Mitochondrial Complex IV Deficiency Nuclear Type 11 Caused by a Novel Start-Lost Variant in the COX20 Gene
Abstract
1. Introduction
2. Materials and Methods
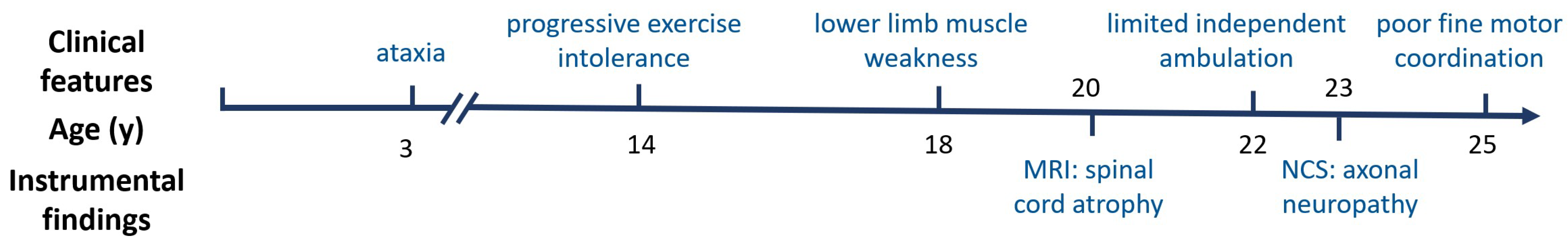
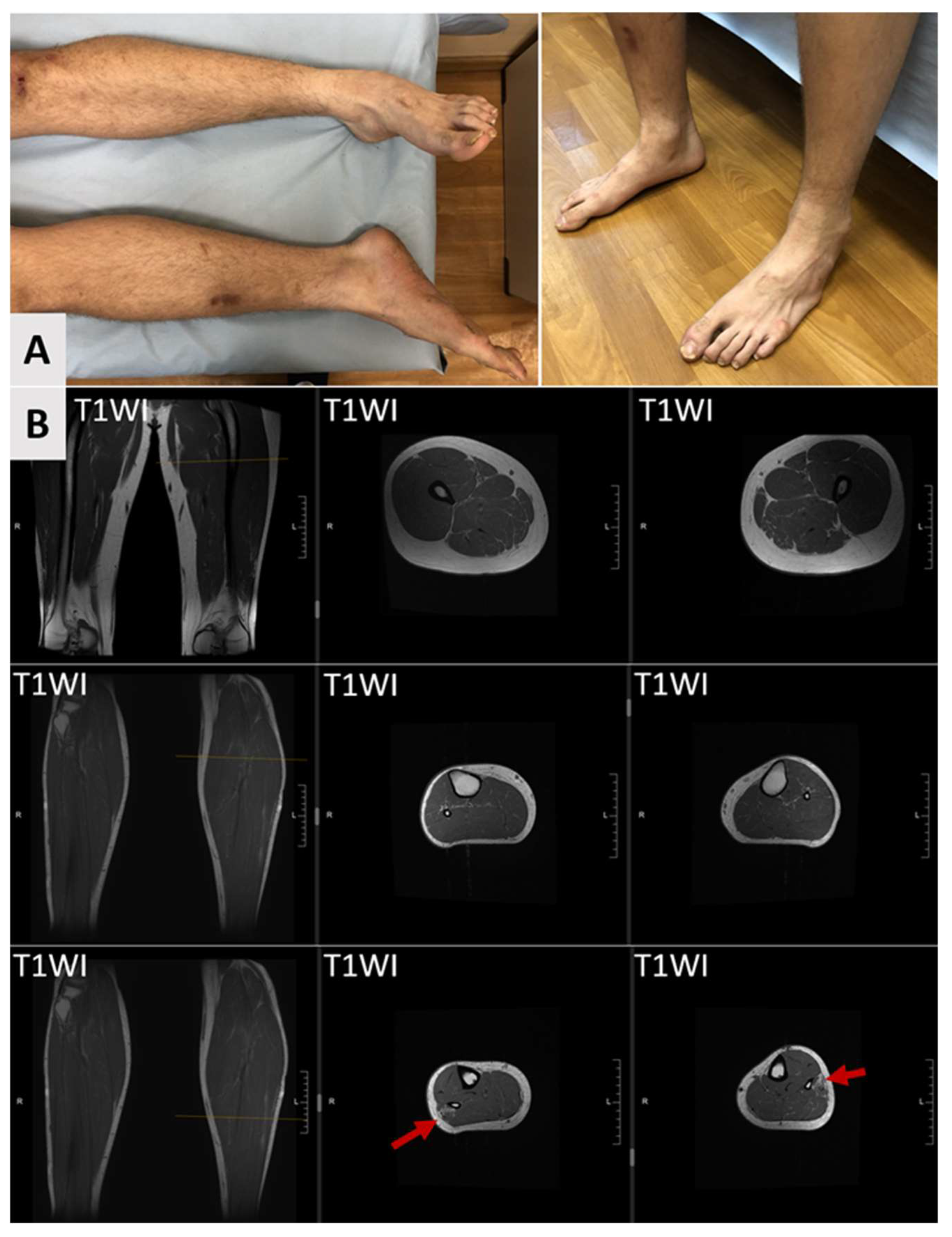
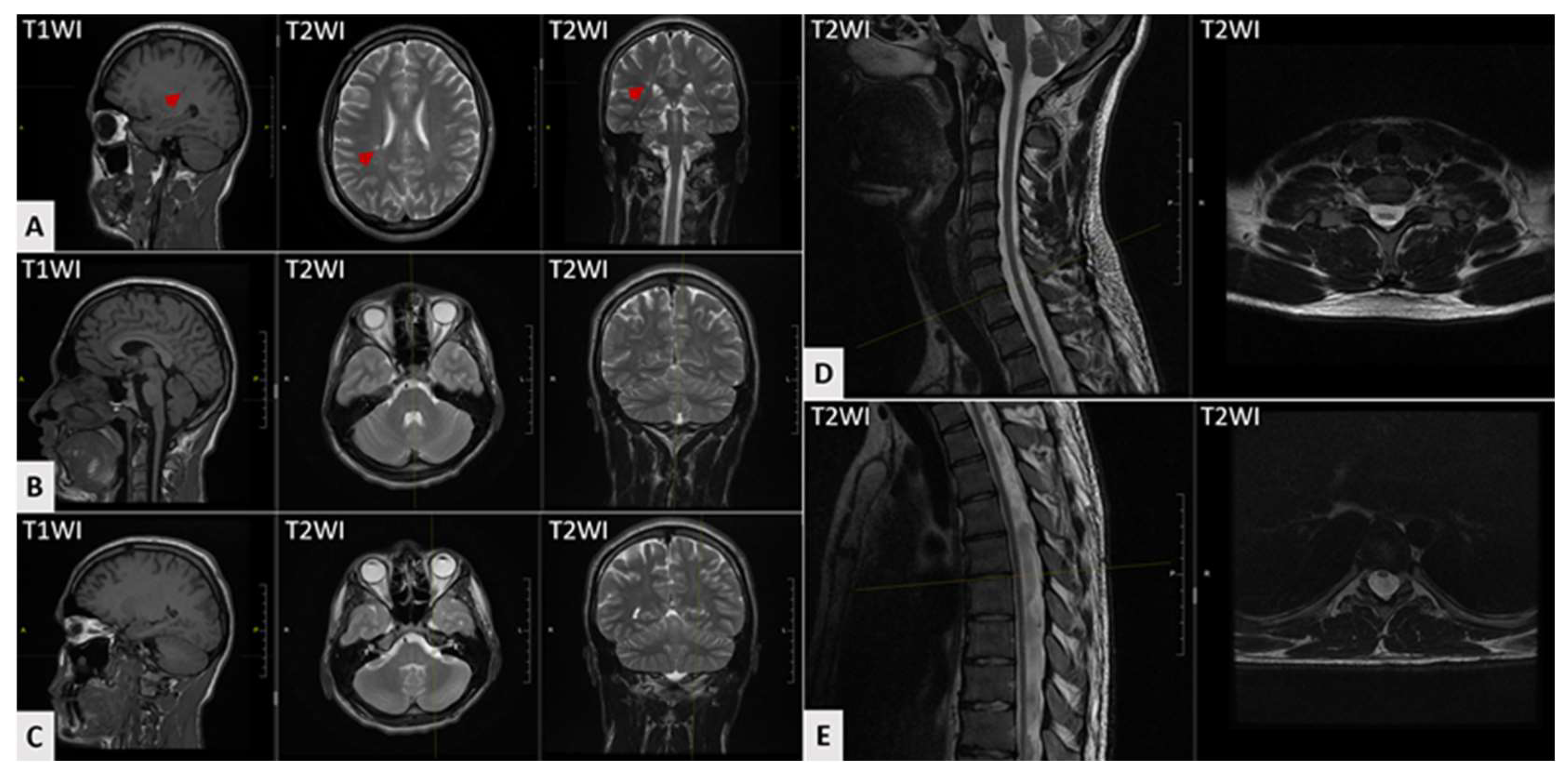
3. Case Report
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timón-Gómez, A.; Nývltová, E.; Abriata, L.A.; Vila, A.J.; Hosler, J.; Barrientos, A. Mitochondrial Cytochrome c Oxidase Biogenesis: Recent Developments. Semin. Cell Dev. Biol. 2018, 76, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ji, T.; Lian, Y.; Wang, S.; Chen, X.; Li, S.; Yin, Y.; Dong, X. Observation of Novel COX20 Mutations Related to Autosomal Recessive Axonal Neuropathy and Static Encephalopathy. Hum. Genet. 2019, 138, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-L.; Ma, Y.; Yu, H.; Wei, Q.; Li, J.-Q.; Liu, G.-L.; Li, H.-F.; Chen, L.; Chen, D.-F.; Bai, G.; et al. Bi-Allelic Loss of Function Variants in COX20 Gene Cause Autosomal Recessive Sensory Neuronopathy. Brain 2021, 144, 2457–2470. [Google Scholar] [CrossRef] [PubMed]
- Ban, R.; Kopajtich, R.; Lv, J.; Stenton, S.L.; Shimura, M.; Wang, Z.; Yuan, Y.; Wang, J.; Han, X.; Liu, Z.; et al. The Phenotypic Spectrum of COX20-Associated Mitochondrial Disorder. Brain 2022, 145, e125–e127. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Guo, D.; Zhang, X.; Ji, K.; Lv, H.; Zhang, Y.; Chen, Z.; Ma, J.; Fang, Y.; Liu, Y. Compound Heterozygous COX20 Variants Impair the Function of Mitochondrial Complex IV to Cause a Syndrome Involving Ophthalmoplegia and Visual Failure. Front. Neurol. 2022, 13, 873943. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y. Clinical and Genetic Characteristics of Children with COX20-Associated Mitochondrial Disorder: Case Report and Literature Review. BMC Med. Genomics 2023, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, R.; Wanschers, B.F.J.; Nijtmans, L.G.; Rodenburg, R.J.; Zschocke, J.; Dikow, N.; van den Brand, M.A.M.; Hendriks-Franssen, M.G.M.; Gilissen, C.; Veltman, J.A.; et al. A Mutation in the FAM36A Gene, the Human Ortholog of COX20, Impairs Cytochrome c Oxidase Assembly and Is Associated with Ataxia and Muscle Hypotonia. Hum. Mol. Genet. 2013, 22, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Naess, K.; Bruhn, H.; Stranneheim, H.; Freyer, C.; Wibom, R.; Mourier, A.; Engvall, M.; Nennesmo, I.; Lesko, N.; Wredenberg, A.; et al. Clinical Presentation, Genetic Etiology, and Coenzyme Q10 Levels in 55 Children with Combined Enzyme Deficiencies of the Mitochondrial Respiratory Chain. J. Pediatr. 2021, 228, 240–251.e2. [Google Scholar] [CrossRef] [PubMed]
- Ozcanyuz, D.G.; Incecik, F.; Herguner, O.M.; Mungan, N.O.; Bozdogan, S.T. Dysarthria, Ataxia, and Dystonia Associated with COX20 (FAM36A) Gene Mutation: A Case Report of a Turkish Child. Ann. Indian Acad. Neurol. 2020, 23, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Logan, R.; Elson, M.J.; Luke, R.R.; Verma, S. Expanding the Genotype–Phenotype Correlation of Childhood Sensory Polyneuropathy of Genetic Origin. Sci. Rep. 2020, 10, 16184. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.G.; Tiongson, E.; Diaz, F.; Haude, K.; Panzer, K.; Collier, A.; Kim, J.; Adams, D.; Tifft, C.J.; Cui, H.; et al. Novel Pathogenic COX20 Variants Causing Dysarthria, Ataxia, and Sensory Neuropathy. Ann. Clin. Transl. Neurol. 2018, 6, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Jayadev, S.; Bird, T.D. Hereditary Ataxias: Overview. Genet. Med. 2013, 15, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Shchagina, O.; Orlova, M.; Murtazina, A.; Filatova, A.; Skoblov, M.; Dadali, E. Evaluation of Pathogenicity and Causativity of Variants in the MPZ and SH3TC2 Genes in a Family Case of Hereditary Peripheral Neuropathy. Int. J. Mol. Sci. 2023, 24, 9786. [Google Scholar] [CrossRef] [PubMed]
- Rezende, T.J.R.; Adanyaguh, I.; Barsottini, O.G.P.; Bender, B.; Cendes, F.; Coutinho, L.; Deistung, A.; Dogan, I.; Durr, A.; Fernandez-Ruiz, J.; et al. Genotype-Specific Spinal Cord Damage in Spinocerebellar Ataxias: An ENIGMA-Ataxia Study. J. Neurol. Neurosurg. Psychiatry 2024, 95, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, J.L.; Barsottini, O.G.P. Spinal Cord Atrophy in Spinocerebellar Ataxia Type 1. Arq. Neuropsiquiatr. 2013, 71, 977. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martins, C.R.; Martinez, A.R.M.; de Rezende, T.J.R.; Branco, L.M.T.; Pedroso, J.L.; Barsottini, O.G.P.; Lopes-Cendes, I.; França, M.C. Spinal Cord Damage in Spinocerebellar Ataxia Type 1. Cerebellum 2017, 16, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Lukas, C.; Hahn, H.K.; Bellenberg, B.; Hellwig, K.; Globas, C.; Schimrigk, S.K.; Köster, O.; Schöls, L. Spinal Cord Atrophy in Spinocerebellar Ataxia Type 3 and 6: Impact on Clinical Disability. J. Neurol. 2008, 255, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
| Clinical Picture | Frequency |
|---|---|
| Age at examination, years (range, mean) | 1–32 (16) |
| Age of onset, years (range, mean) | 0–17 (4) |
| Global developmental delay | 38% (14/32) |
| Intellectual disability | 28% (9/32) |
| Gait disturbance | 65% (20/31) |
| Wheelchair dependency | 26% (8/31) |
| Could not walk without support | 9% (3/31) |
| Ataxia | 97% (28/29) |
| Dysarthria | 58% (18/31) |
| Dystonia | 28% (9/32) |
| Choreoathetosis | 9% (3/32) |
| Muscle hypotonia | 39% (12/31) |
| Seizures | 3% (1/32) |
| Pyramidal signs | 16% (5/31) |
| Limb muscle hypertonia | 13% (6/31) |
| Peripheral motor–sensory neuropathy | 53% (16/30) |
| Muscle weakness | 53% (17/32) |
| Distal muscular hypotrophy/atrophy | 16% (5/31) |
| Foot drop | 23% (7/31) |
| Foot deformity | 42% (13/31) |
| Sensory neuropathy | 33% (10/30) |
| Reduced vibration sense | 81% (9/11) |
| Hyperalgesia | 27% (9/11) |
| Areflexia/hyporeflexia | 74% (23/31) |
| Hyperreflexia | 3% (1/31) |
| Hearing impairment | 6% (2/31) |
| Ptosis | 3% (1/31) |
| Strabismus or ophtalmoparesis | 10% (3/31) |
| Visual impairment | 13% (4/31) |
| Brain and spinal cord MRI | |
| MRI: cerebral atrophy | 28% (5/18) |
| MRI: cerebellar atrophy | 17% (3/18) |
| MRI: T2-hyperintensities at cerebral white matter | 5% (1/18) |
| MRI: T2-hyperintensities at thalamus | 5% (1/18) |
| MRI: spinal cord atrophy | 44% (8/18) |
| Nerve conduction study | |
| NCS: CMAP reduced/absent | 78% (18/23) |
| NCS: SNAP reduced/absent | 100% (24/24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuchina, A.; Borovikov, A.; Sidorova, O.; Orlova, M.; Ryzhkova, O.; Zaigrin, I.; Murtazina, A. Mitochondrial Complex IV Deficiency Nuclear Type 11 Caused by a Novel Start-Lost Variant in the COX20 Gene. Genes 2025, 16, 1069. https://doi.org/10.3390/genes16091069
Kuchina A, Borovikov A, Sidorova O, Orlova M, Ryzhkova O, Zaigrin I, Murtazina A. Mitochondrial Complex IV Deficiency Nuclear Type 11 Caused by a Novel Start-Lost Variant in the COX20 Gene. Genes. 2025; 16(9):1069. https://doi.org/10.3390/genes16091069
Chicago/Turabian StyleKuchina, Anna, Artem Borovikov, Olga Sidorova, Maria Orlova, Oxana Ryzhkova, Igor Zaigrin, and Aysylu Murtazina. 2025. "Mitochondrial Complex IV Deficiency Nuclear Type 11 Caused by a Novel Start-Lost Variant in the COX20 Gene" Genes 16, no. 9: 1069. https://doi.org/10.3390/genes16091069
APA StyleKuchina, A., Borovikov, A., Sidorova, O., Orlova, M., Ryzhkova, O., Zaigrin, I., & Murtazina, A. (2025). Mitochondrial Complex IV Deficiency Nuclear Type 11 Caused by a Novel Start-Lost Variant in the COX20 Gene. Genes, 16(9), 1069. https://doi.org/10.3390/genes16091069





