Genetic and Sonographic Insights into First-Trimester Fetal Cystic Hygroma: A Retrospective 30-Year Analysis Using 3D/4D Ultrasound and Cytogenetic Evaluation in Croatia (1993–2023)
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
| Study | Number of Cases | Mean Gestational Age (Weeks) | Total |
|---|---|---|---|
| Reus et al., 1987 [41] | 1 | 12 | 1/1 (100.0%) |
| Pons et al., 1989 [42] | 4 | 11 | 4/4 (100.0%) |
| Bronshtein et al., 1989 [34] | 70 | 12.4 | 10/70 (14.3%) |
| Cullen et al., 1990 [27] | 30 | 10.6 | 15/30 (50.0%) |
| Podobnik et al., 1991 [11] | 2 | 12.4 | 2/2 (100.0%) |
| Shulman et al., 1992 [29] | 32 | 11.2 | 15/32 (46.9%) |
| Rietke et al., 1992 [35] | 22 | 13.1 | 7/22 (31.8%) |
| Johnson et al., 1993 [6] | 68 | 11.9 | 41/68 (60.3%) |
| Malone et al., 2005 [20] | 134 | 12.5 | 67/134 (50.0%) |
| Podobnik et al., 2008 [22] | 35 | 11.8 | 17/35 (48.6%) |
| Scholl et al., 2016 [25] | 212 | 12.3 | 128/212 (60.4%) |
| Sparks et al., 2020 [23] | 127 | 13 | 37/127 (30% with pathological CMA) (29.1%) |
| Malone et al., 2021 [7] | 410 | 12.8 | 230/410 (56.1%) |
| Current study | 405 | 11.5 | 210/405 (51.9%) |
| Total | 1552 | 11.8 | 784/1552 (50.5%) |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buijtendijk, M.F.; Bet, B.B.; Leeflang, M.M.; Shah, H.; Reuvekamp, T.; Goring, T.; Docter, D.; Timmerman, M.G.; Dawood, Y.; Lugthart, M.A.; et al. Diagnostic accuracy of ultrasound screening for fetal structural abnormalities during the first and second trimester of pregnancy in low-risk and unselected populations. Cochrane Database Syst. Rev. 2024, 2024, CD014715. [Google Scholar] [CrossRef] [PubMed]
- Rosati, P.; Guariglia, L. Transvaginal ultrasound detection of septated and non-septated cystic hygroma in early pregnancy. Fetal Diagn. Ther. 1997, 12, 132–135. [Google Scholar] [CrossRef]
- Rosati, P.; Guariglia, L. Prognostic value of ultrasound findings of fetal cystic hygroma detected in early pregnancy by transvaginal sonography. Ultrasound Obstet. Gynecol. 2000, 16, 245–250. [Google Scholar] [CrossRef]
- Podobnik, M.; Singer, Z.; Podobnik-Šarkanji, S.; Bulić, M. First-trimester diagnosis of cystic hygromata by transvaginal sonography. Ultrasound Obstet. Gynecol. 1992, 2, 124–125. [Google Scholar] [CrossRef]
- Bernsthein, H.S.; Filly, R.A.; Goldberg, J.D.; Golbus, M.S. Prognosis of fetuses with a cystic hygroma. Prenat. Diagn. 1991, 11, 349–356. [Google Scholar] [CrossRef]
- Johnson, M.P.; Johnson, A.; Holzgreve, W.; Isada, N.B.; Wapner, R.J.; Treadwell, M.C.; Heegeret, S.; Evans, M.I. First-trimester simple hygroma: Cause and outcome. Am. J. Obstet. Gynecol. 1993, 168, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Malone, C.M.; Mullers, S.; Kelliher, N.; Dalrymple, J.; O’Beirnes, J.; Flood, K.; Malone, F. Euploid first-trimester cystic hygroma: A more benign entity than previously thought? Fetal Diagn. Ther. 2021, 48, 667–671. [Google Scholar] [CrossRef]
- Kohli, E.; Sawal, A.; Kohli, G. Nuchal cystic hygroma in fetus: A case report. Cureus 2024, 16, e56018. [Google Scholar] [CrossRef]
- Tsegaye, M.A.; Adugna, A.N.; Abchale, A.B.; Ayano, S.M.; Tesfay, R.H. A rare case report: An early second trimester cystic hygroma with hydrops fetalis. Radiol. Case Rep. 2024, 20, 632–636. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zheng, J.Q.; Zhang, H.P. A case report of Turner syndrome associated with fetal nuchal cystic hygroma and bilateral syndactyly of the hands and feet. Ital. J. Pediatr. 2019, 45, 85. [Google Scholar] [CrossRef] [PubMed]
- Podobnik, M.; Singer, Z.; Bulić, M.; Kukura, V. Transabdominal placental biopsy in the second and third trimester of pregnancy. J. Perinat. Med. 1991, 19, 455–463. [Google Scholar] [CrossRef]
- Phupong, V.; Sittisomwong, T. Spontaneous resolution of cystic hygroma in 47, XYY fetus. Arch. Gynecol. Obstet. 2007, 276, 77–80. [Google Scholar] [CrossRef]
- Noia, G.; Pellegrino, M.; Masini, L.; Visconti, D.; Manzoni, C.; Chiaradia, G.; Caruso, A. Fetal cystic hygroma: The importance of natural history. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 407–413. [Google Scholar] [CrossRef]
- Chen, Y.N.; Chen, C.P.; Lin, C.J.; Chen, S.W. Prenatal ultrasound evaluation and outcome of pregnancy with fetal cystic hygromas and lymphangiomas. J. Med. Ultrasound 2017, 25, 12–15. [Google Scholar] [CrossRef]
- Lugli, L.; Rossi, C.; Berardi, A.; Pugliese, M.; Ceccarelli, P.L.; Sileo, F.G.; Chiossi, G.; Contu, G.; Calabrese, O.; La Marca, A.; et al. Prenatal multidisciplinary counseling for fetal congenital anomalies: A narrative review. Int. J. Gynaecol. Obstet. 2025, 169, 498–510. [Google Scholar] [CrossRef]
- Kordjalik, P.; Szmyd, B.; Karuga, F.F.; Daszkiewicz, G.; Strzelecka, I.; Respondek-Liberska, M. The value of fetal heart evaluation in fetuses with rare congenital lymphangiomas: A cohort study from a single tertiary center across two decades (1999–2020). J. Clin. Med. 2022, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.C.; Pitzegerd, P.G.; Desa, D.; Filly, R.A.; Golbus, M.S.; Adzick, N.S.; Harrison, M.R. Cervical cystic hygroma in the fetus: Clinical spectrum and outcome. J. Pediatr. Surg. 1990, 25, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Iskender, C.; Tarım, E.; Cok, T.; Yalcinkaya, C.; Kalaycı, H.; Sahin, F. Fetal axillary cystic hygroma: A novel association with triple X syndrome. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94, 955–957. [Google Scholar] [CrossRef] [PubMed]
- Van der Putte, S.C.J. Lymphatic malformation in human fetuses: A study of fetuses with Turner’s syndrome or status Bonnevie-Ullrich. Virchows Arch. 1977, 376, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Malone, F.D.; Ball, R.H.; Nyberg, D.H. First-trimester septated cystic hygroma: Prevalence, natural history and pediatric outcome. Obstet. Gynecol. 2005, 106, 288–294. [Google Scholar] [CrossRef]
- Tsai, H.F.; Kang, L.; Tsai, P.Y.; Cheng, Y.C.; Yzu, C.H.; Chang, C. Prenatal diagnosis of fetal cystic hygroma using three-dimensional ultrasound in 2000–2011. J. Med. Ultrasound 2012, 20, 155–161. [Google Scholar] [CrossRef]
- Podobnik, M.; Singer, Z.; Podobnik-Šarkanji, S.; Bulić, M. First trimester diagnosis of cystic hygromata using transvaginal ultrasound and cytogenetic evaluation. J. Perinat. Med. 1995, 2, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.N.; Lianoglou, B.R.; Adami, R.R.; Pluym, I.D.; Holliman, K.; Duffy, J.; Downum, S.L.; Patel, S.; Faubel, A.; Boe, N.M.; et al. Exome sequencing for prenatal diagnosis in nonimmune hydrops fetalis. N. Engl. J. Med. 2020, 383, 1746–1756. [Google Scholar] [CrossRef]
- Norton, E.; Ziffle, J.; Lianoglou, R.; Devine, P.; Sparks, N. Exome sequencing vs. targeted gene panels for the evaluation of nonimmune hydrops fetalis. Am. J. Obstet. Gynecol. 2022, 226, 128–139. [Google Scholar] [CrossRef]
- Scholl, J.; Chasen, S.T. First-trimester cystic hygroma: Does early detection matter? Prenat. Diagn. 2016, 36, 432–436. [Google Scholar] [CrossRef]
- Sherer, D.M.; Hsieh, V.; Hall, A.; Gerren, A.; Walters, E.; Dalloul, M. Current perspectives of prenatal cell-free DNA screening in clinical management of first-trimester septated cystic hygroma. Int. J. Womens Health 2022, 14, 1499–1518. [Google Scholar] [CrossRef]
- Cullen, M.T.; Green, J.; Whetham, J.; Saafia, C.; Gabrielli, S.; Hobins, J.C. Transvaginal ultrasonographic detection of congenital anomalies in the first trimester. Am. J. Obstet. Gynecol. 1990, 163, 466–476. [Google Scholar] [CrossRef]
- Falcon, O.; Cavoretto, P.; Peralta, C.F.; Csapo, B.; Nicolaides, K.H. Fetal head-to-trunk volume ratio in chromosomally abnormal fetuses at 11 + 0 to 13 + 6 weeks of gestation. Ultrasound Obstet. Gynecol. 2005, 26, 755–760. [Google Scholar] [CrossRef]
- Shulman, L.P.; Emerson, D.S.; Felker, R.E.; Philips, O.P.; Simpson, J.E.; Ellias, S. High frequency of cytogenetic abnormalities in fetuses with cystic hygroma diagnosed in first trimester. Obstet. Gynecol. 1992, 80, 80–82. [Google Scholar]
- Schreurs, L.; Lanno, L.; De CVan Schoubroeck, D.; Deviend, K.; Richter, J. First-trimester cystic hygroma colli: Retrospective analysis in a tertiary center. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 8, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Chevernak, F.A.; Isaacson, G.; Blakemore, K.J.; Breg, W.R.; Hobbins, J.C.; Berkowitz, R.L.; Tortora, M.; Mayden, K.; Mahoney, M.J. Fetal cystic hygroma: Cause and natural history. N. Engl. J. Med. 1983, 309, 822–825. [Google Scholar] [CrossRef]
- Abramowitz, J.C.; Ersof, S.L.; Doyle, D.L.; Smith, D.; Levy, D.L. Congenital cystic hygroma of the neck diagnosed prenatally: Outcome with normal and abnormal karyotype. Prenat. Diagn. 1989, 9, 321–325. [Google Scholar] [CrossRef]
- Cohen, M.M.; Schwartz, S.; Schwartz, M.F.; Smith, D.; Lavy, D.L. Antenatal detection of cystic hygroma. Obstet. Gynecol. Surv. 1989, 44, 481–486. [Google Scholar] [CrossRef]
- Bronshtein, M.; Rotem, S.; Yoffe, N.; Blumenfeld, Z. First-trimester and early second-trimester diagnosis of nuchal cystic hygroma by transvaginal sonography: Diverse prognosis of the septated from nonseptated lesion. Am. J. Obstet. Gynecol. 1989, 161, 78–81. [Google Scholar] [CrossRef]
- Rietke, M.; Van Zalen-Sprock, M.G.; John, M.G.; Van Gut, P.; Herman, P.; Van Gejin, H.P. First-trimester diagnosis of cystic hygroma: Course and outcome. Am. J. Obstet. Gynecol. 1992, 167, 94–98. [Google Scholar] [CrossRef]
- Miller, D.T.; Adam, M.P.; Aradhya, S.; Biesecker, L.G.; Brothman, A.R.; Carter, N.P.; Church, D.M.; Crolla, J.A.; Eichler, E.E.; Epstein, C.J.; et al. Consensus statement: Chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am. J. Hum. Genet. 2010, 86, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Batzir, N.A.; Shohat, M.; Maya, I. Chromosomal microarray analysis (CMA)—A clinical diagnostic tool in the prenatal and postnatal settings. Pediatr. Endocrinol. Rev. 2015, 13, 448–454. [Google Scholar] [PubMed]
- Mellis, R.; Eberhardt, R.Y.; Hamilton, S.J.; McMullan, D.J.; Kilby, M.D.; Maher, E.R.; Hurles, M.; Giordano, J.L.; Aggarwal, V.; Goldstein, D.B. Fetal exome sequencing for isolated increased nuchal translucency: Should we be doing it? BJOG Int. J. Obstet. Gynaecol. 2022, 129, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.; Nazaré, B.; Canavarro, M.C. Parental psychological distress and quality of life after a prenatal or postnatal diagnosis of congenital anomaly: A controlled comparison study with parents of healthy infants. Disabil. Health J. 2012, 5, 67–74. [Google Scholar] [CrossRef]
- Bekkhus, M.; Skreden, M.; Skari, H.; Skreden, E.; Emblem, R.; Haugen, G.; Malt, U.F. Paternal psychological stress after detection of fetal anomaly during pregnancy: A prospective longitudinal observational study. Front. Psychol. 2020, 11, 1848. [Google Scholar] [CrossRef]
- Reuss, A.; Pijpers, L.; Schampers, P.T.; Wladimiroff, J.W.; Sachs, E.S. The importance of chorionic villus sampling after first trimester diagnosis of cystic hygroma. Prenat. Diagn. 1987, 7, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Pons, J.C.; Diallo, A.A.; Eydoux, P.; Rais, S.; Doumerc, S.; Frydman, R.; Papiernik, E. Chorionic villus sampling after first trimester diagnosis of fetal cystic hygroma colli. Eur. J. Obstet. Gynecol. Reprod. Biol. 1989, 33, 141–146. [Google Scholar] [CrossRef] [PubMed]
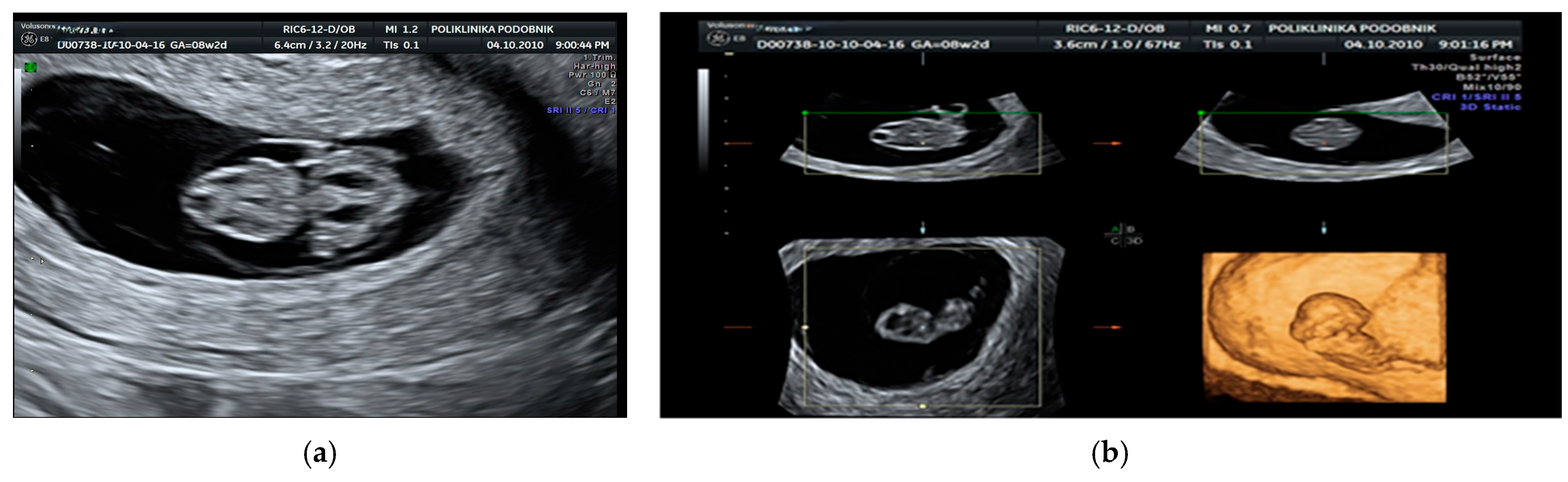
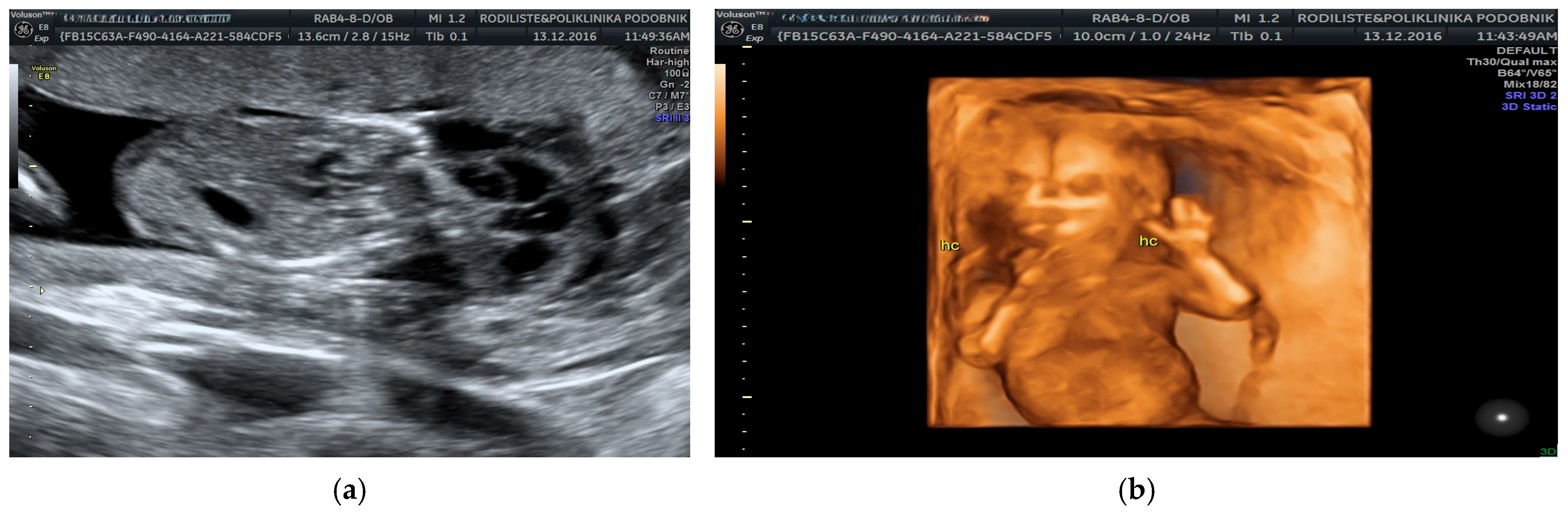

| Category | Count (N) | Percentage (%) |
|---|---|---|
| Total chromosomal abnormalities | 210 | 100 (51.9 as a fraction of all pregnancies) |
| Trisomy 21 | 80 | 38.1 |
| Trisomy 18 | 47 | 22.4 |
| Trisomy 13 | 33 | 16.6 |
| Turner syndrome (45,X) | 30 | 14.2 |
| Turner mosaic | 3 | 1.4 |
| Copy number variants (total) | 5 | 2.3 |
| 10q26.12q26.3 deletion | 1 | 0.4 |
| 5q21.1q34 deletion | 1 | 0.4 |
| 22q11.2 deletion | 1 | 0.4 |
| 22q11.2 duplication | 1 | 0.4 |
| 46,XX, del(2q13) | 1 | 0.4 |
| Other syndromes | 7 | 3.3 |
| Noonan syndrome | 5 | 2.4 |
| Roberts syndrome | 1 | 0.4 |
| Cornelia de Lange syndrome | 1 | 0.4 |
| Structural anomalies (in abnormal karyotypes) | 15 | 7.1 |
| Hydrops, effusion, ascites | 6 | 2.9 |
| Hydrocephalus | 2 | 1.0 |
| Arthrogryposis | 1 | 0.4 |
| Agenesis of corpus callosum | 1 | 0.4 |
| Diaphragmatic hernia | 1 | 0.4 |
| Meromelia | 1 | 0.4 |
| Tetralogy of Fallot | 2 | 1.0 |
| Bilateral hydronephrosis | 1 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podobnik, P.; Meštrović, T.; Podobnik, M.; Lončar, I.; Bertović-Žunec, I.; Kurdija, K.; Jelčić, D.; Srebreniković, Z.; Podobnik-Šarkanji, S. Genetic and Sonographic Insights into First-Trimester Fetal Cystic Hygroma: A Retrospective 30-Year Analysis Using 3D/4D Ultrasound and Cytogenetic Evaluation in Croatia (1993–2023). Genes 2025, 16, 980. https://doi.org/10.3390/genes16080980
Podobnik P, Meštrović T, Podobnik M, Lončar I, Bertović-Žunec I, Kurdija K, Jelčić D, Srebreniković Z, Podobnik-Šarkanji S. Genetic and Sonographic Insights into First-Trimester Fetal Cystic Hygroma: A Retrospective 30-Year Analysis Using 3D/4D Ultrasound and Cytogenetic Evaluation in Croatia (1993–2023). Genes. 2025; 16(8):980. https://doi.org/10.3390/genes16080980
Chicago/Turabian StylePodobnik, Petra, Tomislav Meštrović, Mario Podobnik, Igor Lončar, Ivan Bertović-Žunec, Kristian Kurdija, Dženis Jelčić, Zlata Srebreniković, and Slava Podobnik-Šarkanji. 2025. "Genetic and Sonographic Insights into First-Trimester Fetal Cystic Hygroma: A Retrospective 30-Year Analysis Using 3D/4D Ultrasound and Cytogenetic Evaluation in Croatia (1993–2023)" Genes 16, no. 8: 980. https://doi.org/10.3390/genes16080980
APA StylePodobnik, P., Meštrović, T., Podobnik, M., Lončar, I., Bertović-Žunec, I., Kurdija, K., Jelčić, D., Srebreniković, Z., & Podobnik-Šarkanji, S. (2025). Genetic and Sonographic Insights into First-Trimester Fetal Cystic Hygroma: A Retrospective 30-Year Analysis Using 3D/4D Ultrasound and Cytogenetic Evaluation in Croatia (1993–2023). Genes, 16(8), 980. https://doi.org/10.3390/genes16080980







