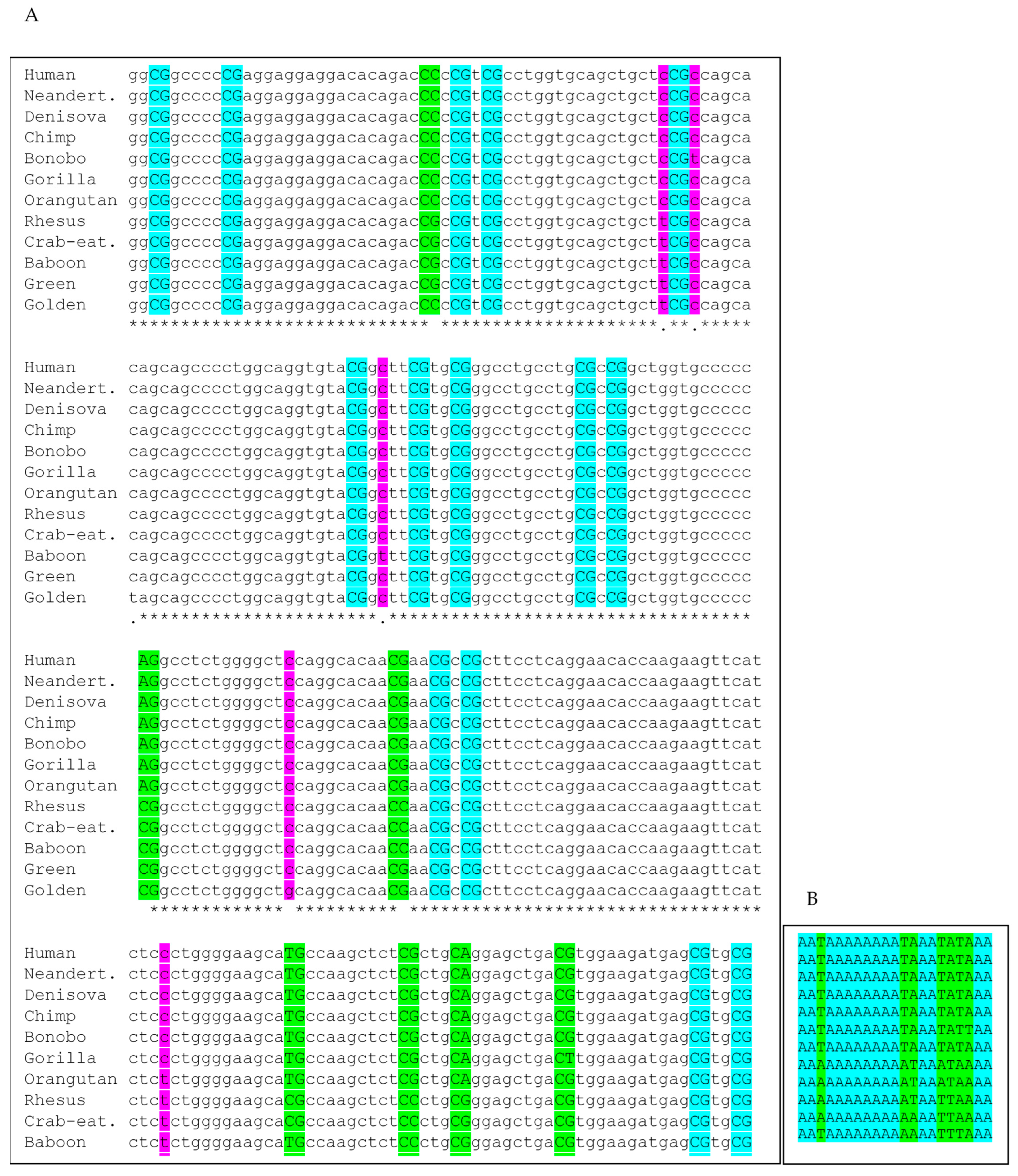
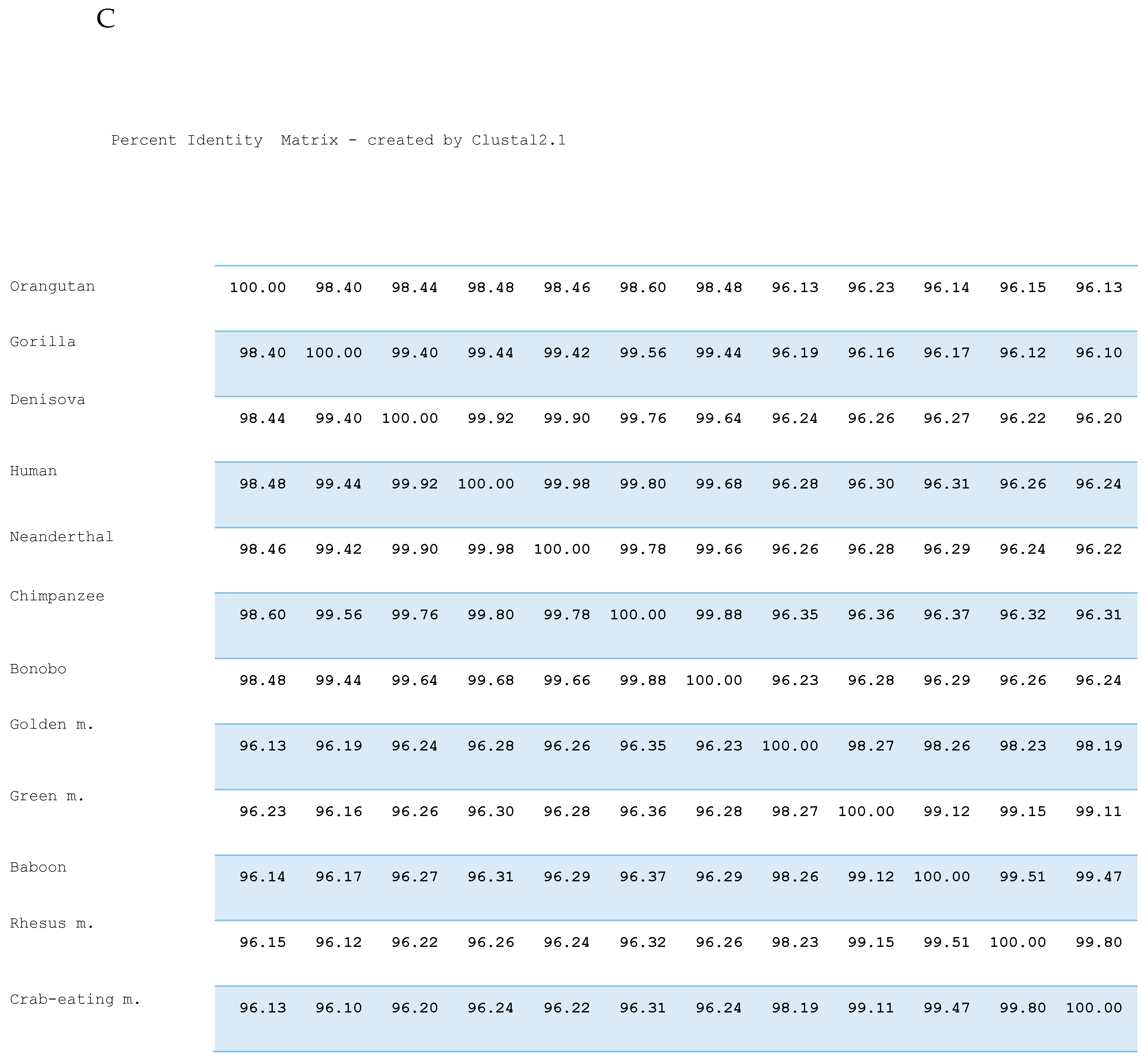
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waddington, C.H. The Strategy of the Genes, 1st ed.; Routledge: London, UK, 1957. [Google Scholar]
- Waddington, C.H. Canalization of development and genetic assimilation of acquired characters. Nature 1959, 183, 1654–1655. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.P.; Wolffe, A.P. Methylation-induced repression—Belts, braces, and chromatin. Cell 1999, 99, 451–454. [Google Scholar] [CrossRef]
- Zampieri, M.; Ciccarone, F.; Calabrese, R.; Franceschi, C.; Bürkle, A.; Caiafa, P. Reconfiguration of DNA methylation in aging. Mech. Ageing Dev. 2015, 151, 60–70. [Google Scholar] [CrossRef]
- Vavouri, T.; Peinado, M.A. CpG Islands; Springer: New York, NY, USA, 2018; Volume 1766, ISBN 978-1-4939-7767-3. [Google Scholar]
- Schulz, W.A. Molecular Biology of Human Cancers, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2023; Chapter 8.2. [Google Scholar]
- Shen, J.C.; Rideout, W.M., 3rd; Jones, P.A. The rate of hydrolytic deamination of 5-methylcytosine in double-stranded DNA. Nucleic Acids Res. 1994, 22, 972–976. [Google Scholar] [CrossRef]
- Sved, J.; Bird, A. The expected equilibrium of the CpG dinucleotide in vertebrate genomes under a mutation model. Proc. Natl. Acad. Sci. USA 1990, 87, 4692–4696. [Google Scholar] [CrossRef]
- Fryxell, K.J.; Moon, W.J. CpG mutation rates in the human genome are highly dependent on local GC content. Mol. Biol. Evol. 2005, 22, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, T. Detection and interpretation of altered methylation patterns in cancer cells. Nat. Rev. Cancer 2005, 5, 223–231. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Jones, P.A. The DNA methylation paradox. Trends Genet. 1999, 15, 34–37. [Google Scholar] [CrossRef]
- Gelfman, S.; Cohen, N.; Yearim, A.; Ast, G. DNA-methylation effect on cotranscriptional splicing is dependent on GC architecture of the exon-intron structure. Genome Res. 2013, 23, 789–799. [Google Scholar] [CrossRef]
- Choi, J.K. Contrasting chromatin organization of CpG islands and exons in the human genome. Genome Biol. 2010, 11, R70. [Google Scholar] [CrossRef]
- Angeloni, A.; Bogdanovic, O. Sequence determinants, function, and evolution of CpG islands. Biochem. Soc. Trans. 2021, 49, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Cain, J.A.; Montibus, B.; Oakey, R.J. Intragenic CpG Islands and Their Impact on Gene Regulation. Front. Cell Dev. Biol. 2022, 10, 832348. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, B.E.; Mikkelsen, T.S.; Xie, X.; Kamal, M.; Huebert, D.J.; Cuff, J.; Fry, B.; Meissner, A.; Wernig, M.; Plath, K.; et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 2006, 125, 315–326. [Google Scholar] [CrossRef]
- Klose, R.J.; Bird, A.P. Genomic DNA methylation: The mark and its mediators. Trends Biochem. Sci. 2006, 31, 89–97. [Google Scholar] [CrossRef]
- Smith, Z.D.; Meissner, A. DNA methylation: Roles in mammalian development. Nat. Rev. Genet. 2013, 14, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Deb-Rinker, P.; Ly, D.; Jezierski, A.; Sikorska, M.; Walker, P.R. Sequential DNA methylation of the Nanog and Oct-4 upstream regions in human NT2 cells during neuronal differentiation. J. Biol. Chem. 2005, 280, 6257–6260. [Google Scholar] [CrossRef]
- Li, J.Y.; Pu, M.T.; Hirasawa, R.; Li, B.-Z.; Huang, Y.-N.; Zeng, R.; Jing, N.-H.; Chen, T.; Li, E.; Sasaki, H.; et al. Synergistic function of DNA methyltransferases Dnmt3a and Dnmt3b in the methylation of Oct4 and Nanog. Mol. Cell. Biol. 2007, 27, 8748–8759. [Google Scholar] [CrossRef]
- Rowland, T.J.; Bonham, A.J.; Cech, T.R. Allele-specific proximal promoter hypomethylation of the telomerase reverse transcriptase gene (TERT) associates with TERT expression in multiple cancers. Mol. Oncol. 2020, 14, 2358–2374. [Google Scholar] [CrossRef]
- Ott, P.; Araúzo-Bravo, M.J.; Hoffmann, M.J.; Poyet, C.; Bendhack, M.L.; Santourlidis, S.; Erichsen, L. Differential DNA Methylation of THOR and hTAPAS in the Regulation of hTERT and the Diagnosis of Cancer. Cancers 2022, 14, 4384. [Google Scholar] [CrossRef]
- Ghanjati, F.; Santourlidis, S. Effect on Multipotency and Phenotypic Transition of Unrestricted Somatic Stem Cells from Human Umbilical Cord Blood after Treatment with Epigenetic Agents. Stem Cells Int. 2016, 2016, 7643218. [Google Scholar] [CrossRef]
- Santourlidis, S.; Wernet, P.; Ghanjati, F.; Graffmann, N.; Springer, J.; Kriegs, C.; Zhao, X.; Brands, J.; Araúzo-Bravo, M.J.; Neves, R.; et al. Unrestricted somatic stem cells (USSC) from human umbilical cord blood display uncommitted epigenetic signatures of the major stem cell pluripotency genes. Stem Cell Res. 2011, 6, 60–69. [Google Scholar] [CrossRef]
- Athanasiadou, R.; de Sousa, D.; Myant, K.; Merusi, C.; Stancheva, I.; Bird, A.; Feil, R. Targeting of de novo DNA methylation throughout the Oct-4 gene regulatory region in differentiating embryonic stem cells. PLoS ONE 2010, 5, e9937. [Google Scholar] [CrossRef]
- Auclair, G.; Guibert, S.; Bender, A.; Weber, M. Ontogeny of CpG island methylation and specificity of DNMT3 methyltransferases during embryonic development in the mouse. Genome Biol. 2014, 15, 545. [Google Scholar] [CrossRef] [PubMed]
- Rizzino, A.; Wuebben, E.L. Sox2/Oct4: A delicately balanced partnership in pluripotent stem cells and embryogenesis. Biochim. Biophys. Acta 2016, 1859, 780–791. [Google Scholar] [CrossRef]
- Batista, L.F. Telomere biology in stem cells and reprogramming. Prog. Mol. Biol. Transl. Sci. 2014, 125, 67–88. [Google Scholar]
- Cong, Y.S.; Wright, W.E.; Shay, J.W. Human telomerase and its regulation. Microbiol. Mol. Biol. Rev. 2002, 66, 407–425. [Google Scholar] [CrossRef]
- Santourlidis, S.; Araúzo-Bravo, M.J.; Brodell, R.T.; Hassan, M.; Bendhack, M.L. hTERT Epigenetics Provides New Perspectives for Diagnosis and Evidence-Based Guidance of Chemotherapy in Cancer. Int. J. Mol. Sci. 2024, 25, 7331. [Google Scholar] [CrossRef] [PubMed]
- Serakinci, N.; Mega Tiber, P.; Orun, O. Chromatin modifications of hTERT gene in hTERT-immortalized human mesenchymal stem cells upon exposure to radiation. Eur. J. Med. Genet. 2018, 61, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Han, Y.; Guan, X.; Hong, Y.; Meng, J.; Ding, S.; Long, Q.; Yi, W. Regulation and clinical potential of telomerase reverse transcriptase (TERT/hTERT) in breast cancer. Cell Commun. Signal. 2023, 21, 218. [Google Scholar] [CrossRef]
- Penev, A.; Bazley, A.; Shen, M.; Boeke, J.D.; Savage, S.A.; Sfeir, A. Alternative splicing is a developmental switch for hTERT expression. Mol. Cell 2021, 81, 2349–2360.e6. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Giudice, L.C. Developmental regulation of telomerase activity in human fetal tissues during gestation. Mol. Hum. Reprod. 1997, 3, 769–773. [Google Scholar] [CrossRef]
- Armstrong, L.; Saretzki, G.; Peters, H.; Wappler, I.; Evans, J.; Hole, N.; von Zglinicki, T.; Lako, M. Overexpression of telomerase confers growth advantage, stress resistance, and enhanced differentiation of ESCs toward the hematopoietic lineage. Stem Cells 2005, 23, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Catacchio, C.R.; Hillier, L.W.; Porubsky, D.; Li, R.; Sulovari, A.; Fernandes, J.D.; Montinaro, F.; Gordon, D.S.; Storer, J.M.; et al. A high-quality bonobo genome refines the analysis of hominid evolution. Nature 2021, 594, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Arsuaga, J.L.; Martínez, I.; Arnold, L.J.; Aranburu, A.; Gracia-Téllez, A.; Sharp, W.D.; Quam, R.M.; Falguères, C.; Pantoja-Pérez, A.; Bischoff, J.; et al. Neandertal roots: Cranial and chronological evidence from Sima de los Huesos. Science 2014, 344, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Prüfer, K.; Racimo, F.; Patterson, N.; Jay, F.; Sankararaman, S.; Sawyer, S.; Heinze, A.; Renaud, G.; Sudmant, P.H.; De Filippo, C.; et al. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 2014, 505, 43–49. [Google Scholar] [CrossRef]
- Reich, D.; Green, R.E.; Kircher, M.; Krause, J.; Patterson, N.; Durand, E.Y.; Viola, B.; Briggs, A.W.; Stenzel, U.; Johnson, P.L.F.; et al. Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature 2010, 468, 1053–1060. [Google Scholar] [CrossRef]
- Rogers, A.R.; Harris, N.S.; Achenbach, A.A. Neanderthal-Denisovan ancestors interbred with a distantly related hominin. Sci. Adv. 2020, 6, eaay5483. [Google Scholar] [CrossRef]
- Krause, J.; Fu, Q.; Good, J.M.; Viola, B.; Shunkov, M.V.; Derevianko, A.P.; Pääbo, S. The complete mitochondrial DNA genome of an unknown hominin from southern Siberia. Nature 2010, 464, 894–897. [Google Scholar] [CrossRef]
- Kent, W.J. BLAT—The BLAST-like alignment tool. Genome Res. 2002, 12, 656–664. [Google Scholar]
- Katoh, K.; Misakwa, K.; Kei-ichi, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Michener, C.D.; Sokal, R.R. A quantitative approach to a problem in classification. Evolution 1957, 11, 130–162. [Google Scholar] [CrossRef]
- Robinson, O.; Dylus, D.; Dessimoz, C. Phylo.io: Interactive Viewing and Comparison of Large Phylogenetic Trees on the Web. Mol. Biol. Evol. 2016, 33, 2163–2166. [Google Scholar] [CrossRef]
- Raaum, R.L.; Sterner, K.N.; Noviello, C.M.; Stewart, C.-B.; Disotell, T.R. Catarrhine primate divergence dates estimated from complete mitochondrial genomes. J. Hum. Evol. 2005, 48, 237–257. [Google Scholar] [CrossRef]
- Santourlidis, S. Phylo-Epigenetics in Phylogeny Analyses and Evolution. Genes 2024, 15, 1198. [Google Scholar] [CrossRef]
- Polak, P.; Querfurth, R.; Arndt, P.F. The evolution of transcription-associated biases of mutations across vertebrates. BMC Evol. Biol. 2010, 18, 187. [Google Scholar] [CrossRef] [PubMed]
- Heath, T.A.; Hedtke, S.M.; Hillis, D.M. Taxon Sampling and the Accuracy of Phylogenetic Analyses. J. Syst. Evol. 2008, 46, 239–257. [Google Scholar]
- Steiper, M.E.; Young, N.M. Molecular-clock analyses estimate the Cercopithecoides—Hominoide split at 32 million years ago. Proc. Natl. Acad. Sci. USA 2006, 103, 8870–8875. [Google Scholar]
- Guschanski, K.; Krause, J.; Sawyer, S.; Valente, L.M.; Bailey, S.; Finstermeier, K.; Sabin, R.; Gilissen, E.; Sonet, G.; Nagy, Z.T.; et al. Next-generation museomics disentangles one of the largest primate radiations. Syst. Biol. 2013, 62, 539–554. [Google Scholar] [CrossRef]
- Scally, A.; Dutheil, J.Y.; Hillier, L.W.; Jordan, G.E.; Goodhead, I.; Herrero, J.; Hobolth, A.; Lappalainen, T.; Mailund, T.; Marques-Bonet, T.; et al. Insights into hominid evolution from the gorilla genome sequence. Nature 2012, 483, 169–175. [Google Scholar] [CrossRef]
- Stone, A.C.; Battistuzzi, F.U.; Kubatko, L.S.; Perry, G.H.; Trudeau, E.; Lin, H.; Kumar, S. More reliable estimates of divergence times in Pan using complete mtDNA sequences and accounting for population structure. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 3277–3288. [Google Scholar] [CrossRef]
- Zemojtel, T.; Kiełbasa, S.M.; Arndt, P.F.; Behrens, S.; Bourque, G.; Vingron, M. CpG deamination creates transcription factor-binding sites with high efficiency. Genome Biol. Evol. 2011, 3, 1304–1311. [Google Scholar] [CrossRef]
- Pértille, F.; Da Silva, V.H.; Johansson, A.M.; Lindström, T.; Wright, D.; Coutinho, L.L.; Jensen, P.; Guerrero-Bosagna, C. Mutation dynamics of CpG dinucleotides during a recent event of vertebrate diversification. Epigenetics 2019, 14, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Smith, A. Capturing pluripotency. Cell 2008, 132, 532–536. [Google Scholar] [CrossRef]
- Erichsen, L.; Thimm, C.; Santourlidis, S. Methyl Group Metabolism in Differentiation, Aging, and Cancer. Int. J. Mol. Sci. 2022, 23, 8378. [Google Scholar] [CrossRef]
- Lister, R.; Pelizzola, M.; Dowen, R.H.; Hawkins, R.D.; Hon, G.; Tonti-Filippini, J.; Nery, J.R.; Lee, L.; Ye, Z.; Ngo, Q.-M.; et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 2009, 462, 315–322. [Google Scholar] [CrossRef]
- Wolff, G.L.; Kodell, R.L.; Moore, S.R.; Cooney, C.A. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998, 12, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Jirtle, R.L. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Mol. Cell. Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef]
- Takahashi, Y.; Valencia, M.M.; Yu, Y.; Ouchi, Y.; Takahashi, K.; Shokhirev, M.N.; Lande, K.; Williams, A.E.; Fresia, C.; Kurita, M.; et al. Transgenerational inheritance of acquired epigenetic signatures at CpG islands in mice. Cell 2023, 186, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Horsthemke, B.; Bird, A. Loss of CpG island immunity to DNA methylation induced by mutation. Epigenet. Chromatin 2023, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Branciamore, S.; Rodin, A.S.; Riggs, A.D.; Rodin, S.N. Enhanced evolution by stochastically variable modification of epigenetic marks in the early embryo. Proc. Natl. Acad. Sci. USA 2014, 111, 6353–6358. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life; John Murray: London, UK, 1859. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santourlidis, S. Phylo-Epigenetic Conservation and CpG Erosion in OCT4, SOX2, and hTERT Intragenic CpG Islands: A Waddingtonian Perspective on Mammalian Developmental Evolution. Genes 2025, 16, 1102. https://doi.org/10.3390/genes16091102
Santourlidis S. Phylo-Epigenetic Conservation and CpG Erosion in OCT4, SOX2, and hTERT Intragenic CpG Islands: A Waddingtonian Perspective on Mammalian Developmental Evolution. Genes. 2025; 16(9):1102. https://doi.org/10.3390/genes16091102
Chicago/Turabian StyleSantourlidis, Simeon. 2025. "Phylo-Epigenetic Conservation and CpG Erosion in OCT4, SOX2, and hTERT Intragenic CpG Islands: A Waddingtonian Perspective on Mammalian Developmental Evolution" Genes 16, no. 9: 1102. https://doi.org/10.3390/genes16091102
APA StyleSantourlidis, S. (2025). Phylo-Epigenetic Conservation and CpG Erosion in OCT4, SOX2, and hTERT Intragenic CpG Islands: A Waddingtonian Perspective on Mammalian Developmental Evolution. Genes, 16(9), 1102. https://doi.org/10.3390/genes16091102





