Selecting Tolerant Maize Hybrids Using Factor Analytic Models and Environmental Covariates as Drought Stress Indicators
Abstract
1. Introduction
2. Materials and Methods
3. Results
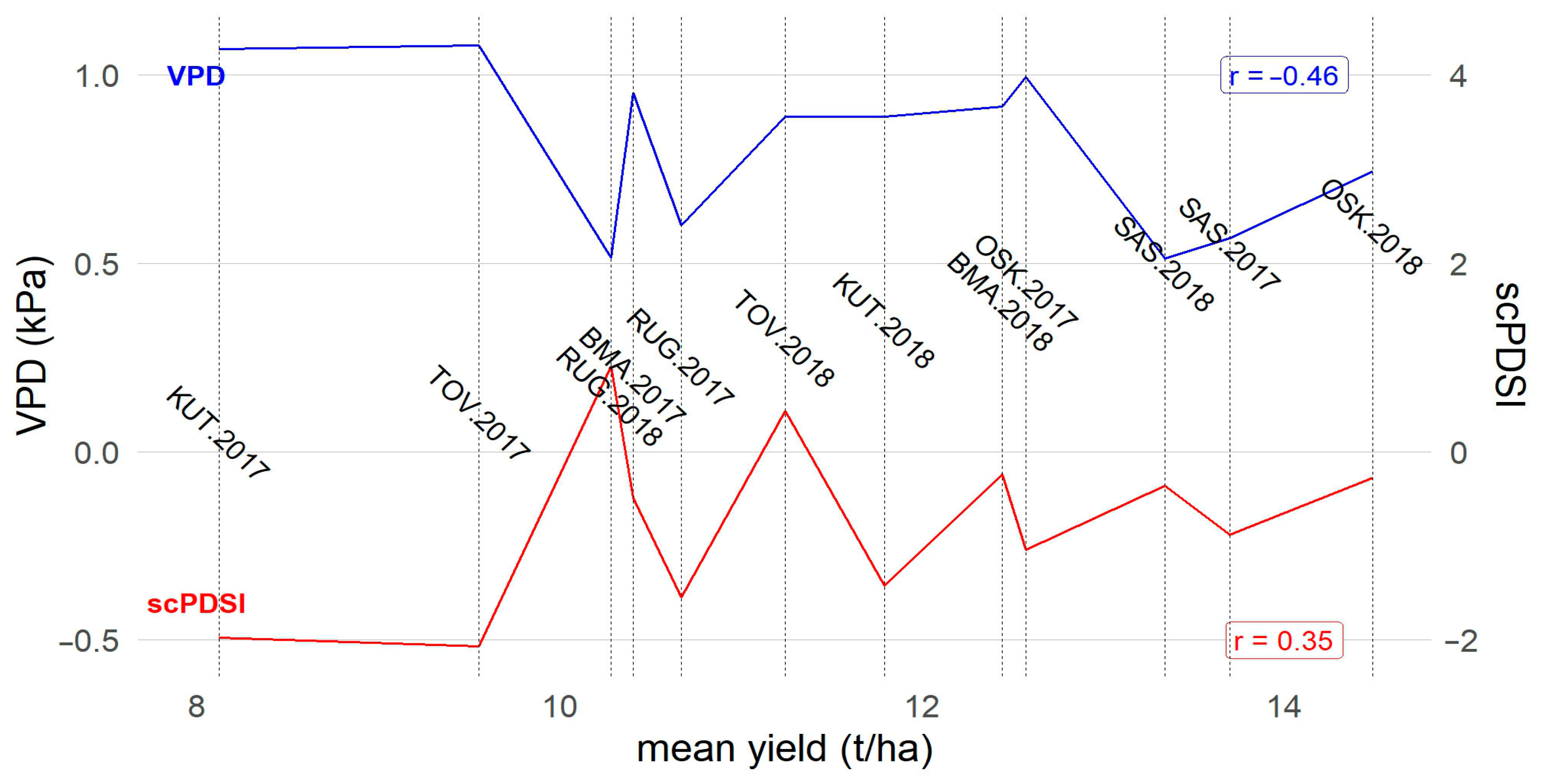
3.1. Environmental Characterization
3.2. MET Analysis
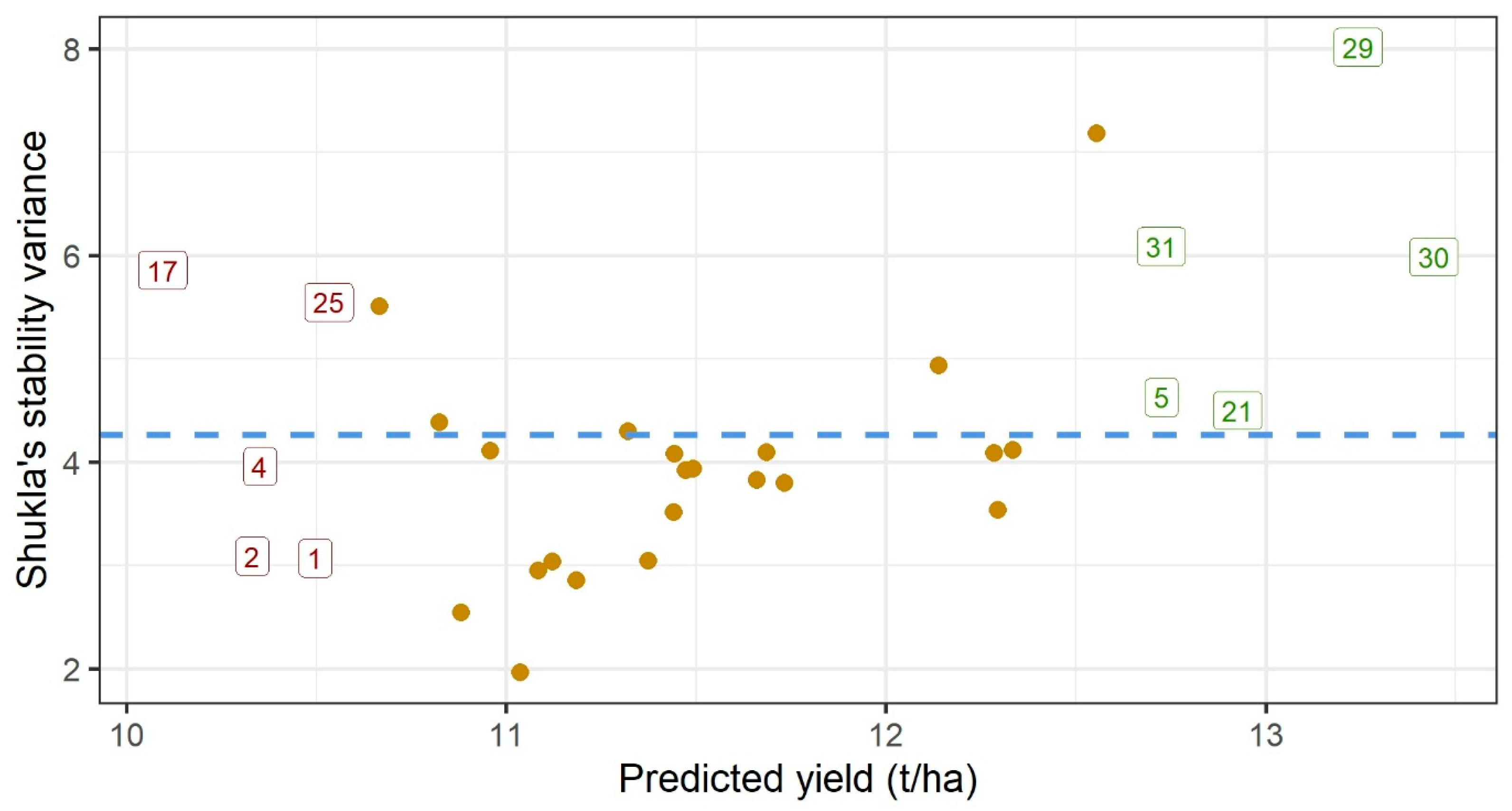
3.3. Yield Stability
3.4. Correlations with Environmental and Genotypic Covariates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edmeades, G.O. Progress in Achieving and Delivering Drought Tolerance in Maize—An Update; ISAAA: Ithaca, NY, USA, 2013. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Summary for Policymakers. In Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2023; pp. 3–32. [Google Scholar] [CrossRef]
- Barker, T.; Campos, H.; Cooper, M.; Dolan, D.; Edmeades, G.; Habben, J.; Schussler, J.; Wright, D.; Zinselmeier, C. Improving drought tolerance in maize. Plant Breed. Rev. 2005, 25, 173–253. [Google Scholar] [CrossRef]
- Xu, Y. Envirotyping for deciphering environmental impacts on crop plants. Theor. Appl. Genet. 2016, 129, 653–673. [Google Scholar] [CrossRef]
- Yue, H.; Gauch, H.G.; Wei, J.; Xie, J.; Chen, S.; Peng, H.; Bu, J.; Jiang, X. Genotype by Environment Interaction Analysis for Grain Yield and Yield Components of Summer Maize Hybrids across the Huanghuaihai Region in China. Agriculture 2022, 12, 602. [Google Scholar] [CrossRef]
- Bänziger, M.; Setimela, P.S.; Hodson, D.; Vivek, B. Breeding for improved drought tolerance in maize adapted to southern Africa Proceedings. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004. [Google Scholar]
- Palmer, W.C. Meteorological Drought; US Department of Commerce; Weather Bureau: Silver Spring, MD, USA, 1965; Volume 45, pp. 1–58.
- Wells, N.; Goddard, S.; Hayes, M.J. A self-calibrating Palmer drought severity index. J. Clim. 2004, 17, 2335–2351. [Google Scholar] [CrossRef]
- van der Schrier, G.; Barichivich, J.; Briffa, K.R.; Jones, P.D. A scPDSI-based global data set of dry and wet spells for 1901–2009. J. Geophys. Res. Atmos. 2013, 118, 4025–4048. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, X.; Pan, Y.; Bai, J.; Li, S. Performance of different drought indices for agriculture drought in the North China Plain. J. Arid Land 2018, 10, 507–516. [Google Scholar] [CrossRef]
- Pandžić, K.; Likso, T.; Curić, O.; Mesić, M.; Pejić, I.; Pasarić, Z. Drought indices for the Zagreb-Grič Observatory with an overview of drought damage in agriculture in Croatia. Theor. Appl. Climatol. 2020, 142, 555–567. [Google Scholar] [CrossRef]
- Pandžić, K.; Likso, T.; Pejić, I.; Šarčević, H.; Pecina, M.; Šestak, I.; Tomšić, D.; Strelec Mahović, N. Application of the self-calibrated palmer drought severity index and standardized precipitation index for estimation of drought impact on maize grain yield in Pannonian part of Croatia. Nat. Hazards 2022, 113, 1237–1262. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements—FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998; Volume 300, p. D05109. [Google Scholar]
- Gholipoor, M.; Choudhary, S.; Sinclair, T.R.; Messina, C.D.; Cooper, M. Transpiration response of maize hybrids to atmospheric vapour pressure deficit. J. Agron. Crop Sci. 2013, 199, 155–160. [Google Scholar] [CrossRef]
- Choudhary, S.; Guha, A.; Kholova, J.; Pandravada, A.; Messina, C.D.; Cooper, M.; Vadez, V. Maize, sorghum, and pearl millet have highly contrasting species strategies to adapt to water stress and climate change-like conditions. Plant Sci. 2020, 295, 110297. [Google Scholar] [CrossRef]
- Galić, V.; Franić, M.; Jambrović, A.; Ledenčan, T.; Brkić, A.; Zdunić, Z.; Šimić, D. Genetic correlations between photosynthetic and yield performance in maize are different under two heat scenarios during flowering. Front. Plant Sci. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Nwayor, I.J.; Robeson, S.M.; Ficklin, D.L.; Maxwell, J.T. A Multiscalar Standardized Vapor Pressure Deficit Index for Drought Monitoring and Impacts. Int. J. Climatol. 2024, 44, 5825–5838. [Google Scholar] [CrossRef]
- Perdomo, J.A.; Carmo-Silva, E.; Hermida-Carrera, C.; Flexas, J.; Galmés, J. Acclimation of biochemical and diffusive components of photosynthesis in rice, wheat, and maize to heat and water deficit: Implications for modelling photosynthesis. Front. Plant Sci. 2016, 7, 1719. [Google Scholar] [CrossRef]
- Yang, Z.; Sinclair, T.R.; Zhu, M.; Messina, C.D.; Cooper, M.; Hammer, G.L. Temperature effect on transpiration response of maize plants to vapour pressure deficit. Environ. Exp. Bot. 2012, 78, 157–162. [Google Scholar] [CrossRef]
- Rosielle, A.A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non-stress environment. Crop Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Fernandez, G.C.J. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and other Food Crops in Temperature and Water Stress, Tainan, Taiwan, 13–18 August 1992; Kuo, C.G., Ed.; [Google Scholar]
- Schneider, K.A.; Rosales-Serna, R.; Ibarra-Perez, F.; Cazares-Enriquez, B.; Acosta-Gallegos, J.A.; Ramirez-Vallejo, P.; Wassimi, N.; Kelly, J.D. Improving common bean performance under drought stress. Crop Sci. 1997, 37, 43–50. [Google Scholar] [CrossRef]
- Hao, Z.F.; Li, X.H.; Su, Z.J.; Xie, C.X.; Li, M.S.; Liang, X.L.; Weng, J.F.; Zhang, D.G.; Li, L.; Zhang, S.H. A proposed selection criterion for drought resistance across multiple environments in maize. Breed. Sci. 2011, 61, 101–108. [Google Scholar] [CrossRef]
- Farshadfar, E.; Poursiahbidi, M.M.; Safavi, S.M. Assessment of drought tolerance in land races of bread wheat based on resistance/tolerance indices. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 143–158. [Google Scholar]
- Sedri, M.H.; Amini, A.; Golchin, A. Evaluation of nitrogen effects on yield and drought tolerance of rainfed wheat using drought stress indices. J. Crop Sci. Biotechnol. 2019, 22, 235–242. [Google Scholar] [CrossRef]
- Grzesiak, S.; Hordyńska, N.; Szczyrek, P.; Grzesiak, M.T.; Noga, A.; Szechyńska-Hebda, M. Variation among wheat (Triticum aestivum L.) genotypes in response to the drought stress: I–selection approaches. J. Plant Interact. 2019, 14, 30–44. [Google Scholar] [CrossRef]
- Smith, A.B.; Cullis, B.R. Plant breeding selection tools built on factor analytic mixed models for multi-environment trial data. Euphytica 2018, 214, 143. [Google Scholar] [CrossRef]
- Dias, K.O.D.G.; Gezan, S.A.; Guimarães, C.T.; Nazarian, A.; da Costa e Silva, L.; Parentoni, S.N.; de Oliveira Guimarães, P.E.; de Oliveira Anoni, C.; Pádua, J.M.V.; de Oliveira Pinto, M.; et al. Improving accuracies of genomic predictions for drought tolerance in maize by joint modeling of additive and dominance effects in multi-environment trials. Heredity 2018, 121, 24–37. [Google Scholar] [CrossRef]
- Mengesha, W.; Menkir, A.; Meseka, S.; Bossey, B.; Afolabi, A.; Burgueno, J.; Crossa, J. Factor analysis to investigate genotype and genotype× environment interaction effects on pro-vitamin A content and yield in maize synthetics. Euphytica 2019, 215, 180. [Google Scholar] [CrossRef]
- Krause, M.D.; Dias, K.O.dG.; dos Santos, J.P.R.; dos Santos, J.P.R.; de Oliveira, A.A. Boosting predictive ability oftropical maize hybrids via genotype-by-environment interaction under multivariate GBLUP models. Crop Sci. 2020, 60, 3049–3065. [Google Scholar] [CrossRef]
- Chaves, S.F.; Evangelista, J.S.; Trindade, R.S.; Dias, L.A.; Guimarães, P.E.; Guimarães, L.J.; Alaves, R.S.; Lopes Bhering, L.; Dias, K.O.G. Employing factor analytic tools for selecting high-performance and stable tropical maize hybrids. Crop Sci. 2023, 63, 1114–1125. [Google Scholar] [CrossRef]
- Oliveira, I.C.M.; Guilhen, J.H.S.; de Oliveira Ribeiro, P.C.; Gezan, S.A.; Schaffert, R.E.; Simeone, M.L.F.; Damasceno, C.M.B.; Carneiro, J.E.D.E.S.; Carneiro, P.C.S.; Parrella, R.A.D.A.C.; et al. Genotype-by-environment interaction and yield stability analysis of biomass sorghum hybrids using factor analytic models and environmental covariates. Field Crops Res. 2020, 257, 107929. [Google Scholar] [CrossRef]
- The VSNi Team. CycDesigN. VSN International Limited. 2025. Available online: https://www.vsni.co.uk/software/cycdesign (accessed on 25 June 2025).
- Gholipoor, M.; Prasad, P.V.; Mutava, R.N.; Sinclair, T.R. Genetic variability of transpiration response to vapor pressure deficit among sorghum genotypes. Field Crops Res. 2010, 119, 85–90. [Google Scholar] [CrossRef]
- Gezan, S.A.; Galli, G.; Murray, D. ASRtriala: An R Package with Complementary Functions for Single and Multi-Environment Trial Analyses, Version 1.0.0; VSN International Ltd.: Hemel Hempstead, UK, 2022. [Google Scholar]
- The VSNi Team. _asreml: Fits Linear Mixed Models Using REML_. R Package Version 4.2.0.257. VSN International Limited. 2025. Available online: https://www.vsni.co.uk/software/asreml-r (accessed on 25 June 2025).
- Cullis, B.R.; Smith, A.B.; Coombes, N.E. On the design of early generation variety trials with correlated data. J. Agric. Biol. Environ. Stat. 2006, 11, 381–393. [Google Scholar] [CrossRef]
- Smith, A.; Cullis, B.; Gilmour, A. Applications: The analysis of crop variety evaluation data in Australia. Aust. N. Z. J. Stat. 2001, 43, 129–145. [Google Scholar] [CrossRef]
- Gogel, B.; Smith, A.; Cullis, B. Comparison of a one-and two-stage mixed model analysis of Australia’s National Variety Trial Southern Region wheat data. Euphytica 2018, 214, 44. [Google Scholar] [CrossRef]
- Smith, A.B.; Ganesalingam, A.; Kuchel, H.; Cullis, B.R. Factor analytic mixed models for the provision of grower information from national crop variety testing programs. Theor. Appl. Genet. 2015, 128, 55–72. [Google Scholar] [CrossRef]
- Matongera, N.; Ndhlela, T.; van Biljon, A.; Labuschagne, M. Genotype x environment interaction and yield stability of normal and biofortified maize inbred lines in stress and non-stress environments. Cogent Food Agric. 2023, 9, 2163868. [Google Scholar] [CrossRef]
- Adnan, A.A.; Diels, J.; Jibrin, J.M.; Kamara, A.Y.; Shaibu, A.S.; Craufurd, P.; Menkir, A. CERES-Maize model for simulating genotype-by-environment interaction of maize and its stability in the dry and wet savannas of Nigeria. Field Crops Res. 2020, 253, 107826. [Google Scholar] [CrossRef]
- Rezende, W.S.; Beyene, Y.; Mugo, S.; Ndou, E.; Gowda, M.; Sserumaga, J.P.; Asea, G.; Ngolinda, I.; Jumbo, M.; Oikeh, S.O.; et al. Performance and yield stability of maize hybrids in stress-prone environments in eastern Africa. Crop J. 2020, 8, 107–118. [Google Scholar] [CrossRef]
- Madhumal Thayil, V.; Zaidi, P.H.; Seetharam, K.; Rani Das, R.; Viswanadh, S.; Ahmed, S.; Miah, M.A.; Koirala, K.B.; Tripathi, M.P.; Arshad, M.; et al. Genotype-by-Environment Interaction Effects under Heat Stress in Tropical Maize. Agronomy 2020, 10, 1998. [Google Scholar] [CrossRef]
- Coulibaly, M.; Bodjrenou, G.; Fassinou Hotègni, N.V.; Akohoue, F.; Agossou, C.A.; Azon, C.F.; Matro, X.; Bello, S.; Adjé, C.O.A.; Sanou, J.; et al. Genotype by environment interaction and stability analysis of three agronomic traits in Kersting’s groundnut (Macrotyloma geocarpum) using factor analytic modeling and environmental covariates. Crop Sci. 2024, 64, 2095–2115. [Google Scholar] [CrossRef]
- Filho, J.S.S.; Oliveira, I.C.M.; Pastina, M.M.; Campos, M.D.S.; de Oliveira, E.J. Genotype x environment interaction in cassava multi-environment trials via analytic factor. PLoS ONE 2024, 19, e0315370. [Google Scholar] [CrossRef]
- Tolhurst, D.J.; Gaynor, R.C.; Gardunia, B.; Hickey, J.M.; Gorjanc, G. Genomic selection using random regressions on known and latent environmental covariates. Theor. Appl. Genet. 2022, 135, 3393–3415. [Google Scholar] [CrossRef]



| Model | No. of Var. Comp. | logLik | AIC |
|---|---|---|---|
| fa1 | 24 | −214.93 | 477.86 |
| fa2 | 35 | −194.68 | 459.35 |
| fa3 | 43 | −187.81 | 461.62 |
| fa4 | 51 | −180.11 | 462.22 |
| fa5 | 59 | −174.09 | 466.18 |
| Location | Year | FA1 | FA2 | Cum. % Gen. Var. |
|---|---|---|---|---|
| Beli Manastir | 2017 | 0.61 | 0.34 | 56.01 |
| 2018 | 1.05 | −0.22 | 78.87 | |
| Kutjevo | 2017 | 0.41 | 0.28 | 38.15 |
| 2018 | 0.79 | −0.25 | 57.45 | |
| Osijek | 2017 | 1.09 | 0.21 | 49.10 |
| 2018 | 0.77 | −0.38 | 75.76 | |
| Rugvica | 2017 | 0.92 | 0.65 | 90.35 |
| 2018 | 1.04 | −0.62 | 80.88 | |
| Šašinovec | 2017 | 1.07 | 0.37 | 72.08 |
| 2018 | 1.14 | −0.40 | 29.92 | |
| Tovarnik | 2017 | 0.85 | 0.51 | 72.77 |
| 2018 | 0.65 | −0.22 | 45.05 |
| Covariate | FA1 | FA2 |
|---|---|---|
| Tmin (°C) | −0.44 | −0.11 |
| Tmax (°C) | −0.32 | 0.14 |
| Tavg (°C) | −0.44 | −0.10 |
| Rainfall (mm) | 0.41 | −0.22 |
| Relative humidity (%) | 0.59 | −0.33 |
| VPD | −0.58 | 0.34 |
| scPDSI | 0.27 | −0.74 |
| Index | β1 (FA1) | β2 (FA2) |
|---|---|---|
| GMP | 0.95 | 0.03 |
| RDY | 0.05 | −0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepinac, D.; Pejić, I.; Pandžić, K.; Likso, T.; Šarčević, H.; Šimić, D.; Bukan, M.; Buhiniček, I.; Jambrović, A.; Marković, B.; et al. Selecting Tolerant Maize Hybrids Using Factor Analytic Models and Environmental Covariates as Drought Stress Indicators. Genes 2025, 16, 754. https://doi.org/10.3390/genes16070754
Stepinac D, Pejić I, Pandžić K, Likso T, Šarčević H, Šimić D, Bukan M, Buhiniček I, Jambrović A, Marković B, et al. Selecting Tolerant Maize Hybrids Using Factor Analytic Models and Environmental Covariates as Drought Stress Indicators. Genes. 2025; 16(7):754. https://doi.org/10.3390/genes16070754
Chicago/Turabian StyleStepinac, Domagoj, Ivan Pejić, Krešo Pandžić, Tanja Likso, Hrvoje Šarčević, Domagoj Šimić, Miroslav Bukan, Ivica Buhiniček, Antun Jambrović, Bojan Marković, and et al. 2025. "Selecting Tolerant Maize Hybrids Using Factor Analytic Models and Environmental Covariates as Drought Stress Indicators" Genes 16, no. 7: 754. https://doi.org/10.3390/genes16070754
APA StyleStepinac, D., Pejić, I., Pandžić, K., Likso, T., Šarčević, H., Šimić, D., Bukan, M., Buhiniček, I., Jambrović, A., Marković, B., Jukić, M., & Gunjača, J. (2025). Selecting Tolerant Maize Hybrids Using Factor Analytic Models and Environmental Covariates as Drought Stress Indicators. Genes, 16(7), 754. https://doi.org/10.3390/genes16070754









