1. Introduction
Verticillium wilt (VW), caused by the fungus
V. dahliae, poses a significant threat to cotton production worldwide [
1,
2]. Owing to the intrinsic characteristics of
V. dahliae [
3], its host range is extremely wide, including over 200 economically crucial crops, especially cotton, eggplant, strawberry, tomato, and potato [
4,
5]. These crops are highly susceptible to VW, and, while significant advancements have been made in the identification of the pathogen, understanding its pathogenesis, and developing control strategies, the prevention and management of these diseases still face numerous challenges. Therefore, investigation of the molecular mechanisms underlying cotton VW not only facilitates the breeding of resistant cultivars but also provides valuable insights and potential solutions for enhancing the resistance of a wide range of economically important crops to
V. dahliae.
In plant–pathogen interactions, vesicle trafficking plays a pivotal role in nearly all strategies of plant immune responses subsequent to pathogen attack. Intracellular vesicles mainly originate from endocytosis, whereas extracellular vehicles (EVs), which include exosomes and ectosomes, also constitute significant components of the cellular landscape [
6]. Pathogens secrete vesicles laden with effector proteins, and, in response, plant cells also emit extracellular vehicles (EVs) into the apoplastic space. These EVs are rich in diverse bioactive molecules, including small RNAs (sRNAs), cell wall-degrading enzymes, and proteins that are responsive to both biotic and abiotic stresses [
7]. During the intricate interplay between plants and fungi, the exchange of vesicles containing specific cargo is a common occurrence. Fungal pathogens exploit vesicle trafficking to deliver effector proteins into host cells, thereby manipulating the host’s immune responses to facilitate their colonization and infection [
8]. When fungi form haustoria, plants transport vesicles to the haustorial site to deliver materials that form the haustorial membrane, thereby restricting pathogen invasion [
9,
10]. In the interaction between plants and pathogens, vesicle trafficking plays an essential role in plant immune responses, with a substantial portion of this trafficking being mediated by endocytosis. In this context, RAB GTPases are indispensable for vesicle transport, including the delivery of vesicular effector proteins to infection sites and their subsequent interactions among effector proteins [
11,
12]. Additionally, vesicle-associated membrane proteins in plants play a crucial role in resisting fungal pathogens [
6].
Endocytosis is a highly conserved process in all eukaryotic organisms, facilitating the internalization of extracellular molecules into the cell through vesicles formed by the invagination of the plasma membrane [
13]. This process is pivotal for plant cell signal transduction, intercellular communication, the establishment of cell polarity, and the maintenance of cellular homeostasis [
14]. In plant cells, two primary forms of endocytosis have been characterized: clathrin-mediated endocytosis (CME) and clathrin-independent endocytosis (CIE). The latter category includes processes such as phagocytosis, macropinocytosis, and caveolin-dependent endocytosis [
14,
15,
16].
In recent years, with the increasing research on vesicle trafficking mediated by Rab proteins, the gene families of various Rab GTPases in plants have been extensively studied. In eukaryotic cells, the plant endomembrane system is involved in various intracellular and intercellular biological functions, with vesicle trafficking serving as a crucial carrier for information exchange and material transport. RAB GTPases are key components of membrane trafficking mechanisms, functioning as molecular switches to regulate transport vesicles through reversible transitions between active and inactive states. It has been reported that the human genome encodes nearly 70 Rab GTPases, while the
Arabidopsis thaliana (A. thaliana) genome encodes 57 Rab GTPases. In plants, the RAB members consist of eight categories: RABA, RABB, RABC, RABD, RABE, RABF, RABG, and RABH, corresponding to animal RAB 11, RAB 2, RAB 18, RAB 1, RAB 8, RAB 5, RAB 7, and RAB 6, respectively [
17]. These eight groups are mostly conserved in green plants, with basal plants also harboring additional members, such as RAB23 [
18,
19]. Among them, RAB11 (RABA) is a conserved subfamily containing 26 RAB members, which are further divided into six subgroups (RABA1–RABA6), with RABA1 being the largest subgroup of RABA.
Rab11 (RABA) encompasses a diverse array of functions within plant cells, including involvement in apical growth, cytokinesis, pollen tube elongation, regulation of transport from the trans-Golgi network (TGN) to the plasma membrane, participation in endocytosis, maintenance of TGN morphology and function, and contribution to cell plate formation. In addition to these roles, Rab11 members modulate salt stress tolerance by participating in the trans-Golgi network and plasma membrane, are implicated in auxin signaling, and are engaged in polarized secretion in root hair cells [
17,
20]. These functions are crucial for the normal physiological activities of plant cells, particularly in response to environmental stimuli and during the regulation of cell division [
21]. In addition to the aforementioned functions, research on Rab11 has revealed its close association with plant–pathogen interactions. The budded virions (BV) of Autographa californica multiple nucleopolyhedrovirus (AcMNPV) enter host cells via clathrin-mediated endocytosis. Studies have shown that RAB11 is involved in this process. Downregulation of Rab11 expression by dominant-negative (DN) mutation significantly reduced BV entry into Spodoptera frugiperda cells (Sf9), suggesting that plant Rab11-mediated endocytosis may be exploited by the virus BV as a means of host cell entry. However, the precise mechanism remains to be elucidated [
22]. In contrast, in rice, transgenic plants overexpressing
OsRab11 exhibited resistance to bacterial pathogens such as Pseudomonas syringae pv. tomato DC3000, through the induction of jasmonic acid (JA)-responsive genes. This observation indicates that
OsRab11 is involved in the plant’s defense against pathogens [
23].
3. Results
3.1. Comprehensive Analysis of Downregulated Genes in G. hirsutum Following Infection with V. dahliae
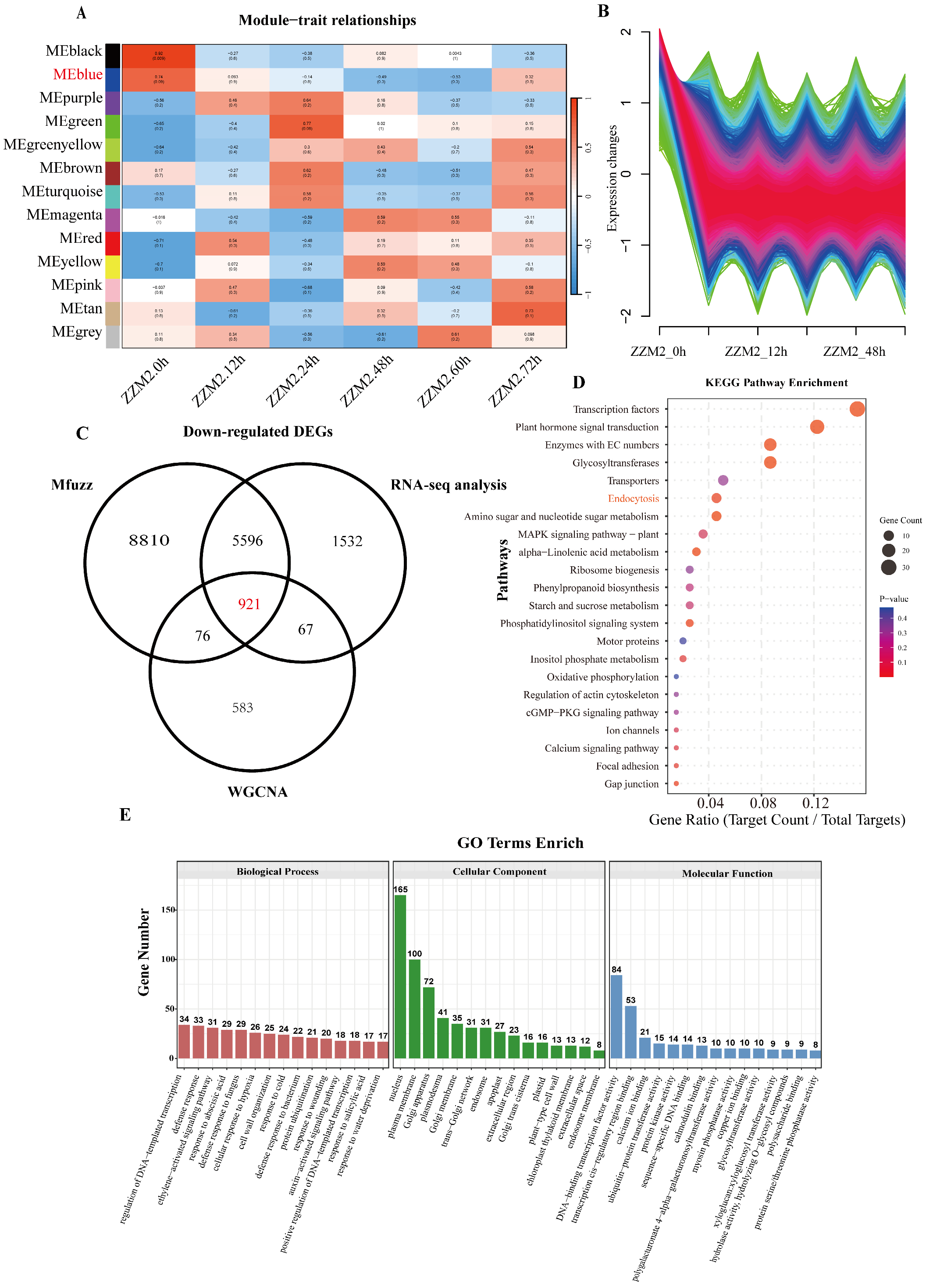
We employed a comprehensive transcriptomic analysis approach, integrating Mfuzz, WGCNA, and RNA-seq analyses, to identify genes with similar expression patterns. By taking the intersection of these three analyses, we conducted a more thorough and in-depth exploration to identify key genes. This integrative approach provides valuable insights into the complex regulatory mechanisms of gene expression and their roles in biological processes.
In this study, transcriptome sequencing was performed on the resistant cultivar Zhongzhimian 2 (ZZM2) of G. hirsutum at three periods (0, 24, and 72 hpi) following infection with the V. dahliae (Vd592). Additionally, we conducted transcriptome sequencing and comparative analysis of ZZM2 at two periods (24 and 72 hpi) of infection with Vd592. Using the criteria of |Log2 (fold-change)| ≥ 1 and false discovery rate (FDR) < 0.05, a total of 8116 downregulated differentially expressed genes (DDEGs) were identified at the 24 and 72 hpi periods.
The transcriptome of ZZM2 at different infection periods after
V. dahliae inoculation was analyzed by weighted gene co-expression network analysis (WGCNA) to identify co-expressed gene modules. WGCNA was employed to delineate the temporal associations between co-expression modules and the distinct infection periods of ZZM2, and to assign differentially expressed genes to their cognate modules. In this study, when the absolute correlation coefficient was ≥0.25 and the
p-value was <0.05, the tissues were defined as tissue-specific modules, resulting in the identification of 13 tissue-specific modules. According to the expression of downregulated genes in the RNA-seq data, the blue module was found to be closely associated with the genes of interest. The final alignment yielded 1647 genes in the blue module (
Figure 1A).
Fuzzy C-means clustering with Mfuzz (Mfuzz) was applied to analyze the expression trends of the transcriptome of ZZM2 infected by
V. dahliae at different infection periods. After removing low-quality data, the fuzzy C-means algorithm was used to retain data points with ACore ≥ 0.5 as significant genes. The expression data were placed into a series of clusters. Based on the expression of downregulated genes in the RNA-seq data, we selected the clusters with the highest similarity in expression pattern (
Figure 1B). As a result, a total of 15,403 genes were identified that exhibited high expression levels at 0 h, followed by a decrease in expression levels and stabilization.
Finally, the intersection of the DEGs obtained from RNA-seq, the 1647 genes from the WGCNA blue module, and the 15,403 genes from the Mfuzz analysis yielded 921 co-expressed genes.
The intersection of these three sets of genes yielded 921 DDEGs, which were subjected to KEGG analysis. The results indicate significant enrichment of transcription factors (TFs) and plant hormone signal transduction pathways, with a considerable number of genes involved (
Figure 1C). Additionally, endocytosis-related genes also showed a certain degree of enrichment. GO analysis revealed that these genes were significantly enriched in biological process categories such as ethylene-activated signaling pathways, defense responses, responses to abscisic acid, defense responses to fungi, defense responses to bacteria, responses to cold, positive regulation of transcription from DNA template, responses to wounding, and auxin-activated signaling pathways (
Figure 1D). These findings suggest that the molecular response mechanisms of cotton to VW may involve enhancing disease resistance through the regulation of ethylene signaling pathways, plant hormone signaling pathways, and gene transcription. KEGG analysis revealed that a large number of genes were involved in pathways including biological metabolism, signal transduction, transport proteins and endocytosis pathways (
Figure 1E).
Further analysis focused on genes related to the endocytosis pathway. We identified nine genes that are both co-expressed and involved in the endocytosis pathway (
Figure 2A). Upon gene identification, Gohir.A03G097101 and Gohir.D02G115600 were identified as uncharacterized proteins in
G. hirsutum; Gohir.A05G232300 and Gohir.D05G234500 were identified as
G. hirsutum phosphatidylinositol 4-phosphate 5-kinase 1 (GhPIP5K1); Gohir.A12G037800 was identified as
G. hirsutum protein Early-responsive to dehydration 7 (GhERD7); and Gohir.D08G111200 was identified as
G. hirsutum probable ADP-ribosylation factor GTPase-activating protein AGD6 (GhAGD6). Additionally, three of these genes were found to belong to the
G. hirsutum ras-related protein RABA1c (
Rab11) gene family and were renamed in this study as follows: Gohir.A06G133200 (GhRab11A06-1), Gohir.A10G034800 (GhRab11A10-3), and Gohir.D06G138600 (GhRab11D06-2). Subsequently, we conducted a comprehensive genome-wide identification of the
Rab11 family and further investigated the expression profiles of
Rab11 family genes in ZZM2 in response to infection by
V. dahliae.
We analyzed the transcriptomic data of the
Rab11 family in ZZM2 at different periods of infection with
V. dahliae and observed that the expression levels of some
Rab11 genes were downregulated (
Figure 2B). To further elucidate the downregulation mechanism of
Rab11 genes, we conducted transcriptomic analyses of the
Rab11 family in three
G. hirsutum cultivars, namely ZZM2, J1, and XLZ, at various periods post-infection with
V. dahliae (see
Figure S1 in Supplementary Materials). Interestingly, although all three cultivars belong to
G. hirsutum, only the disease-resistant cultivar ZZM2 exhibited consistent downregulation of some
Rab11 family genes, by contrast, the susceptible cultivars J1 and XLZ showed no such pattern.
3.2. Identification and Features of Rab11 Genes in Cotton
To identify the
Rab11 genes in
G. hirsutum and
G. barbadense, we conducted a genome-wide prediction by performing BLAST analysis of the
Rab11 gene from
A. thaliana using publicly available genome data. To investigate the evolutionary relationships of Rab11 proteins between
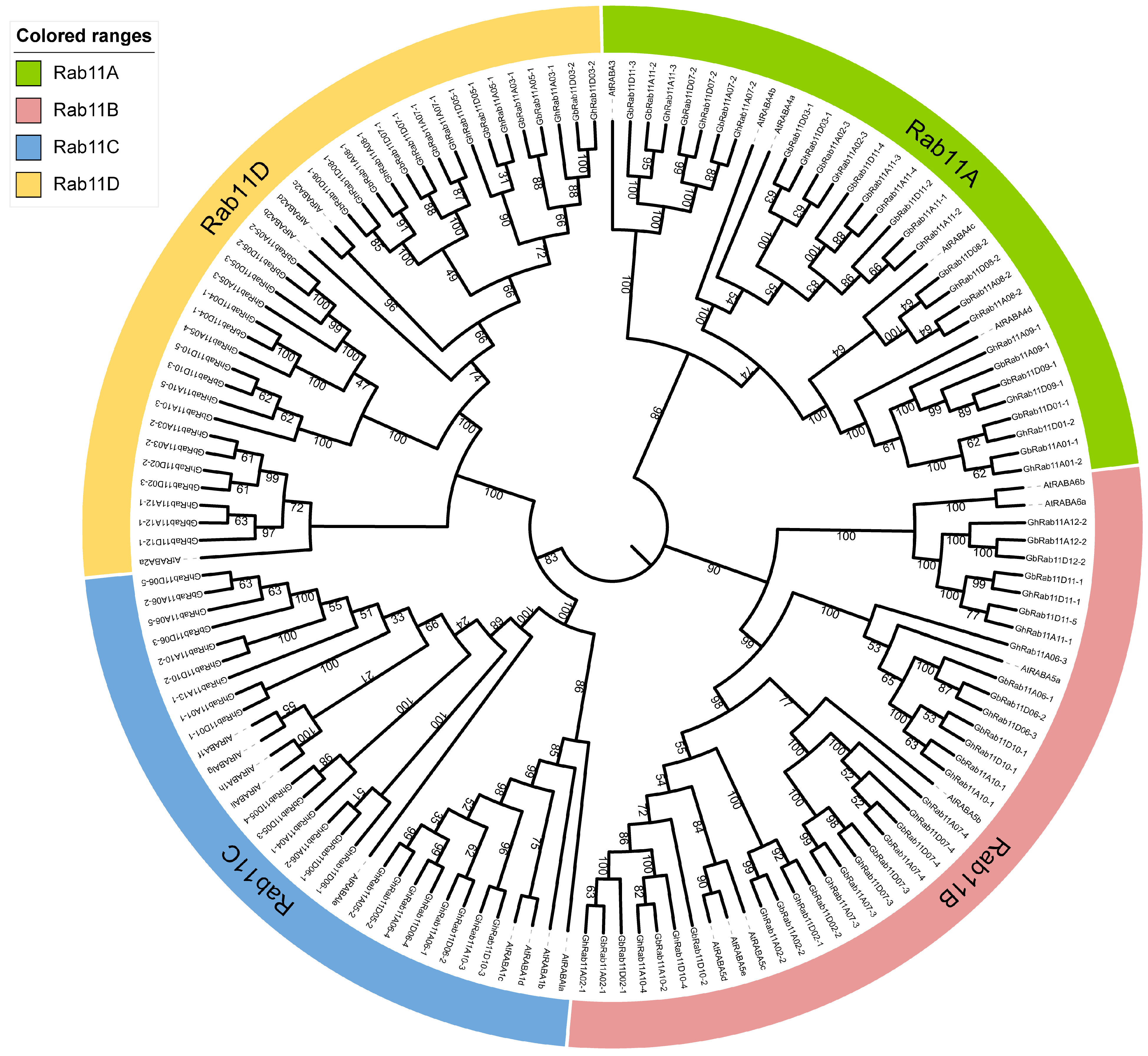
A. thaliana and cotton, we used MEGA 11 software to compare the protein sequences and construct a neighbor-joining (NJ) tree with 1000 bootstrap replicates, as a result, a phylogenetic tree was obtained (
Figure 3). The Poisson model was determined to be the most suitable for phylogenetic tree construction. The constructed phylogenetic tree revealed four distinct sub-clades, namely
Rab11A,
Rab11B, and
Rab11C. We identified 65
GhRab11 genes in
G. hirsutum and 55
GbRab11 genes in
G. barbadense.
Given that
G. hirsutum and
G. barbadense are allo-tetraploids, their gene numbers far exceed those of
A. thaliana, a simple diploid model plant. Driven by species-specific evolutionary differences and functional requirements, the Rab11 protein family, as a member of the small G-protein family, encompasses Rab11A, Rab11B, Rab11C, and Rab11D. These proteins play indispensable roles in intracellular membrane trafficking and are involved in various processes of cotton growth and development, such as cotton fiber development regulation, root tip polar growth, and stress resistance. However, it remains unclear which specific genes are involved in these mechanisms. In particular, there is a paucity of research on the Rab11 protein family in the resistant cotton cultivar ZZM2. We further analyzed the physicochemical properties of Rab11 proteins using the ExPASy website. The results (see
Table S1 in Supplementary Materials) showed that the number of amino acids in
G. hirsutum Rab11 proteins mostly ranged from 200 (GhRab11A13-1) to 236 (GhRab11D07-2), with molecular weights between 18.17 and 28.13 kDa. The predicted isoelectric points (pI) ranged from 4.83 (GhRab11D07-3) to 8.78 (GhRab11A02-3), indicating that the encoded Rab11 proteins may have higher solubility in neutral or weakly acidic environments. The instability index ranged from 17.05 (GhRab11A10-2) to 43.39 (GhRab11A10-5), with most values falling between 20 and 40. This suggests that these proteins may have a certain degree of stability in the cellular environment. Proteins with moderate instability indices may undergo dynamic structural changes within cells, making them suitable for biological processes that require rapid responses. The aliphatic index ranged from 75.53 (GhRab11D10-4) to 92.15 (GhRab11A10-5), with most values between 80 and 90. A higher aliphatic index indicates stronger hydrophobicity of the protein, which may confer greater stability in hydrophobic environments. The average hydrophobicity values ranged from −0.438 (GhRab11A02-1) to −0.173 (GhRab11D10-2), all of which were negative, indicating that these proteins are overall hydrophilic. These are likely to participate in water-soluble reactions within cells, such as enzyme catalysis and signal transduction.
3.3. Chromosomal Localization and Collinearity Relationship Analysis
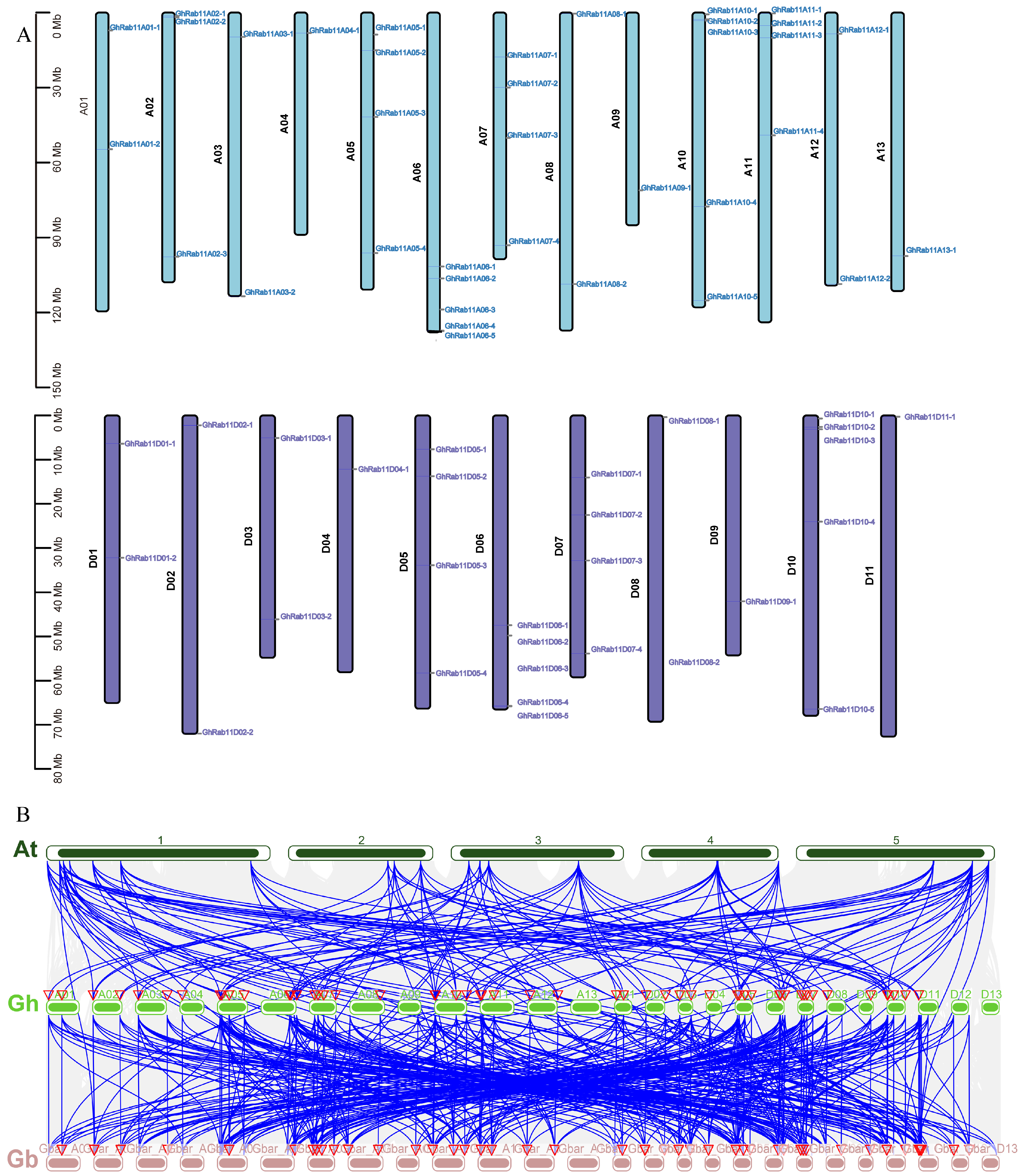
The chromosomal distribution of
GhRab11 is established based on genomic locations (
Figure 4A). In
G. hirsutum, a total of 65
Rab11 genes are unevenly distributed across 24 chromosomes, with 13 on the A subgenome and 11 on the D subgenome. Specifically, 36
GhRab11 genes are located on chromosomes of the A subgenome, and 29
GhRab11 genes are situated on chromosomes of the D subgenome. The comparative genomic collinearity analysis of
A. thaliana,
G. hirsutum, and
G. barbadense illustrates a high degree of collinearity between them
(Figure 4B). Extensive collinear relationships were identified between chromosomes 1–5 of
A. thaliana and the A and D subgenomes of both cotton species. Notably, the
Rab11 genes in
G. hirsutum and
G. barbadense exhibited a high degree of collinearity, further supporting the notion that they share a common ancestor and that their genomic structures have been largely conserved throughout evolution. Observations of multiple chromosomal translocations involving
Rab11 genes in both cotton species and
A. thaliana suggest that these genes may have undergone frequent reorganization and selective pressures in response to long-term environmental adaptation. These translocation events could have facilitated the diversification of
Rab11 genes in cotton, enhancing their functional adaptability under varying environmental conditions. Additionally, these genomic alterations may be closely associated with the evolution of morphological, physiological, and reproductive traits in cotton, playing a pivotal role in the plant’s adaptation to changing environments. Further investigation into Rab11 will aid in a deeper understanding of the mechanisms by which
Rab11 genes have evolved in cotton and how they respond to and adapt to fluctuating environmental conditions.
3.4. Promoter Cis-Regulatory Elements and Gene Structure
We carried out a comprehensive investigation into the structural and functional variety of the
Rab11 gene family, examining aspects such as element distribution, evolutionary relationships, motif distribution, and CDS structure (
Figure 5). By predicting the cis-acting elements and regulatory elements (CREs) in the
GhRab11 promoters and referring to
Figure 5A,B,D, the following results were obtained. All
GhRab11 promoters contain a substantial number of light-responsive elements (including Box 4, G-box, GT1-motif, I-box, LAMP-element, TGACG-motif, WUN-motif, and GATA-motif). This finding strongly suggests that light signaling plays a pivotal role in regulating the expression of the
Rab11 gene family, which is likely closely associated with vital physiological processes such as photomorphogenesis and light-controlled growth in plants. In addition, we have identified numerous elements that are closely related to plant hormone responsiveness, including those responsive to gibberellin (GA), methyl jasmonate (MeJA), abscisic acid (ABA), and auxin (AuX). The majority of these promoters also harbor hormone-responsive elements, such as those responsive to gibberellin (GA-motif, GARE-motif), methyl jasmonate (TGA-element, CGTCA-motif), abscisic acid (ABRE), and auxin (AuxRR-core). As crucial signaling molecules within plants, plant hormones are indispensable in regulating various stages of plant growth and development as well as in responding to environmental stresses. The presence of these hormone-responsive elements indicates that the expression of the
Rab11 gene family may be finely regulated by multiple hormones, thereby participating in the complex physiological regulatory network of plants. In addition to the aforementioned elements, several other functional elements are present. The CCAAT-box, a promoter element, is one of the recognition and binding sites for RNA polymerase II, participating in the initiation and regulation of gene transcription. It is extensively found in the promoter regions of eukaryotic genes. The Myb site, as an MYB binding site, is involved in plant responses to a variety of signals, including light, hormones, and stress signals, and regulates the expression of genes related to plant growth and development as well as stress adaptation. The TC-rich repeats, acting as a silencer element, are involved in the negative regulation of gene expression, possibly by affecting chromatin structure to repress gene transcription. The long terminal repeat (LTR), mainly found in genomic elements such as retrotransposons, may play roles in genome structure and evolution and may also be involved in the regulation of gene expression. The presence of these elements provides strong molecular evidence for the potential functions of the
Rab11 gene family in enabling plants to cope with biotic and abiotic stresses. These elements may activate or repress the transcription of
Rab11 genes, allowing plants to rapidly adjust their physiological and metabolic processes under stress conditions, thereby enhancing their adaptability and stress resistance and ensuring their survival and reproduction in the complex and changing natural environment.
In the category related to abiotic and biotic stress responses, we have also identified a cultivar of elements that are closely connected to stress responses. For instance, elements involved in low-temperature responsiveness and those involved in defense and stress responses. The existence of these elements provides strong molecular evidence for the potential function of the Rab11 gene family in enabling plants to cope with adverse environmental conditions such as drought, low temperature, and pathogen attacks. These elements may activate or repress the transcription of Rab11 genes, allowing plants to rapidly adjust their physiological and metabolic processes under stress conditions, enhance their adaptability and stress resistance, and thus ensure their survival and reproduction in the complex and variable natural environment. Additionally, drought-induced MYB binding sites (MBS, MBSI) are present. The diversity of CREs in the GhRab11 promoters suggests that these genes play important roles in regulating responses to various environmental conditions.
3.5. TF Prediction and PPI in G. hirsutum of DEGs
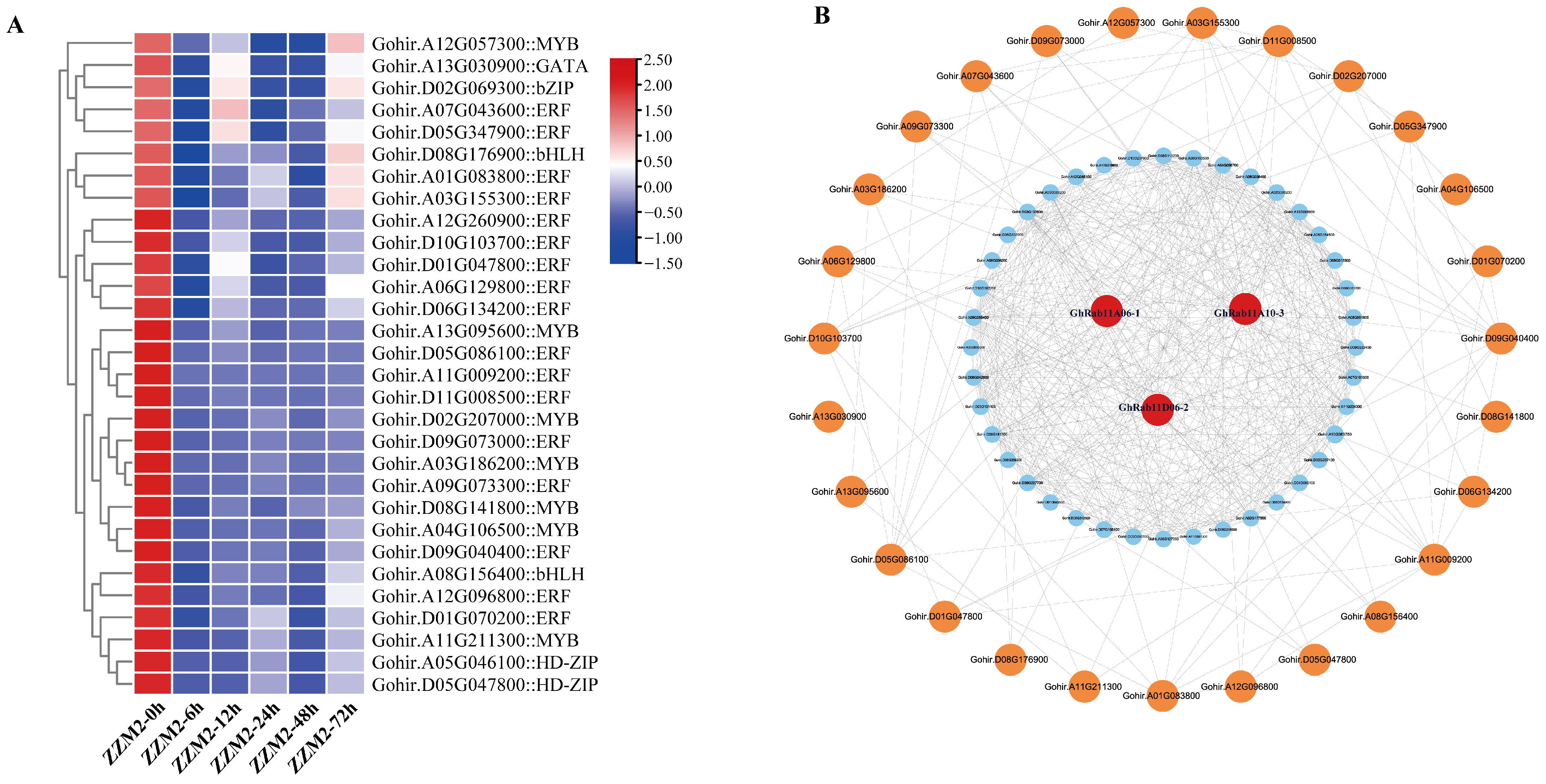
In order to predict the functional modules of the three target genes, based on the mechanism that TFs function usually in clusters combined with PPI for functional module prediction studies, we performed TF prediction and PPI in the
G. hirsutum of DEGs. Based on the comprehensive analysis of downregulated genes in
G. hirsutum following infection with
V. dahliae, The prediction results of the TFs are presented in the following heatmap (
Figure 6A). Protein–protein interaction (PPI) prediction analysis was conducted using three genes—Gohir.A06G133200 (GhRab11A06-1), Gohir.A10G034800 (GhRab11A10-3), and Gohir.D06G138600 (GhRab11D06-2)—from the endocytosis pathways (
Figure 6B). PPI predictive analysis has determined that a total of 30 genes do not directly interact with GhRab11, including GhPIP5K1 (phosphatidylinositol 4-phosphate 5-kinase 1) involved in phosphoinositide signaling, GhAGD6 (probable ADP-ribosylation factor GTPase-activating protein AGD6) associated with small GTPase regulation, GhERD7 (early-responsive to dehydration 7) and dehydration-responsive element-binding proteins—GhDREB1A and GhDREB1D-like—participating in dehydration stress responses, as well as four ethylene-responsive TFs—GhERF3, GhERF4, GhERF5, and GhERF024. Additionally, the interactome encompasses the G-box-binding factor GhGBF4 and members of the Myeloblastosis (MYB)—(GhMYB20, GhMYB30, GhMYB78, GhMYB106)—and Myelocytomatosis (MYC) (GhMYC2-like) transcription factor families. These interacting proteins provide critical targets for elucidating the molecular mechanisms underlying
G. hirsutum defense against
V. dahliae.
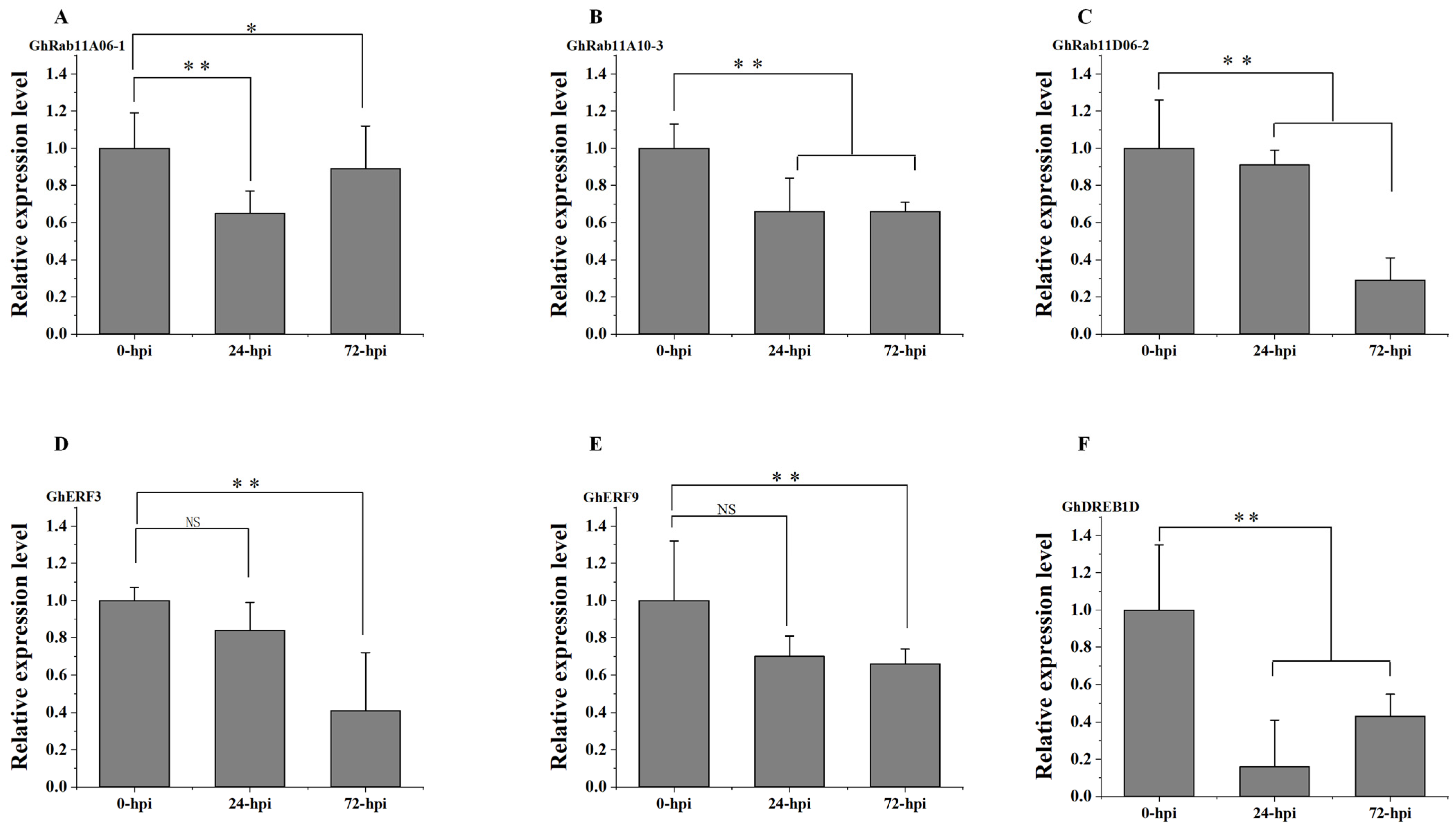
3.6. Validation of Expression Patterns of Rab11-Related Transcription Factors in G. hirsutum
To verify the reliability of the transcriptomic data, we performed qRT-PCR validation on the expression levels of the three
Rab11 genes—GhRab11A06-1 (
Figure 7A), GhRab11A10-3 (
Figure 7B), and GhRab11D06-2 (
Figure 7C)—and three TFs—Gohir.A01G083800 (GhERF3) (
Figure 7D), Gohir.A03G155300 (GhERF9) (
Figure 7E), and Gohir.A12G260900 (GhDREB1D) (
Figure 7F)—which do not directly interact with the three
Rab11 genes. These results indicate that the relative expression levels of the three
Rab11s and the three TFs at the three periods (0, 24 and 72 hpi) after
V. dahliae infection are consistent with the transcriptome data analysis. Compared with 0 hpi, their expression levels were markedly reduced at both 24 hpi and 72 hpi.
5. Discussion
Combining the qRT-PCR validation experiments with the similar results of RNA-seq analyses, and given that genes with similar expression patterns may be functionally related or co-regulated in the pathway, we categorized the 30 genes predicted by the TFs to investigate the functions of the two types of TFs that accounted for a higher percentage of the TFs and found the following.
The ethylene-responsive TFs GhERF3, GhERF4, GhERF5, and GhERF024 belong to the ERF subfamily, with typical AP2/ERF structural domains [
28]. These regulate target gene expression by recognizing GCC-box (AGCCGCC) or DRE/CRT elements. Most ERF TFs activate or repress the expression of downstream defense genes through positive or negative regulation, thereby enhancing plant responses to biotic and abiotic stresses. They play a key role in pathogen defense signaling. The potato StERF94 transcription factor belongs to the ERF family of proteins, and overexpression enhanced the expression of PR-associated defense genes, thereby enhancing potato resistance to
Fusarium solani [
29]. It has also been shown that potato StERF94 overexpression increased the tolerance of transgenic plants, especially in leaves, to drought, high temperature and combined stresses [
30]. In cotton, GhERF1b is positively regulated by the GhMPK9-GhRAF39_1-GhWRKY40a module, which enhances the plant immune response by upregulating the expression of pathogenesis-related (PR) genes (PR1, PR2, PR3), and cotton is more susceptible to
V. dahliae after silencing of GhERF1b by VIGS [
31]. The ERF subfamily appears to increase plant disease resistance through positive regulation in
G. hirsutum–V. dahliae interactions. However, the expression of GhERF subfamily genes fluctuates and decreases within 72 h of infection with
V. dahliae in the disease-resistant cotton variety ZZM2, a phenomenon that deserves further in-depth study.
Promoter cis-regulatory element analysis combined with TF prediction revealed that the MYB binding site (MBS) is a drought-responsive element recognized by MYB TFs, and that MYB mediates the abscisic acid (ABA)-dependent drought response through the ABA-responsive element (ABRE). GhMYB20, GhMYB30, GhMYB78, GhMYB106 belong to the MYB TFs, which are a large gene family in plants and are widely involved in a variety of biological processes [
32]. It has been shown by functional validation that GH_A11G1361 (GhMYB4) can participate in cotton phenol biosynthesis by regulating several key genes in the cotton phenol synthesis pathway [
33]. Gossypol, a canonical terpenoid phytoalexin in
G. hirsutum, is rapidly synthesized and accumulates upon pathogen challenge or environmental stress, exhibiting broad-spectrum antibacterial, antifungal, and antiviral activities. As a central component of the chemical defense arsenal against biotic stress in cotton, gossypol biosynthesis is therefore hypothesized to be modulated by MYB TFs to enhance resistance to
V. dahliae. Nevertheless, this hypothesis remains to be rigorously tested.
Investigations into Rab11 have been extensively conducted in animals. Among Rab small G proteins, Rab11 directs the exocytic and recycling processes, thus controlling both the secretion and composition of plasma membrane [
34]. In particular, Rab11 small GTPases are involved in the exocytic transport of lipids, receptors and transporters [
35,
36,
37,
38,
39,
40,
41,
42]. Given the crucial role of Rab11 in cellular transport processes, it has become a target for hijacking and exploitation by pathogens. Within the first line of defense, which is composed of epithelial and endothelial barriers, Bacillus anthracis and Cholera toxin disrupt the integrity of the membrane barrier and induce tissue disintegration by interfering with the Rab11-mediated transport of cell junction components [
43,
44]. During viral infections, the transport function of Rab11 is either exploited or subverted by pathogens. For instance, Salmonella typhimurium enhances its invasion efficiency by utilizing Rab11-mediated transport processes [
45]. Hantavirus accomplishes viral budding or release by hijacking Rab11-mediated recycling endosomes [
41,
46]. In contrast, Shigella flexneri disrupts Rab11-mediated transport of antimicrobial peptides [
47] and mediates an early transport defect interfering with the arrival of cargo at prevacuoles.
In the study of vacuolar transport pathways in tobacco leaf epidermal cells, it was found that mutant Rab11 mediates early transport defects, affecting the delivery of cargo to the prevacuolar compartment [
48]. Concurrently, the Rab11 protein is essential for transport from the trans-Golgi network (TGN) to the plasma membrane (PM) [
49]. The research on the interaction between Rab11 and pathogens in plants is still not fully adequate. However, based on some research conclusions, during the long-term coevolution of plants and pathogens, plants have continuously evolved complex defense mechanisms to resist pathogen invasion, while pathogens have correspondingly developed a cultivar of strategies to evade or disrupt the plant defense system. Among these, the Rab11 protein plays a key role in vesicular transport within plant cells. It is not only involved in the normal growth and development of plants and responses to abiotic stress, but also plays an important role in the interaction between plants and pathogens. Studies have shown that pathogens can interfere with the function of Rab11 by secreting effector proteins, thereby disrupting the plant immune response. For example, we found that Rab11 in plants interacts with the Phytophthora infestans effector RXLR24, during which the effector RXLR24 interferes with the secretion and transport of the host cell’s pathogenesis-related protein PR1, thereby disrupting the host immune response [
21]. OsRab11 regulates the transport of intracellular vesicles to affect the transmission of JA-related signaling molecules, thereby activating the expression of downstream defense genes [
23]. The interaction between pathogens and the Rab11 protein is an important aspect of the mutual game between plants and pathogens during their long-term coevolution, reflecting the complex molecular-level struggle between plants and pathogens.
Overall, especially in the study of biotic stresses on plants, Rab11 has been attracting attention, and it is interesting to note that downregulation of Rab11 expression in plant–virus interactions showed that plants were more resistant to disease, while plants overexpressing OsRab11 in rice–fungus interactions were more resistant to disease. Therefore, we speculate that Rab11 may play a “double-edged sword” role in plant–pathogen interactions. In this study, it was found that Rab11 was affected by V. dahliae. This result, combined with the current research status of Rab11 interaction between plants and pathogens, leads us to speculate that the function of plants in ZZM2—actively regulating the gene expression of Rab11—is reduced by V. dahliae infection. This reduction takes place in order to avoid this expression becoming a tool for pathogens to invade the host, instead becoming a source of disease resistance. The present study lays the foundation for elucidating the role of Rab11 genes in VW resistance in cotton through the TF prediction and PPI of three GhRab11 genes. In this study, the expression profile and potential regulatory network of the GhRab11 family under VW stress were systematically mapped for the first time, which lays the foundation for elucidating its role in the vesicular transport–immunity cross-pathway, and provides candidate genes and regulatory elements for cotton disease resistance breeding, which is of great significance in providing a theoretical basis and genetic resources for cotton disease resistance breeding.