Genome-Wide Association Study of Chlorophyll Fluorescence and Hyperspectral Indices in Drought-Stressed Young Plants in Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Phenotyping
2.3. GWAS and Canidate Genes
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.-H.; Lee, B.-M. Effects of Climate Change and Drought Tolerance on Maize Growth. Plants 2023, 12, 3548. [Google Scholar] [CrossRef] [PubMed]
- Galić, V.; Mlinarić, S.; Marelja, M.; Zdunić, Z.; Brkić, A.; Mazur, M.; Begović, L.; Šimić, D. Contrasting Water Withholding Responses of Young Maize Plants Reveal Link Between Lipid Peroxidation and Osmotic Regulation Corroborated by Genetic Analysis. Front. Plant Sci. 2022, 13, 804630. [Google Scholar] [CrossRef] [PubMed]
- Ribaut, J.-M.; Betran, J.; Monneveux, P.; Setter, T. Drought Tolerance in Maize. In Handbook of Maize: Its Biology; Bennetzen, J.L., Hake, S.C., Eds.; Springer: New York, NY, USA, 2009; pp. 311–344. ISBN 978-0-387-79417-4. [Google Scholar]
- Li, D.; Quan, C.; Song, Z.; Li, X.; Yu, G.; Li, C.; Muhammad, A. High-Throughput Plant Phenotyping Platform (HT3P) as a Novel Tool for Estimating Agronomic Traits From the Lab to the Field. Front. Bioeng. Biotechnol. 2021, 8, 623705. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, Y.; Du, J.; Guo, X.; Wen, W.; Gu, S.; Wang, J.; Fan, J. Crop Phenomics: Current Status and Perspectives. Front. Plant Sci. 2019, 10, 714. [Google Scholar] [CrossRef]
- Liu, M.; Qi, H.; Zhang, Z.P.; Song, Z.W.; Kou, T.J.; Zhang, W.J.; Yu, J.L. Response of Photosynthesis and Chlorophyll Fluorescence to Drought Stress in Two Maize Cultivars. Afr. J. Agric. Res. 2012, 7, 4751–4760. [Google Scholar] [CrossRef]
- Kondić-Špika, A.; Mikić, S.; Mirosavljević, M.; Trkulja, D.; Marjanović Jeromela, A.; Rajković, D.; Radanović, A.; Cvejić, S.; Glogovac, S.; Dodig, D.; et al. Crop Breeding for a Changing Climate in the Pannonian Region: Towards Integration of Modern Phenotyping Tools. J. Exp. Bot. 2022, 73, 5089–5110. [Google Scholar] [CrossRef]
- Li, Y.; Song, H.; Zhou, L.; Xu, Z.; Zhou, G. Tracking Chlorophyll Fluorescence as an Indicator of Drought and Rewatering across the Entire Leaf Lifespan in a Maize Field. Agric. Water Manag. 2019, 211, 190–201. [Google Scholar] [CrossRef]
- Badr, A.; Brüggemann, W. Special Issue in Honour of Prof. Reto J. Strasser—Comparative Analysis of Drought Stress Response of Maize Genotypes Using Chlorophyll Fluorescence Measurements and Leaf Relative Water Content. Photosynthetica 2020, 58, 638–645. [Google Scholar] [CrossRef]
- Römer, C.; Wahabzada, M.; Ballvora, A.; Pinto, F.; Rossini, M.; Panigada, C.; Behmann, J.; Léon, J.; Thurau, C.; Bauckhage, C.; et al. Early Drought Stress Detection in Cereals: Simplex Volume Maximisation for Hyperspectral Image Analysis. Functional Plant Biol. 2012, 39, 878–890. [Google Scholar] [CrossRef]
- Asaari, M.S.M.; Mertens, S.; Dhondt, S.; Inzé, D.; Wuyts, N.; Scheunders, P. Analysis of Hyperspectral Images for Detection of Drought Stress and Recovery in Maize Plants in a High-Throughput Phenotyping Platform. Comput. Electron. Agric. 2019, 162, 749–758. [Google Scholar] [CrossRef]
- Mertens, S.; Verbraeken, L.; Sprenger, H.; Demuynck, K.; Maleux, K.; Cannoot, B.; De Block, J.; Maere, S.; Nelissen, H.; Bonaventure, G.; et al. Proximal Hyperspectral Imaging Detects Diurnal and Drought-Induced Changes in Maize Physiology. Front. Plant Sci. 2021, 12, 640914. [Google Scholar] [CrossRef]
- Vukadinović, L.; Galić, V.; Brkić, A.; Jambrović, A.; Šimić, D. Comparing Chlorophyll Fluorescence and Hyperspectral Indices in Drought-Stressed Young Plants in a Maize Diversity Panel. Agronomy 2025, 15, 1604. [Google Scholar] [CrossRef]
- Sheoran, S.; Kaur, Y.; Kumar, S.; Shukla, S.; Rakshit, S.; Kumar, R. Recent Advances for Drought Stress Tolerance in Maize (Zea mays L.): Present Status and Future Prospects. Front. Plant Sci. 2022, 13, 872566. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Silva, L. Light Ray Tracing through a Leaf Cross Section. Appl. Opt. 1973, 12, 2950–2954. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Guo, T.; Dzievit, M.J.; Yu, X.; Liu, P.; Price, K.P.; Yu, J. Genetic Dissection of Seasonal Vegetation Index Dynamics in Maize through Aerial Based High-Throughput Phenotyping. Plant Genome 2021, 14, e20155. [Google Scholar] [CrossRef]
- Mérida-García, R.; Gálvez, S.; Solís, I.; Martínez-Moreno, F.; Camino, C.; Soriano, J.M.; Sansaloni, C.; Ammar, K.; Bentley, A.R.; Gonzalez-Dugo, V.; et al. High-Throughput Phenotyping Using Hyperspectral Indicators Supports the Genetic Dissection of Yield in Durum Wheat Grown under Heat and Drought Stress. Front. Plant Sci. 2024, 15, 1470520. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, D.; Liu, M.; Cui, Z.; Sun, D.; Li, C.; Zhang, A.; Cao, H.; Ruan, Y. Genome-Wide Association Study Identified Novel SNPs Associated with Chlorophyll Content in Maize. Genes 2023, 14, 1010. [Google Scholar] [CrossRef]
- Liu, P.; Xiang, C.; Liu, K.; Yu, H.; Liao, Z.; Shen, Y.; Liu, L.; Ma, L. Genome-Wide Association Study Reveals Genetic Basis and Candidate Genes for Chlorophyll Content of Leaves in Maize (Zea mays L.). PeerJ 2024, 12, e18278. [Google Scholar] [CrossRef]
- Alexander, D.H.; Lange, K. Enhancements to the ADMIXTURE Algorithm for Individual Ancestry Estimation. BMC Bioinform. 2011, 12, 246. [Google Scholar] [CrossRef]
- Ganal, M.W.; Durstewitz, G.; Polley, A.; Bérard, A.; Buckler, E.S.; Charcosset, A.; Clarke, J.D.; Graner, E.-M.; Hansen, M.; Joets, J.; et al. A Large Maize (Zea mays L.) SNP Genotyping Array: Development and Germplasm Genotyping, and Genetic Mapping to Compare with the B73 Reference Genome. PLoS ONE 2011, 6, e28334. [Google Scholar] [CrossRef]
- Money, D.; Gardner, K.; Migicovsky, Z.; Schwaninger, H.; Zhong, G.-Y.; Myles, S. LinkImpute: Fast and Accurate Genotype Imputation for Nonmodel Organisms. G3 (Bethesda) 2015, 5, 2383–2390. [Google Scholar] [CrossRef]
- Strasser, R.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation; Taylor & Francis: Abingdon, UK, 2000; p. 445. ISBN 978-0-7484-0821-4. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS; NASA: Washington, DC, USA, 1974. [Google Scholar]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and Model Inversion Methods with Narrowband Optical Indices for Chlorophyll Content Estimation in Closed Forest Canopies with Hyperspectral Data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef]
- Kauth, R.; Thomas, G. The Tasselled Cap—A Graphic Description of the Spectral-Temporal Development of Agricultural Crops as Seen by LANDSAT. In LARS Symposia; IEEE: West Lafayette, IN, USA, 1976. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N. Remote Estimation of Chlorophyll Content in Higher Plant Leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral Vegetation Indices and Novel Algorithms for Predicting Green LAI of Crop Canopies: Modeling and Validation in the Context of Precision Agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for Association Mapping of Complex Traits in Diverse Samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Monier, B.; Casstevens, T.M.; Bradbury, P.J.; Buckler, E.S. rTASSEL: An R Interface to TASSEL for Analyzing Genomic Diversity. J. Open Source Softw. 2022, 7, 4530. [Google Scholar] [CrossRef]
- Gao, X.; Starmer, J.; Martin, E.R. A Multiple Testing Correction Method for Genetic Association Studies Using Correlated Single Nucleotide Polymorphisms. Genet. Epidemiol. 2008, 32, 361–369. [Google Scholar] [CrossRef]
- Gao, X.; Becker, L.C.; Becker, D.M.; Starmer, J.D.; Province, M.A. Avoiding the High Bonferroni Penalty in Genome-Wide Association Studies. Genet. Epidemiol. 2010, 34, 100–105. [Google Scholar] [CrossRef]
- Dyer, S.C.; Austine-Orimoloye, O.; Azov, A.G.; Barba, M.; Barnes, I.; Barrera-Enriquez, V.P.; Becker, A.; Bennett, R.; Beracochea, M.; Berry, A.; et al. Ensembl 2025. Nucleic Acids Res. 2025, 53, D948–D957. [Google Scholar] [CrossRef]
- Woodhouse, M.R.; Cannon, E.K.; Portwood, J.L.; Harper, L.C.; Gardiner, J.M.; Schaeffer, M.L.; Andorf, C.M. A Pan-Genomic Approach to Genome Databases Using Maize as a Model System. BMC Plant Biol. 2021, 21, 385. [Google Scholar] [CrossRef]
- Šimić, D.; Lepeduš, H.; Jurković, V.; Antunović, J.; Cesar, V. Quantitative Genetic Analysis of Chlorophyll a Fluorescence Parameters in Maize in the Field Environments. J. Integr. Plant Biol. 2014, 56, 695–708. [Google Scholar] [CrossRef]
- Galic, V.; Franic, M.; Jambrovic, A.; Ledencan, T.; Brkic, A.; Zdunic, Z.; Simic, D. Genetic Correlations Between Photosynthetic and Yield Performance in Maize Are Different Under Two Heat Scenarios During Flowering. Front. Plant Sci. 2019, 10, 566. [Google Scholar] [CrossRef]
- Fracheboud, Y.; Jompuk, C.; Ribaut, J.M.; Stamp, P.; Leipner, J. Genetic Analysis of Cold-Tolerance of Photosynthesis in Maize. Plant Mol. Biol. 2004, 56, 241–253. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Zhang, N.; Wang, X.; Zhang, Y.; Ding, Y.; Kuai, B.; Huang, X.; Tuberosa, R. Mapping and Validation of the Quantitative Trait Loci for Leaf Stay-Green-Associated Parameters in Maize. Plant Breed. 2017, 136, 188–196. [Google Scholar] [CrossRef]
- Ali, W.; Grzybowski, M.; Torres-Rodríguez, J.V.; Li, F.; Shrestha, N.; Mathivanan, R.K.; de Bernardeaux, G.; Hoang, K.; Mural, R.V.; Roston, R.L.; et al. Quantitative Genetics of Photosynthetic Trait Variation in Maize. J. Exp. Bot. 2025, eraf198. [Google Scholar] [CrossRef]
- Gudi, S.; Saini, D.K.; Halladakeri, P.; Singh, G.; Singh, S.; Kaur, S.; Goyal, P.; Srivastava, P.; Mavi, G.S.; Sharma, A. Genome-Wide Association Study Unravels Genomic Regions Associated with Chlorophyll Fluorescence Parameters in Wheat (Triticum aestivum L.) under Different Sowing Conditions. Plant Cell Rep. 2023, 42, 1453–1472. [Google Scholar] [CrossRef]
- Brkić, A.; Vila, S.; Šimić, D.; Jambrović, A.; Zdunić, Z.; Salaić, M.; Brkić, J.; Volenik, M.; Galić, V. In-Season Predictions Using Chlorophyll a Fluorescence for Selecting Agronomic Traits in Maize. Plants 2025, 14, 1216. [Google Scholar] [CrossRef]
- Galić, V.; Anđelković, V.; Kravić, N.; Grčić, N.; Ledenčan, T.; Jambrović, A.; Zdunić, Z.; Nicolas, S.; Charcosset, A.; Šatović, Z.; et al. Genetic Diversity and Selection Signatures in a Gene Bank Panel of Maize Inbred Lines from Southeast Europe Compared with Two West European Panels. BMC Plant Biol. 2023, 23, 315. [Google Scholar] [CrossRef]
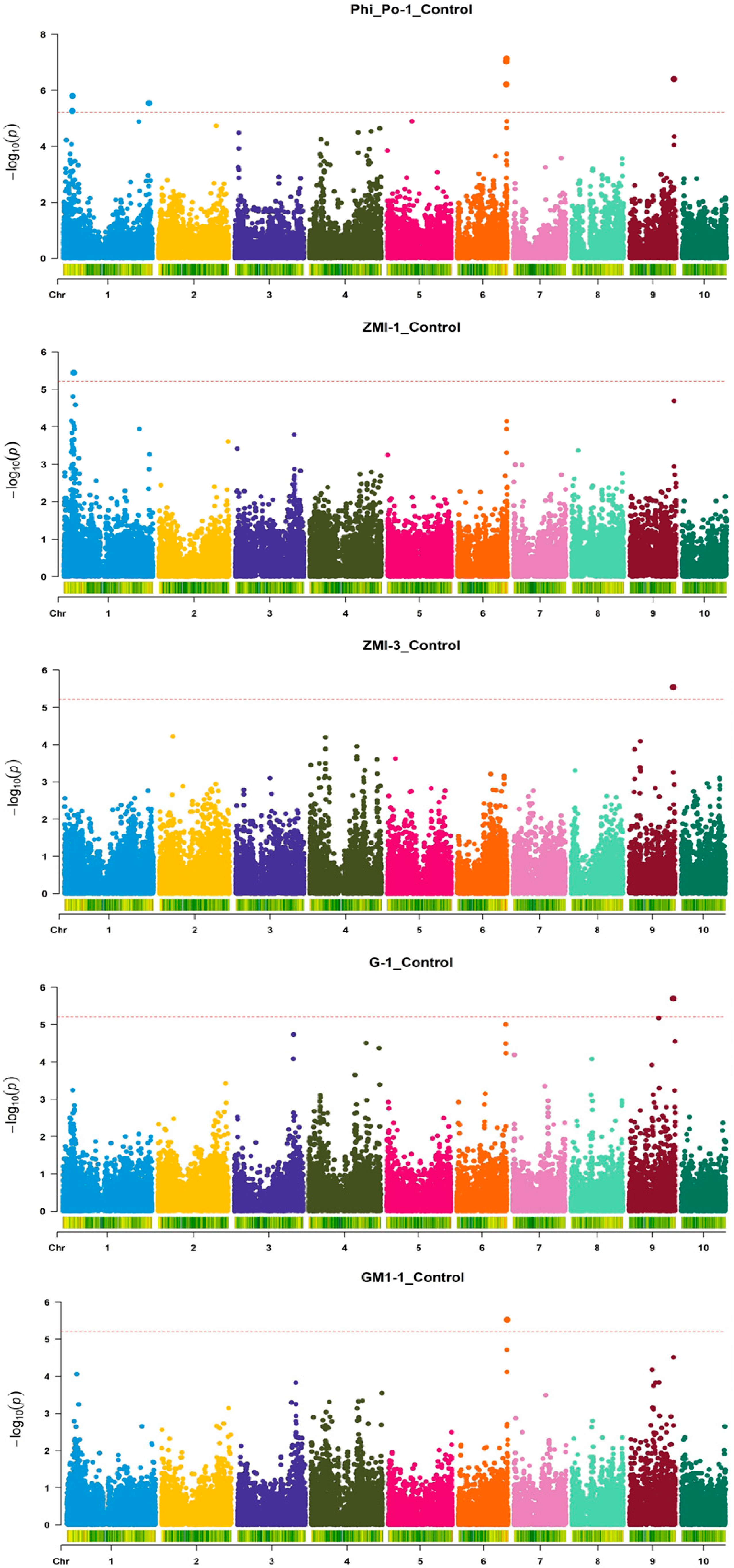

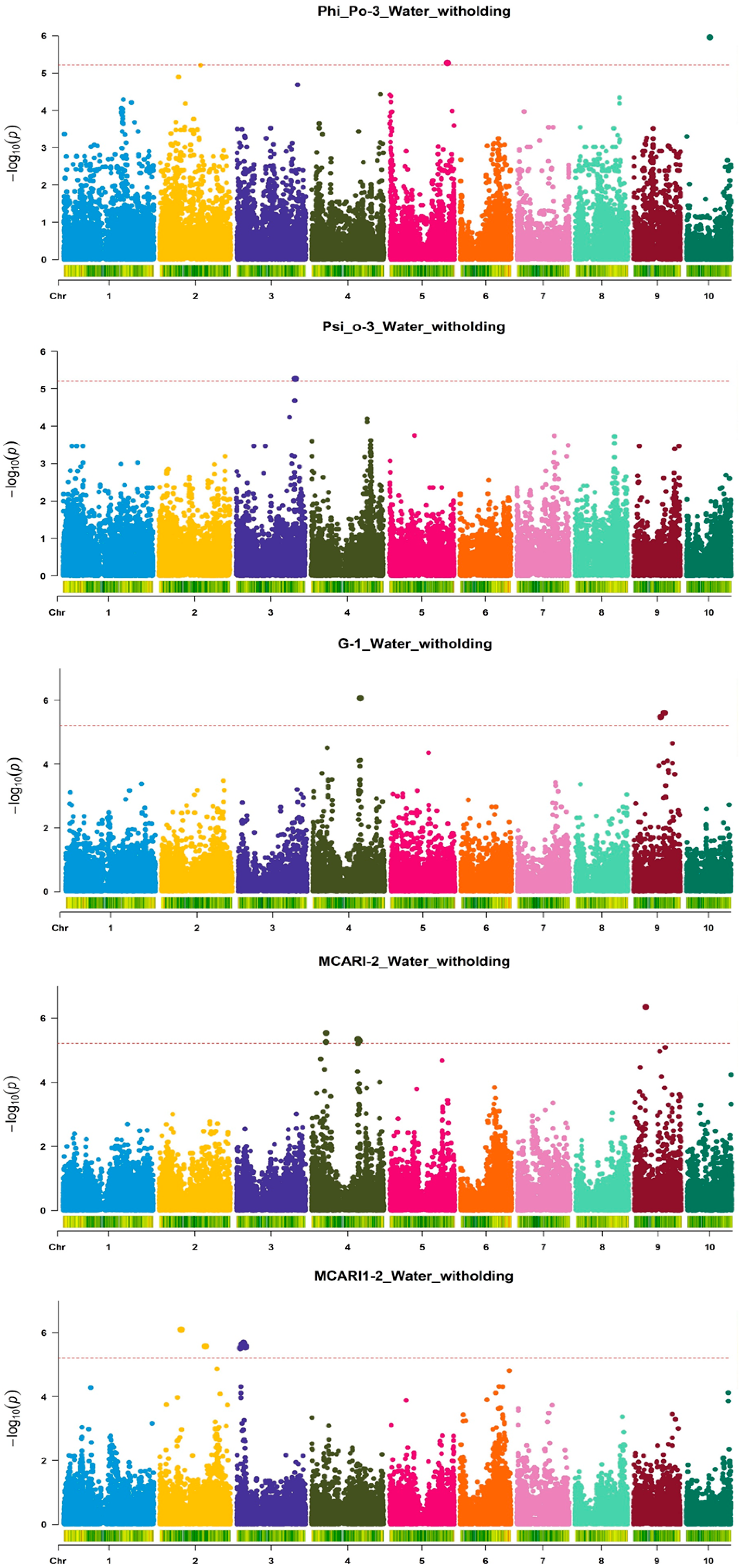
| Index | Treatment | Measurement | Mean | SD 1 | Min | Max | CV |
|---|---|---|---|---|---|---|---|
| ChlF indices | |||||||
| Phi_Po | Control | 1 | 0.81 | 0.02 | 0.69 | 0.84 | 2.30 |
| 2 | 0.79 | 0.02 | 0.69 | 0.84 | 2.93 | ||
| 3 | 0.77 | 0.03 | 0.60 | 0.84 | 4.52 | ||
| Water Withholding | 1 | 0.81 | 0.02 | 0.67 | 0.85 | 2.30 | |
| 2 | 0.77 | 0.04 | 0.57 | 1.21 | 5.53 | ||
| 3 | 0.62 | 0.18 | 0.00 | 1.22 | 29.12 | ||
| Psi_o | Control | 1 | 0.60 | 0.05 | 0.25 | 0.72 | 8.46 |
| 2 | 0.56 | 0.06 | 0.23 | 0.69 | 11.17 | ||
| 3 | 0.52 | 0.09 | 0.13 | 0.70 | 18.24 | ||
| Water Withholding | 1 | 0.59 | 0.06 | 0.30 | 0.71 | 9.59 | |
| 2 | 0.53 | 0.07 | 0.21 | 0.68 | 12.82 | ||
| 3 | 0.42 | 0.12 | 0.01 | 0.80 | 28.91 | ||
| NIR indices | |||||||
| NDVI | Control | 1 | 0.41 | 0.03 | 0.12 | 0.50 | 8.02 |
| 2 | 0.40 | 0.03 | 0.19 | 0.47 | 7.35 | ||
| 3 | 0.37 | 0.05 | 0.06 | 0.49 | 12.31 | ||
| Water Withholding | 1 | 0.40 | 0.03 | 0.28 | 0.47 | 6.58 | |
| 2 | 0.37 | 0.04 | 0.13 | 0.49 | 10.87 | ||
| 3 | 0.33 | 0.06 | 0.09 | 0.46 | 18.75 | ||
| ZMI | Control | 1 | 1.78 | 0.14 | 1.13 | 2.17 | 8.10 |
| 2 | 1.69 | 0.15 | 1.14 | 2.12 | 9.14 | ||
| 3 | 1.56 | 0.19 | 0.89 | 2.04 | 12.14 | ||
| Water Withholding | 1 | 1.73 | 0.13 | 1.32 | 2.20 | 7.63 | |
| 2 | 1.63 | 0.19 | 1.02 | 2.12 | 11.52 | ||
| 3 | 1.45 | 0.23 | 0.91 | 2.04 | 15.80 | ||
| UVIS indices | |||||||
| MCARI | Control | 1 | 1718 | 479 | 137 | 5288 | 28 |
| 2 | 1972 | 588 | 486 | 4694 | 30 | ||
| 3 | 2489 | 915 | 330 | 6627 | 37 | ||
| Water Withholding | 1 | 1844 | 461 | 518 | 4028 | 25 | |
| 2 | 1974 | 760 | 57 | 5821 | 38 | ||
| 3 | 2148 | 893 | 124 | 6414 | 42 | ||
| MCARI1 | Control | 1 | 12,668 | 1081 | 3376 | 16,277 | 9 |
| 2 | 13,052 | 1199 | 3195 | 16,842 | 9 | ||
| 3 | 13,520 | 1723 | 0 | 19,422 | 13 | ||
| Water Withholding | 1 | 12,862 | 1128 | 4328 | 16,797 | 9 | |
| 2 | 12,534 | 1721 | 0 | 17,700 | 14 | ||
| 3 | 11,025 | 2804 | 372 | 17,800 | 25 | ||
| Greenness | Control | 1 | 1.44 | 0.07 | 1.14 | 1.79 | 4.92 |
| 2 | 1.48 | 0.09 | 1.20 | 1.83 | 5.74 | ||
| 3 | 1.54 | 0.11 | 1.22 | 1.91 | 7.16 | ||
| Water Withholding | 1 | 1.45 | 0.07 | 1.21 | 1.73 | 4.52 | |
| 2 | 1.46 | 0.10 | 1.10 | 1.87 | 6.68 | ||
| 3 | 1.40 | 0.13 | 1.05 | 1.74 | 9.27 | ||
| GM1 | Control | 1 | 1.31 | 0.08 | 0.97 | 1.50 | 5.86 |
| 2 | 1.25 | 0.09 | 0.93 | 1.56 | 7.37 | ||
| 3 | 1.17 | 0.11 | 0.75 | 1.47 | 9.31 | ||
| Water Withholding | 1 | 1.29 | 0.08 | 0.95 | 1.48 | 5.83 | |
| 2 | 1.21 | 0.11 | 0.82 | 1.47 | 8.89 | ||
| 3 | 1.18 | 0.11 | 0.82 | 1.49 | 9.70 | ||
| GM2 | Control | 1 | 1.65 | 0.09 | 1.17 | 1.86 | 5.56 |
| 2 | 1.58 | 0.11 | 1.09 | 1.96 | 6.74 | ||
| 3 | 1.50 | 0.14 | 0.85 | 1.81 | 9.54 | ||
| Water Withholding | 1 | 1.62 | 0.09 | 1.12 | 1.84 | 5.71 | |
| 2 | 1.52 | 0.14 | 0.90 | 1.85 | 9.04 | ||
| 3 | 1.40 | 0.18 | 0.90 | 1.85 | 12.76 | ||
| SNP | Position (bp) | Index-Measurement_Treatment 1 | Gene | Description | GO Domain 2 |
|---|---|---|---|---|---|
| SYN6732 | 8,131,266 | Phi_Po-1_C | Zm00001eb002960 | Aconitate hydratase (Aconitase) (EC 4.2.1.3) | MF; CC; BP |
| Zm00001eb002980 | 2Fe-2S ferredoxin-type domain-containing protein | MF | |||
| Zm00001eb002990 | Scarecrow-like protein 6 | MF; CC; BP | |||
| SYN11901 | 8,510,819 | Phi_Po-1_C | Zm00001eb003140 | Eisosome protein SEG2 | BP |
| Zm00001eb003150 | MHD1 domain-containing protein | MF; CC; BP | |||
| Zm00001eb003170 | Proteasome component3; Protein NRT1/PTR FAMILY 5.2 | MF; CC; BP | |||
| SYN5905 | 9,984,596 | Phi_Po-1_C | Zm00001eb003680 | ARM repeat superfamily protein | CC |
| Zm00001eb003690 | Tankyrase 1 | MF; CC; BP | |||
| PZE-101206617 | 255,801,405 | Phi_Po-1_C; ZMI-1_C | Zm00001eb050200 | Gibberellin-regulated protein 1 | CC |
| PZE-101206972 | 256,352,663 | NDVI-1_C | Zm00001eb050290 (Orphan251) | Calmodulin binding protein; (Orphans transcription factor) | MF; CC; BP |
| PZE-101213333 | 263,494,471 | NDVI-1_C | Zm00001eb052020 | DUF4378 domain-containing protein | |
| SYN30053 | 297,383,893 | NDVI-1_C | Zm00001eb061650 | Protein TIFY (Jasmonate ZIM domain-containing protein) | CC; BP |
| Zm00001eb061670 | Rubredoxin-like superfamily protein; Rubredoxin-like domain-containing protein | MF; CC; BP | |||
| SYN30050 | 297,452,662 | NDVI-1_C | Zm00001eb061720 | Rubredoxin-like domain-containing protein | MF; CC; BP |
| Zm00001eb061740 | Phytocyanin domain-containing protein | MF; CC; BP | |||
| Zm00001eb061690 | Rubredoxin-like domain-containing protein | MF; CC; BP | |||
| Zm00001eb061730 | Rubredoxin-like domain-containing protein | ||||
| Zm00001eb061670 | Rubredoxin-like superfamily protein; Rubredoxin-like domain-containing protein | MF; CC; BP |
| SNP | Chr | Position (bp) | Index-Measurement_Treatment 1 | Gene | Annotation | GO Domain 2 |
|---|---|---|---|---|---|---|
| PZE-102083803 | 2 | 71,991,689 | MCARI1-2_W | Zm00001eb085350 (dnaJ_4) | J domain-containing protein; DNAJ heat shock N-terminal domain-containing protein | MF; CC; BP |
| Zm00001eb085360 (NFD4_3) | Major facilitator superfamily protein | CC; BP | ||||
| SYN37731; SYN37721 | 3 | 13,322,314; 13,322,419 | MCARI1-2_W | Zm00001eb123160 | Autophagy-related protein 18c | MF; CC; BP |
| Zm00001eb123170 | RING-type domain-containing protein | MF; CC; BP | ||||
| PZE-103025670 | 3 | 18,270,049 | MCARI1-2_W | Zm00001eb124560 (At2g39795_1) | Mitochondrial glycoprotein | CC |
| Zm00001eb124570 (mcfB_1) | Mitochondrial substrate carrier family protein; Mitochondrial substrate carrier family protein (Protein brittle-1) | MF; CC; BP | ||||
| PZE-103031625 | 3 | 23,789,785 | MCARI1-2_W | Zm00001eb125530 | Mannose-6-phosphate isomerase (EC 5.3.1.8) | MF; CC; BP |
| SYN571; SYN572 | 3 | 29,970,155; 29,970,561 | MCARI1-2_W | Zm00001eb126630 | Uncharacterized protein | CC |
| PZE-103145413 | 3 | 200,419,345 | MCARI1-3_C | Zm00001eb152450 | Aspartate aminotransferase (EC 2.6.1.1) | MF; CC; BP |
| Zm00001eb152460 | Rop guanine nucleotide exchange factor 9; PRONE domain-containing protein | MF; CC; BP | ||||
| PZE-103162732 | 3 | 213,380,441 | Psi_o-3_W | Zm00001eb156520 (RAX3_0) | Transcription factor MYB36 | MF |
| PZE-104005660 | 4 | 1,515,154 | MCARI-2_W | Zm00001eb164580 | protein ALTERED XYLOGLUCAN 4 | - |
| PZE-104080257 | 4 | 154,445,958 | MCARI-2_W | Zm00001eb186100 | STI1 domain-containing protein; Hsp70-Hsp90 organizing protein 3 | MF; CC; BP |
| Zm00001eb186110 | Uncharacterized protein | - | ||||
| PZE-104137089 | 4 | 224,139,502 | G-1_W | Zm00001eb202870 | Protein DETOXIFICATION (Multidrug and toxic compound extrusion protein) | MF; CC; BP |
| SNP | Chr | Position (bp) | Index-Measurement_Treatment 1 | Gene | Annotation | GO Domain 2 |
|---|---|---|---|---|---|---|
| PZE-105011679 | 5 | 5,105,787 | Phi_Po-3_W | Zm00001eb213320 | IQ-domain 5 | MF; CC; BP |
| Zm00001eb213330 | Expp1 protein | MF; CC; BP | ||||
| Zm00001eb213350 | t-SNARE coiled-coil homology domain-containing protein | CC; BP | ||||
| SYN14962 | 5 | 6,920,822 | Phi_Po-3_W | Zm00001eb214310 | Protein arginine N-methyltransferase 2 (Type IV protein arginine N-methyltransferase); RMT2 domain-containing protein | CC; BP |
| Zm00001eb214320 (Orphan326) | protein-serine/threonine phosphatase (EC 3.1.3.16) | MF; BP | ||||
| Zm00001eb214330 | SWIb domain-containing protein | CC | ||||
| Zm00001eb214350 | Gibberellin-regulated protein 10 | CC; BP | ||||
| Zm00001eb214360 | aspartate-semialdehyde dehydrogenase (EC 1.2.1.11) | MF; CC; BP | ||||
| Zm00001eb214370 | TOG domain-containing protein | MF | ||||
| Zm00001eb214380 | TIP41-like family protein | CC; BP | ||||
| SYN26542 | 6 | 35,091,443 | MCARI1-3_C | Zm00001eb265310 | Protein kinase domain-containing protein (wakl32—wall associated kinase like32) | MF; CC; BP |
| SYN11972 | 6 | 88,729,282 | GM2-2_C | Zm00001eb271480 | Caffeoyl-CoA 3-O-methyltransferase 1 | MF; CC; BP |
| SYN4313; SYN4314 | 6 | 165,501,556; 165,501,601 | Phi_Po-1_C; GM1-1_C; GM2-1_C | Zm00001eb290700 | Protein kinase domain-containing protein | MF; CC; BP |
| Zm00001eb290720 | Protein kinase domain-containing protein | MF; CC; BP | ||||
| Zm00001eb290730 | Thioredoxin domain-containing protein | MF; CC; BP | ||||
| Zm00001eb290740 | Secreted protein | - | ||||
| SYN4302 | 6 | 165,504,772 | Phi_Po-1_C | see SYN4313; SYN4314 |
| SNP | Chr | Position (bp) | Index-Measurement_Treatment 1 | Gene | Annotation | GO Domain 2 |
|---|---|---|---|---|---|---|
| PZE-109031829 | 9 | 38,308,890 | ZMI-3_C; MCARI-2_W | Zm00001eb380540 | Protein kinase domain-containing protein | MF; BP |
| Zm00001eb380550 | SMAD/FHA domain-containing protein | MF; CC; BP | ||||
| Zm00001eb380560 | Myb/SANT-like domain-containing protein | - | ||||
| PZE-109060136 | 9 | 102,752,415 | G-1_W | Zm00001eb387560 (abhd17c_5) | Alpha/beta-Hydrolases superfamily protein; Serine aminopeptidase S33 domain-containing protein | MF; CC; BP |
| Zm00001eb387550 | Uncharacterized protein | CC | ||||
| Zm00001eb387540 | Intron maturase type II family protein | MF; CC; BP | ||||
| PZE-109091063 | 9 | 138,752,843 | G-1_W | Zm00001eb395380 | GRAB2 protein | MF; BP |
| PZE-109108465 | 9 | 149,818,933 | Phi_Po-1_C; G-1_C; GM2-2_C; MCARI1-1_C; NDVI-1_C | Zm00001eb399040 (SMD3B_0) | Small nuclear ribonucleoprotein Sm D3 (Sm-D3) (snRNP core protein D3) | MF; CC; BP |
| PZE-110000531 | 10 | 1,417,727 | Phi_Po-3_W | Zm00001eb405060 (NH5.1_1) | Regulatory protein NPR5; BTB domain-containing protein | MF; CC; BP |
| Zm00001eb405080 | Scarecrow-like protein 3 | - | ||||
| Zm00001eb405110 | Scarecrow-like protein 3 | MF; CC; BP | ||||
| Zm00001eb405120 | Uncharacterized protein | CC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukadinović, L.; Galić, V.; Mazur, M.; Jambrović, A.; Šimić, D. Genome-Wide Association Study of Chlorophyll Fluorescence and Hyperspectral Indices in Drought-Stressed Young Plants in Maize. Genes 2025, 16, 1068. https://doi.org/10.3390/genes16091068
Vukadinović L, Galić V, Mazur M, Jambrović A, Šimić D. Genome-Wide Association Study of Chlorophyll Fluorescence and Hyperspectral Indices in Drought-Stressed Young Plants in Maize. Genes. 2025; 16(9):1068. https://doi.org/10.3390/genes16091068
Chicago/Turabian StyleVukadinović, Lovro, Vlatko Galić, Maja Mazur, Antun Jambrović, and Domagoj Šimić. 2025. "Genome-Wide Association Study of Chlorophyll Fluorescence and Hyperspectral Indices in Drought-Stressed Young Plants in Maize" Genes 16, no. 9: 1068. https://doi.org/10.3390/genes16091068
APA StyleVukadinović, L., Galić, V., Mazur, M., Jambrović, A., & Šimić, D. (2025). Genome-Wide Association Study of Chlorophyll Fluorescence and Hyperspectral Indices in Drought-Stressed Young Plants in Maize. Genes, 16(9), 1068. https://doi.org/10.3390/genes16091068






